Abstract
The efficiency of gene transfer into human hematopoietic stem cells by oncoretroviral vectors is too low for effective gene therapy of most hematologic diseases. Retroviral vectors based on the nonpathogenic foamy viruses (FV) are an alternative gene-transfer system. In this study, human umbilical cord blood CD34+ cells were transduced with FV vectors by a single 10-h exposure to vector stocks and then injected into sublethally irradiated nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice. At 5–7 weeks after transplantation, high transgene expression rates were observed in engrafted human hematopoietic cells, including over 60% of clonogenic progenitors. Significant transgene silencing did not occur. We developed an approach for expanding human cell populations derived from transplanted mice to show that multiple SCID repopulating cells (SRCs) had been transduced, including some that were capable of both lymphoid and myeloid differentiation. These findings demonstrate for the first time that human pluripotent (lympho-myeloid) hematopoietic stem cells repopulate NOD/SCID mice and can be efficiently transduced by FV vectors.
Spumaviruses or foamy viruses (FV) are nonpathogenic retroviruses with a wide tissue tropism commonly found in mammals (1, 2), although humans are not a natural reservoir of infection (3, 4), and there is no evidence of human-to-human transfer (5, 6). FV vectors transduce nondividing cells more efficiently than oncoretroviral vectors, are not inactivated by human serum, and have a large packaging capacity (7). We previously developed methods for the production of high titer, helper-free FV vectors (8) and found that they could efficiently transduce murine hematopoietic stem cells (HSCs) (9). However, the high HSC transduction rates obtained in mice by other vector types have not been reproduced in primates, prompting us to test FV vectors in human cells.
One model currently used to study human hematopoiesis is xenotransplantation of immunodeficient nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice (10, 11). These mice support multilineage human hematopoiesis for several weeks, and the low transduction rates of SCID repopulating cells (SRCs) by oncoretroviral vectors indicate that it is an excellent preclinical gene therapy model of human repopulating cells (12). SRCs have proliferative capacities, repopulation kinetics, and cell-surface phenotypes that suggest they are true HSCs (12–14); however, multipotential differentiation has not been demonstrated conclusively by analysis of vector integration patterns in lineage-specific progeny cells. Here, we show that FV vectors efficiently transduce SRCs, and that clonal lymphomyeloid repopulation occurs with marked cells as expected for transduced pluripotent HSCs.
Materials and Methods
Hematopoietic Cell Culture and Transductions.
Human CD34+ cells were isolated from umbilical cord blood under an Institutional Review Board-approved protocol by using a CD34+ cell isolation kit as per the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Transductions were performed for 10 h in CH296-coated (5 μg/cm2, Takara Shuzo, Otsu, Japan) 6-well plates with 0.75–1.5 × 106 cells per well in 1.5 ml of concentrated FV vector stock resuspended in DMEM supplemented with 20% (vol/vol) FBS (HyClone), 100 ng/ml flt-3 ligand (FL), and 100 ng/ml stem-cell factor (SCF). After transductions, colony assays were performed in methylcellulose (Methocult H4230; StemCell Technologies, Vancouver) supplemented with 50 ng/ml SCF, 10 ng/ml granulocyte-macrophage colony stimulating factor, 10 ng/ml IL-3, and 2 units/ml erythropoietin. Colonies were scored on a fluorescent microscope 12–14 days later. Pretransplantation marking rates also were determined by flow cytometry of cells grown for 5 days in DMEM with 20% (vol/vol) FBS, 100 ng/ml FL, 100 ng/ml SCF, 20 ng/ml IL-3, and 20 ng/ml IL-6.
Bone marrow was harvested from the femurs, tibias, and iliac crests of mice at 5–7 weeks after transplantation, and nucleated cells were isolated by lysing red blood cells. Colony assays were performed as described above on human CD34+-sorted cells from transplanted mice.
To generate B cell lines, CD19+/green fluorescent protein-positive (GFP+)-sorted cells from transplanted mice were cultured at a concentration of 1–3 × 105 cells per ml in lymphoid growth medium [Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 20% (vol/vol) heat-inactivated (56°C for 30 min) human serum (HS)/100 ng/ml FL/20 ng/ml IL-3/20 ng/ml IL-7] for 7–9 days. Cultures then were divided into 150-μl aliquots in 96-well plates, 50 μl of B95–8 conditioned media [containing Epstein–Barr virus (EBV) and generated from B95–8 cells, as described (15), except that IMDM with 20% (vol/vol) FBS was used] was added to each well, and cytokines were replenished. When media began to acidify, volumes were doubled by the addition of lymphoid growth medium, and cultures were transferred into progressively larger wells. Once cultures were established in 6-well plates, their growth was usually cytokine-independent, and they were maintained in IMDM supplemented with 20% (vol/vol) FBS. Expanded myeloid cells were produced by culturing either CD34+/GFP+ or CD34+/GFP−-sorted cells from transplanted mice at 5 × 104 to 2 × 105 cells per ml in IMDM supplemented with 10% (vol/vol) human serum/50 ng/ml SCF/50 ng/ml FL/20 ng/ml MGDF/10 ng/ml IL-6, as described (16).
All media contained 100 units per ml penicillin G, 100 μg/ml streptomycin, and 1.25 μg/ml amphotericin B. All cytokines were recombinant human.
Vector Stock Production.
FV vector stocks were produced by calcium phosphate cotransfection of 293T cells with packaging plasmid pCGPES and vector plasmid pCGPMscvF and concentrated as described (8, 9, 17). Neither construct encodes the essential FV transactivator Bel1, so replication-competent retrovirus cannot arise during vector production, as previously demonstrated by marker rescue assay (8). Titers were determined by infection of 293T cells with dilutions of vector stock and flow cytometry quantification of GFP expression 72 h later.
Transplantation of Mice.
Transplant recipients were 6–10 week NOD/LtSz-scid/scid mice (NOD/SCID), sublethally irradiated with 350 cGy from a 137Cs source (GammaCell 40, AEC, Kanata, ON, Canada) immediately before tail-vein injection of transduced or control CD34+ human cord blood cells.
Cell Phenotyping and Sorting.
Flow cytometry was performed on FACScan (Becton Dickinson) or Coulter XL-MCL (Beckman Coulter) machines; sterile sorting was performed on a FACSVantage SE (Becton Dickinson) machine. Phycoerythrin (PE)-conjugated antibodies to human CD45, CD33, CD19, CD34 (Becton Dickinson), CD23 (Beckman Coulter), PE-cyanin 5.1-conjugated anti-CD20 (Beckman Coulter), and isotype controls were used according to the manufacturers' instructions.
DNA Studies.
Genomic DNA was isolated with a DNA isolation kit D-5000 (Gentra Systems) as per the manufacturer's instructions, and Southern blots were performed on NsiI- and BclI-digested samples by using standard techniques (18). Vector copy numbers were quantified by PhosphorImager analysis (Molecular Dynamics) and corrected for DNA-loading differences by reprobing blots with a 1.1-kb fragment of the COL1A1 gene. Specific integration site probes were generated by PCR (primers and conditions available on request) of genomic regions flanking mapped vector integration sites. For inverse PCR assays, 1 μg of B cell line DNA was digested with HhaI, MspI, or NlaIII, extracted with phenol/chloroform, and circularized in a volume of 200 μl with 400 units of T4 DNA Ligase (New England Biolabs) overnight at 14°C. Samples were ethanol precipitated and resuspended in 20 μl of TE buffer (10 mM Tris/1 mM EDTA, pH 8.0). Nla III-digested samples were further digested with HindIII and then heat inactivated at 65°C for 20 min before PCR. Nested PCRs were performed on each ligated sample by using Ex-TaqDNA polymerase (Takara Shuzo). The first round was performed with the primers 5′-GGGTGATTGCAATGCTTTCT-3′ and 5′-TGTCTCTCATCCCAGGTACG-3′ under the following conditions: 94°C × 3 min for 1 cycle; 94°C × 1 min, 56°C × 1 min, and 72°C × 2 min for 25 cycles; 72°C × 5 min for 1 cycle. For the second round, PCR products were further amplified for 35 cycles by using either primer set 5′-GGGAGATATCTAGTGATATAAGTGTG-3′ and 5′-GTCCATAAGCTTCCATAGCTG-3′ under the same conditions as above, or with primer set 5′-AAACCGACTTGATTCGAGAACC-3′ and 5′-ATGAGGAGCAGGAGTATTTGGG-3′ and an annealing temperature of 60°C. PCR products were sequenced and results were analyzed by using the BLAT sequence search tool (http://genome.ucsc.edu/) to query the April, 2001 freeze of the human genome database.
Results
Transgene Expression in SRCs.
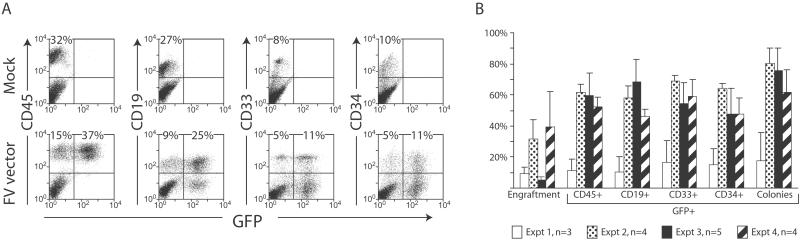
In four independent experiments, we transduced human umbilical cord blood CD34+ cells with the FV vector CGPMscvF (Fig. 1) encoding GFP at multiplicities of infection (mois) of 5–23 by a single 10-h exposure on fibronectin fragment CH296-coated dishes. After transduction, 1.5 × 105 − 3.0 × 105 cells were transplanted into sublethally irradiated NOD/SCID mice. Pretransplantation marking rates of 38–92% were obtained in four different experiments based on GFP expression in liquid cultures and progenitor colonies (Table 1). Engraftment and multilineage marking rates were determined by flow cytometry and progenitor colony assays of bone marrow cells at 5–7 weeks after transplantation (Fig. 2 A and B). In the first experiment, a frozen vector stock was thawed before use, and marking rates were comparatively low. In experiments 2–4, fresh vector stocks were used, and a mean GFP expression rate of 58% was seen in engrafted human hematopoietic cells (CD45+), with similar rates in B cells (CD19+), myeloid cells (CD33+), and primitive progenitors (CD34+). Transduction rates of clonogenic progenitors were somewhat higher, with 62–80% of colonies expressing GFP in experiments 2–4.
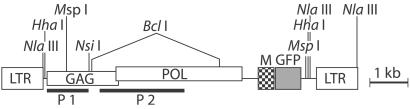
Figure 1.
Foamy virus vector. The structure of the integrated FV vector CGPM scvF is shown. Although gag and pol genes are present, they are not expressed because of lack of the bel-1 transactivator gene (8). GFP reporter gene transcription is driven by the internal murine stem-cell virus (M) promoter (44). Locations of restriction sites and probe fragments (P1 and P2) used in this study are indicated.
Table 1.
Summary of transplantation conditions and pre-transplantation marking rates
| Mice transplanted | CD34 cell source | CD34 purity, % | Cells per mouse | Vector stock | moi | Liquid culture marking, % | Colony marking, % |
|---|---|---|---|---|---|---|---|
| 1 A–C | Fresh, 1 cord | 80 | 1.5 × 105 | Thawed | 6 | 38 | 64 |
| 2 A–D | Thawed, 4 cords | 60 | 2.5 × 105 | Fresh | 18 | 70 | 86 |
| 3 A–E | Fresh, 2 cords | 96 | 3.0 × 105 | Fresh | 23 | 60 | 92 |
| 4 A–D | Thawed, 4 cords | 90 | 3.0 × 105 | Fresh | 5 | 51 | 77 |
Figure 2.
Transgene expression in engrafted human cells. (A) Examples of flow cytometry results from bone marrow cells isolated 6 weeks after transplantation with untransduced (mock) or FV vector-transduced cord blood CD34+ cells. Values shown are the percent of all analyzed cells present in the indicated quadrants. Expression of GFP and human cell-surface markers (CD45, pan-hematopoietic; CD19, B-lymphoid; CD33, myeloid; and CD34, primitive progenitors) were measured. (B) Summary of flow cytometry results from 16 mice transplanted in four separate experiments. Engraftment levels represent the percentage of all bone-marrow cells expressing human CD45. Other values are percent GFP+ human cells expressing each indicated cell-surface marker as determined by flow cytometry or percent GFP+ colonies as determined by progenitor assays.
Southern Blot Analysis of Expanded Lineage-Specific Cell Populations.
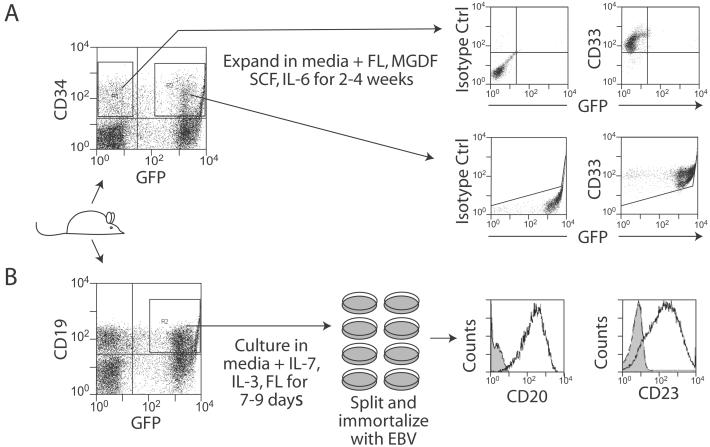
To study vector integration patterns, we expanded both myeloid and lymphoid cells isolated from transplanted mice. Myeloid cells were obtained by sorting CD34+/GFP+ or CD34+/GFP− human cell populations from harvested marrow, and then culturing them in the presence of FL, megakaryocyte growth and development factor, SCF, and IL-6. After 2–4 weeks, total cell numbers increased 10–100 fold, and the surviving cells expressed CD33, demonstrating selective expansion of a myeloid population under these conditions, as described (Fig. 3A; ref. 16). Transduced human B-lymphoid cells were obtained by sorting CD19+/GFP+ cells from harvested marrow, and culturing them in the presence of IL-7, IL-3, and SCF for 7–9 days. The cells then were split into multiple wells and infected with EBV. Over a 1–3 month period, immortalized cell clones eventually grew in many of the infected wells. These CD19+ cell lines also expressed B cell markers CD20 and CD23 (Fig. 3B).
Figure 3.
Generation of expanded lineage-specific cell populations. (A) Expanded GFP+ and GFP− polyclonal myeloid cell populations were generated by ex vivo culture of sorted human CD34+/GFP+ and CD34+/GFP− cells in the indicated cytokines. After expansion, the cell populations expressed the myeloid marker CD33 as shown for two examples with isotype controls. (B) Transduced B cell lymphoid lines were produced by EBV-immortalization of human CD19+/GFP+-sorted cells isolated from transplanted mice and stimulated ex vivo with the indicated cytokines. The immortalized cells expressed B cell markers CD23 and CD20 as shown by flow cytometry examples of a specific cell line. Results are superimposed onto isotype controls (shaded in gray).
Myeloid cell populations and a total of 25 distinct B cell lines were derived from six transplanted mice (2 to 12 B cell lines per mouse). Proviral integration patterns were determined by Southern blot analysis (Fig. 4 A and B), and the polyclonal myeloid populations contained multiple integration sites as expected. Most B cell lines also contained multiple proviruses and, based on the distribution of hybridizing band intensities, appeared to be oligoclonal, with the majority of contributing clones containing more than one provirus. However, 8 of 25 B cell lines could be definitively identified as clonal, either because they contained a single provirus or because the pattern seen on Southern blots was present in more than one cell line, such as cell lines 1 and 3 from mouse 3D, each containing the same six proviruses (Fig. 4A). In the case of mouse 4B, we estimated that the 12 B cell lines generated were derived from 23 different clones containing 1–6 proviruses each, based on groupings of bands with similar intensities on Southern blots (Fig. 4B and data not shown). The presence of multiple integrated proviruses in B cell clones demonstrates the high transduction efficiency of FV vectors.
Figure 4.
DNA analysis of vector integrations and provirus copy number. (A) Southern blot of genomic DNA from expanded human myeloid cells (M) and three B cell lines derived from mouse 3D were digested with BclI or NsiI and hybridized to probe P1 (Fig. 1). B cell lines 1 and 3 have identical patterns, indicating that they represent the same clonal population. (B) Southern blots of genomic DNA from expanded human myeloid cells (M) and 12 B cell lines isolated from mouse 4B. Junction fragments (Fig. 5) found to be present in both lymphoid and myeloid populations are indicated by asterisks. (C) Determination of vector copy number in expanded human GFP+ and GFP− myeloid cells from mice 4B, 4C, and 4D. BclI-digested DNA was hybridized to probe P2 (Fig. 1), identifying a 3.5-kb internal fragment in all integrated proviruses. Dilution standards are mixtures of DNA from diploid human fibroblasts containing a single FV vector provirus with DNA from normal human lymphocytes. Copy numbers (proviruses/diploid genome) determined by PhosphoImager quantitation are indicated below each sample. Correction for DNA loading was based on reprobing the same blot with a fragment of the human COL1A1 gene.
By probing for an internal restriction fragment of the FV vector genome, we found that the expanded GFP+ myeloid cells from mice 4B and 4C contained an average of 3.8 and 2.2 proviruses per cell, respectively (Fig. 4C). Expanded GFP− myeloid cells from the same mice had average copy numbers ≤0.3. Thus, only a small fraction of cells containing the vector failed to express detectable levels of GFP, and significant transgene silencing did not occur. Based on the GFP expression rates of 68–71% in human myeloid cells from these mice, overall proviral copy numbers of 2.6 and 1.6 were obtained in this experiment, which used an moi of 5 transducing particles per cell, further demonstrating efficient gene transfer by FV vectors.
Transduction of Lympho-Myeloid Repopulating Cells.
We compared integration patterns in lymphoid B cell lines and expanded myeloid cells to determine if a cell capable of differentiating down both lineages had been transduced. Based on a comparison of junction fragments on Southern blots, no common lymphoid and myeloid patterns were detected in three of the six mice studied. In the other three animals, 6 of 18 immortalized B cell lines had integration patterns after both BclI and NsiI digestion that potentially matched those seen in expanded myeloid cells (Fig. 4 and data not shown). However, the large number of bands in the myeloid DNA samples made it difficult to determine whether these were the result of common vector integrations. Therefore, these six B cell lines were further characterized by inverse PCR and sequencing of flanking genomic DNA. We were able to amplify and sequence 12 junction fragments from these six cell lines; we identified their chromosomal locations by alignment with the public human genome database (Table 2). The distribution seemed random with respect to chromosomal location and transcriptional activity, in contrast to a previous murine cell study describing preferential FV integration in highly expressed genes (19). In one case, both junction fragments from a single provirus were sequenced (Integration 4, Table 2), and a 4-bp repeat of genomic sequence was found at the integration site, as expected for integrase-mediated foamy virus integration events (19, 20).
Table 2.
Foamy virus vector integration sites
| Integration | Chromosomal location | Nearest gene/EST | Distance |
|---|---|---|---|
| 1 | Chr 3: 94,485,132 | DKFZP5640123 | +135.2 kb |
| 2 | Chr 8: 18,458,369 | LOC51201 | Intron 1 |
| 3 | Chr 6: 6,602198 | LOC51299 | +13.6 kb |
| 4 | Chr 21: 13,461,441 | NRIP1 | −175.3 kb |
| EST: AI79324 | −75.9 kb | ||
| 5 | Chr 1: 134,092,559 | SLC16A1 | −31.6 kb |
| EST: AW293638 | +1.0 kb | ||
| 6 | Chr 17: 73,773,156 | KCNJ16 | −38.1 kb |
| EST: BE777672 | Intron 1 | ||
| 7 | Chr 17: 32,834,004 | HPCA66 | −5.1 kb |
| EST: AW974585 | Intron 1 | ||
| 8 | Chr 20: 32,467,706 | KIAA0255 | Intron 1 |
| 9 | Chr 15: 60,944,087 | DAPK2 | Intron 3 |
| 10 | Chr 13: 35,094,751 | CCNA1 | +100.2 kb |
| 11 | Chr 19: 39,539,889 | CEBPA | +26.6 kb |
| 12 | Chr 10: 46,657,395 | DEPP | +211.6 kb |
| EST: AA401537 | +31.3 kb |
If an EST is closer to the mapped integration site than the nearest known gene, then both are listed.
Based on the genomic sequence at the mapped integration sites, we were able to predict the sizes of FV-hybridizing restriction fragments produced by digestion with BclI and NsiI. In each of the six B cell lines studied, we identified an integration site that produced a hybridizing band of similar size to one seen in the corresponding myeloid cell population. Flanking genomic DNA from each of these junctions was amplified by PCR and used as a probe to identify common junction fragments in lymphoid and myeloid cells. In two of six cases (Integrations 3 and 4, Table 2), a hybridizing fragment of the expected size was present both in DNA from the B cell line containing that junction and in DNA from expanded myeloid cells derived from the same mouse (Fig. 5). The bands were lighter in myeloid cell DNA, as expected for these polyclonal populations. In the remaining four cases, genomic probes identified the expected junction fragments only in the B cell line from which they were mapped, and not in the corresponding myeloid cell populations.
Figure 5.
Identification of common lympho-myeloid vector integration sites. (A) A map of integration site 4 (Table 2) is shown with predicted pre- and postintegration structures of the chromosome 21 insertion site, including BclI (B) and NsiI (N) restriction sites and fragment sizes, genomic (GP1) and FV (P1) probe locations, and the LTR-flanked GFP gene present in the vector provirus. A Southern blot of BclI-digested genomic DNA from expanded myeloid cells (M) and four B cell lines from the same mouse probed with GP1 identifies the 13.1-kb fragment of preintegration loci in all samples, and the 6.3-kb fragment due to FV integration in myeloid cells and B cell line 3 from which it was mapped. (B) A similar analysis of integration site 3 at chromosome 6 is shown, probed with GP2. The 12.9- and 2.7-kb BclI fragments predicted to hybridize to FV probe P1 at integration sites 4 and 3, respectively, can be seen in Fig. 3B (clones 3 and 1, respectively).
Discussion
A fundamental challenge in the gene therapy of hematologic diseases is to improve stem-cell gene transfer rates. Primate HSCs are quiescent and not efficiently transduced by oncoretroviral vectors, which require mitosis for nuclear entry (21) and pseudotyping with nonecotropic envelope proteins that may limit transduction (22). Lentiviral vectors can transduce some nondividing cells (23–25), and a number of groups have demonstrated the ability of these vectors to efficiently transduce SRCs (26–28). However, the enthusiasm generated by these results is tempered by safety concerns. Our prior results with murine HSCs (9) and the results with human cells reported here suggest that vectors based on nonpathogenic FVs are a promising alternative for HSC gene transfer. We found that FV vectors efficiently transduced human SRCs with typical marking rates of over 50% in engrafted human hematopoietic cells. These transduction rates are similar to the best SRC marking results obtained with lentiviral vectors, and the mois needed were lower than those typically used for VSV-pseudotyped lentiviral vectors (26, 28). Importantly, the transduction conditions involved minimal (10 h) ex vivo manipulations that should allow cells to remain in G0/G1 and preserve their repopulating ability (29–31), there was high transgene expression without significant silencing, and in some cases, we could demonstrate the transduction of pluripotent human HSCs with both lymphoid and myeloid potential.
FV vectors may have performed well in these studies because they are able to transduce quiescent cells more efficiently than oncoretroviral vectors (7, 32). This finding could be related to several properties of FVs, including nuclear entry of viral genomes in G1/S phase-arrested cells (33), completion of cDNA synthesis during particle production rather than after infection (34, 35), or persistence of preintegration complexes in nondividing cells until cell proliferation resumes (7). High receptor levels on HSCs also could have promoted efficient transduction, because the currently unidentified cellular receptor(s) for FV seems to be expressed in all mammalian cell types studied to date, including primary hematopoietic cells (1, 9, 36).
The SRC is commonly used as a surrogate for human HSCs in studies of phenotyping, ex vivo expansion, and gene transfer. Based on the number of distinct, transduced B cell lines we generated from individual mice, the percent of transduced SRCs, and the number of transplanted human cells, we calculate that the frequency of SRCs was 1 per 8,000–45,000 cord blood CD34+ cells, a number that is consistent with previous reports (13, 16, 37, 38). Limiting dilution studies and Southern blot analysis of unfractionated human cells in NOD/SCID mice suggest that the SRC is a pluripotent HSC (37, 39, 40). Our study provides the first multilineage marking evidence that at least some engrafted lymphoid and myeloid progeny were derived from the same transplanted SRC. In a prior study employing immunodeficient beige/nude/Xid mice, Nolta et al. (41) used inverse PCR and junction-site sequencing of expanded T-cell and myeloid clones to demonstrate oncoretroviral vector transduction of human pluripotent HSCs in a small percentage of the junctions analyzed. Because technical limitations preclude the identification of clones that are poorly represented in either hematopoietic lineage, both Nolta's study and ours underestimate the true frequency of common junctions. However, these approaches provide definitive evidence that mouse xenotransplantation models can assay human pluripotent HSCs. The NOD/SCID model has been more widely used in recent years because of higher engraftment levels that do not depend on supplementation with human cytokines.
Although pluripotent SRCs clearly exist, it should not be assumed that all human hematopoiesis in immunodeficient mice derives from them. There is growing evidence that primitive lineage-restricted cells can engraft and contribute to hematopoiesis (42, 43). In the case of the NOD/SCID model, there is a skewing of human hematopoiesis toward B lymphoid growth, which may represent repopulation by lymphoid-restricted SRCs, as supported by some of our integration-site findings. All 10 immortalized B cell lines from mouse 4D contained the same clone of cells, and in 8 of these lines, this clone was the only one present (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Despite this concentration of B-lymphopoiesis from a single SRC, the same clone could not be demonstrated in the myeloid population.
Our approach for generating FV-transduced, immortalized B cell lines from transplanted mice is an innovation that can be used to study SRC biology, providing information on common integration sites, SRC frequencies, and provirus copy numbers in individual SRCs. Similar studies of FV-marked SRCs transplanted at limiting dilutions or followed over time may help determine the relative frequencies of lineage-committed vs. pluripotent SRCs in different cell populations and improve our understanding of human hematopoiesis. Combining the SRC assay with integration site analysis also can provide definitive evidence for human HSC transduction, which is difficult to demonstrate experimentally without performing a clinical trial. Future transplantation experiments in large animal models will help determine the therapeutic potential of FV vectors.
Supplementary Material
Acknowledgments
We thank Roli Hirata for expert technical assistance, Vivian Zafiropoulos for help with the NOD/SCID colony, Katy Dougherty for help with flow cytometry, and M. Juliana McElrath for providing the B95-8 cell line. This work was supported by National Institutes of Health Grants K08 DK02980-01, P01 DK55759, PEGT HL66947, and K08 DK02776-02, and the Medical Research Council of Canada (Jesse Davidson Postdoctoral Fellowship).
Abbreviations
- FV
foamy viruses
- NOD/SCID
nonobese diabetic-severe combined immunodeficiency
- SRC
SCID repopulating cell
- HSC
hematopoietic stem cells
- SCF
stem-cell factor
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Linial M L. J Virol. 1999;73:1747–1755. doi: 10.1128/jvi.73.3.1747-1755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flugel R M. J Acquired Immune Defic Syndr. 1991;4:739–750. [PubMed] [Google Scholar]
- 3.Ali M, Taylor G P, Pitman R J, Parker D, Rethwilm A, Cheingsong-Popov R, Weber J N, Bieniasz P D, Bradley J, McClure M O. AIDS Res Hum Retroviruses. 1996;12:1473–1483. doi: 10.1089/aid.1996.12.1473. [DOI] [PubMed] [Google Scholar]
- 4.Schweizer M, Turek R, Hahn H, Schliephake A, Netzer K O, Eder G, Reinhardt M, Rethwilm A, Neumann-Haefelin D. AIDS Res Hum Retroviruses. 1995;11:161–170. doi: 10.1089/aid.1995.11.161. [DOI] [PubMed] [Google Scholar]
- 5.Heneine W, Switzer W M, Sandstrom P, Brown J, Vedapuri S, Schable C A, Khan A S, Lerche N W, Schweizer M, Neumann-Haefelin D, et al. Nat Med. 1998;4:403–407. doi: 10.1038/nm0498-403. [DOI] [PubMed] [Google Scholar]
- 6.Sandstrom P A, Phan K O, Switzer W M, Fredeking T, Chapman L, Heneine W, Folks T M. Lancet. 2000;355:551–552. doi: 10.1016/S0140-6736(99)05292-7. [DOI] [PubMed] [Google Scholar]
- 7.Russell D W, Miller A D. J Virol. 1996;70:217–222. doi: 10.1128/jvi.70.1.217-222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trobridge G D, Russell D W. Hum Gene Ther. 1998;9:2517–2525. doi: 10.1089/hum.1998.9.17-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassilopoulos G, Trobridge G, Josephson N C, Russell D W. Blood. 2001;98:604–609. doi: 10.1182/blood.v98.3.604. [DOI] [PubMed] [Google Scholar]
- 10.Dick J E. Curr Opin Hematol. 1996;3:405–409. doi: 10.1097/00062752-199603060-00002. [DOI] [PubMed] [Google Scholar]
- 11.Greiner D L, Hesselton R A, Shultz L D. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 12.Larochelle A, Vormoor J, Hanenberg H, Wang J C, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, et al. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Wang J C Y, Kapp U, Bonnet D, Dick J E. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cashman J D, Lapidot T, Wang J C, Doedens M, Shultz L D, Lansdorp P, Dick J E, Eaves C J. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- 15.Wall F E, Henkel R D, Stern M P, Jenson H B, Moyer M P. In Vitro Cell Dev Biol Anim. 1995;31:156–159. doi: 10.1007/BF02633976. [DOI] [PubMed] [Google Scholar]
- 16.Piacibello W, Sanavio F, Severino A, Dane A, Gammaitoni L, Fagioli F, Perissinotto E, Cavalloni G, Kollet O, Lapidot T, Aglietta M. Blood. 1999;93:3736–3749. [PubMed] [Google Scholar]
- 17.Trobridge G, Vassilopoulos G, Josephson N, Russell D W. Methods Enzymol. 2002;346:628–648. doi: 10.1016/s0076-6879(02)46082-x. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 19.Neves M, Peries J, Saib A. Res Virol. 1998;149:393–401. doi: 10.1016/s0923-2516(99)80007-7. [DOI] [PubMed] [Google Scholar]
- 20.Enssle J, Moebes A, Heinkelein M, Panhuysen M, Mauer B, Schweizer M, Neumann-Haefelin D, Rethwilm A. J Gen Virol. 1999;80:1445–1452. doi: 10.1099/0022-1317-80-6-1445. [DOI] [PubMed] [Google Scholar]
- 21.Roe T, Reynolds T C, Yu G, Brown P O. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlic D, Girard L J, Jordan C T, Anderson S M, Cline A P, Bodine D M. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 25.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi H, Smith K A, Mosier D E, Verma I M, Torbett B E. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 27.Guenechea G, Gan O I, Inamitsu T, Dorrell C, Pereira D S, Kelly M, Naldini L, Dick J E. Mol Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 28.Woods N B, Fahlman C, Mikkola H, Hamaguchi I, Olsson K, Zufferey R, Jacobsen S E, Trono D, Karlsson S. Blood. 2000;96:3725–3733. [PubMed] [Google Scholar]
- 29.Gothot A, van der Loo J C, Clapp D W, Srour E F. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 30.Glimm H, Oh I H, Eaves C J. Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 31.Peters S O, Kittler E L, Ramshaw H S, Quesenberry P J. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 32.Mergia A, Chari S, Kolson D L, Goodenow M M, Ciccarone T. Virology. 2001;280:243–252. doi: 10.1006/viro.2000.0773. [DOI] [PubMed] [Google Scholar]
- 33.Saib A, Puvion-Dutilleul F, Schmid M, Peries J, de The H. J Virol. 1997;71:1155–1161. doi: 10.1128/jvi.71.2.1155-1161.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moebes A, Enssle J, Bieniasz P D, Heinkelein M, Lindemann D, Bock M, McClure M O, Rethwilm A. J Virol. 1997;71:7305–7311. doi: 10.1128/jvi.71.10.7305-7311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu S F, Baldwin D N, Gwynn S R, Yendapalli S, Linial M L. Science. 1996;271:1579–1582. doi: 10.1126/science.271.5255.1579. [DOI] [PubMed] [Google Scholar]
- 36.Hirata R K, Miller A D, Andrews R G, Russell D W. Blood. 1996;88:3654–3661. [PubMed] [Google Scholar]
- 37.Conneally E, Cashman J, Petzer A, Eaves C. Proc Natl Acad Sci USA. 1997;94:9836–9841. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan C J, Shpall E J, McNulty O, McNiece I, Dick J E, Shultz L D, Keller G. Blood. 1997;90:85–96. [PubMed] [Google Scholar]
- 39.Guenechea G, Gan O I, Dorrell C, Dick J E. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 40.Wang J C, Doedens M, Dick J E. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 41.Nolta J A, Dao M A, Wells S, Smogorzewska E M, Kohn D B. Proc Natl Acad Sci USA. 1996;93:2414–2419. doi: 10.1073/pnas.93.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akashi K, Traver D, Miyamoto T, Weissman I L. Nature (London) 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 43.Kondo M, Weissman I L, Akashi K. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 44.Hawley R G, Lieu F H, Fong A Z, Hawley T S. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.