Abstract
Intracellular bacterial pathogens employ a variety of strategies to invade their eukaryotic host cells. From an ultrastructural standpoint, the processes that bacteria employ to invade their host cells include conventional phagocytosis, coiling phagocytosis, and ruffling/triggered macropinocytosis. In this paper, we describe a novel process by which Francisella tularensis, the agent of tularemia, enters host macrophages. F. tularensis is a remarkably infectious facultative intracellular bacterial parasite—as few as 10 bacteria can cause life-threatening disease in humans. However, the ultrastructure of its uptake and the receptor mechanisms that mediate its uptake have not been reported previously. We have used fluorescence microscopy and electron microscopy to examine the adherence and uptake of a virulent recent clinical isolate of F. tularensis, subspecies tularensis, and the live vaccine strain (LVS), subspecies holarctica, by human macrophages. We show here that both strains of F. tularensis enter human macrophages by a novel process of engulfment within asymmetric, spacious pseudopod loops, a process that differs ultrastructurally from all previously described uptake mechanisms. We demonstrate also that adherence and uptake of F. tularensis by macrophages is strongly dependent upon complement receptors and upon serum with intact complement factor C3 and that uptake requires actin microfilaments. These findings have significant implications for understanding the intracellular biology and virulence of this extremely infectious pathogen.
Francisella tularensis is a nonmotile, nonsporulating, gram-negative coccobacillus that causes zoonotic disease in small animals such as rodents, rabbits, and beavers. Humans acquire tularemia by handling infected animals, by consumption of contaminated food or water, or by the bite of blood-sucking insects. F. tularensis consists of three main subspecies—tularensis, holarctica, and mediasiatica—which differ in their geographic distributions and in their virulence in humans (14). F. tularensis subspecies tularensis, found almost exclusively in North America, is highly virulent for humans. As few as 10 organisms delivered subcutaneously or 25 organisms delivered by inhalation can lead to a severe, potentially lethal, infection in humans (34, 35). F. tularensis subspecies holarctica (found in North America and in Europe) and subspecies mediasiatica (found in Asia) are of lower virulence. Because of its high infectivity and capacity to cause severe morbidity and mortality, F. tularensis subspecies tularensis is classified as a category A potential agent of bioterrorism (12).
Although F. tularensis can be grown in the laboratory on enriched culture media, we (8) and others (3, 15, 20) have shown that F. tularensis bacteria invade and grow productively in macrophages. It is thought that, in natural infections, the bacterium replicates intracellularly within host mononuclear phagocytes (26, 42). After entry of the organism into the macrophages, we have found that F. tularensis initially resides in a phagosome. However, the bacterium arrests the maturation of its phagosome, which acquires some markers of early and late endosomes, but not cathepsin D, and it inhibits the acidification of its phagosome (8). A unique feature of the phagosome is that it is often surrounded by a dense fibrillar coat (8). With more time after infection, the phagosomal membrane is disrupted and the bacterium replicates freely in the cytoplasm of the macrophage (8, 20). While these aspects of intracellular life after entry have been reported, the ultrastructure and mechanisms that mediate uptake of this highly infectious bacterium have not previously been reported.
MATERIALS AND METHODS
Bacteria.
A F. tularensis live vaccine strain (LVS) of subspecies holarctica and a virulent recent clinical isolate (RCI; NY 96-3369) of subspecies tularensis were obtained from the Centers for Disease Control and Prevention (Atlanta, GA). The bacteria were passaged, stored, and scraped from agar plates after overnight culture as previously described (8). Formalin-killed bacteria were prepared as previously described (8). Periodate-treated bacteria were prepared by incubating bacteria with 1% periodic acid for 30 min at room temperature, incubation with 10 mM lysine in phosphate-buffered saline (PBS) for 30 min at room temperature (to quench and cross-link the aldehydes generated by periodate oxidation of carbohydrates), and washing twice with PBS. Salmonella enterica serovar Typhimurium (wild-type strain SL1344) and Shigella flexneri (wild-type strain M90T) were obtained from the laboratory of Jeffrey Miller (University of California, Los Angeles). Prior to infection experiments, S. enterica serovar Typhimurium and S. flexneri were grown overnight on LB plates supplemented with 50 μg/ml streptomycin and on Trypticase soy agar plates, respectively, and the bacteria were scraped into normal saline as described for F. tularensis. A recent clinical isolate of Neisseria gonorrhea was provided by David Bruckner (University of California, Los Angeles).
Human serum, cells, and cell lines.
Human serum was prepared and handled in a manner to preserve complement activity (24). Heat-inactivated serum was prepared by incubation of the serum at 56°C for 30 min. C3-depleted serum was purchased from Quidel Scientific Corporation and purified complement factor C3 from Sigma Chemical Company.
Peripheral blood mononuclear cells were isolated (8), adjusted to 3 × 106 cells/ml in RPMI 1640 with glutamine (Cellgro) and 20% autologous serum, and incubated for 5 days in sterile screw-cap Teflon wells (Savillex Corp., Minnetonka, MN) at 37°C and 5% CO2 prior to use. Teflon wells were chilled on ice, and the mononuclear cells were resuspended, washed, and allowed to adhere to coverslips or plastic tissue culture plates (8) or used in suspension for transmission electron microscopy (TEM) uptake experiments. The University of California-Los Angeles Institutional Review Board approved the participation of normal human blood donors in our research.
THP-1 cells (ATCC TIB 202) were differentiated on glass coverslips for 3 days (8) prior to use. HeLa cells (ATCC CCL-2) were added to glass coverslips in 2-cm2 tissue culture wells at 105 cells/well and cultured to confluency in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum (HI-FBS; Irvine Scientific) for 2 days prior to use.
Examination of adherence or uptake of F. tularensis in macrophage monolayers.
Monolayers of monocyte-derived macrophages (MDM) or phorbol myristate acetate (PMA)-differentiated THP-1 cells on glass coverslips were incubated for 60 min with F. tularensis at a multiplicity of infection (MOI) of 35:1 (bacteria:monocyte) in RPMI medium containing 10% fresh human AB serum, washed with RPMI medium, fixed for 30 min in 2% paraformaldehyde in 0.1 M PIPES [piperazine-N,N′bis(2-ethanesulfonic acid)] containing 6% sucrose, and permeabilized by incubation for 15 min with 0.1% Triton X-100 in PBS containing 10 mM glycine HCl. Nonspecific antibody binding sites were blocked by incubation with 5% goat serum in PBS, and bacteria were stained with rabbit anti-F. tularensis antiserum (Becton Dickinson) (1:1,000) and Oregon green-conjugated goat anti-rabbit immunoglobulin G (IgG) (Molecular Probes). In some experiments, adherent bacteria were distinguished from intracellular bacteria by staining the monolayers with rabbit anti-F. tularensis antibody and Texas red-X-conjugated goat anti-rabbit IgG (Molecular Probes) prior to permeabilization with 0.1% Triton X-100 and staining of the intracellular bacteria with mouse monoclonal antibody to F. tularensis lipopolysaccharide (LPS) (Biogenesis) (5 μg/ml) and Oregon green-conjugated goat anti-mouse IgG. Coverslips were incubated with 2.5 μM DAPI (4′,6′diamidino-2-phenylindole) (Sigma Chemical Company) in PBS for 15 min, washed, mounted with Prolong Antifade Mounting Medium (Molecular Probes), and viewed by epifluorescence and confocal fluorescence microscopy (8).
Treatment of monolayers with monoclonal antibodies to complement receptors.
Human MDM or PMA-differentiated monolayers of THP-1 cells on coverslips were preincubated with IgG1,κ monoclonal antibodies to CD11b or CD18 (BD Pharmingen) (10 μg/ml) individually or in combination (10 μg/ml each) for 30 min at 37°C in RPMI medium containing 10% HI-FBS or with an isotypic control monoclonal antibody (mouse anti-HLA-DR; Biosource) (10 or 20 μg/ml, respectively). Prior to use, the antibodies were dialyzed against RPMI 1640 containing l-glutamine and 20 mM HEPES to remove sodium azide and concentrated by centrifugation in Centricon filtration units (Pharmacia). The medium was replaced with RPMI 1640 containing 10% fresh AB serum, the same monoclonal antibodies, and F. tularensis RCI or LVS at an MOI of 35:1, and the cultures were incubated for an additional 1 h at 37°C, washed, fixed, and stained by immunofluorescence for F. tularensis.
Transmission electron microscopy.
To examine the ultrastructure of uptake of F. tularensis, S. enterica serovar Typhimurium, and S. flexneri by human MDM in suspension or by adherent THP-1 cells, we preopsonized the bacteria with 10% fresh human serum for 15 min at 37°C, chilled the bacteria on ice, and added them to a suspension of ice-cold human MDM or a plate of PMA-differentiated THP-1 cells at an MOI of 1,670:1 (bacteria:macrophage). The use of this relatively high MOI is standard for the examination of uptake at very early time points (≤5 min) by TEM (24). Bacterial uptake profiles are rare in TEM thin sections when lower MOIs are used. The tube or plate was centrifuged sequentially at 215 × g and 860 × g (10 min each) at 4°C, a temperature at which phagocytosis does not occur. For the MDM in suspension, the supernate was removed and the pelleted cells and bacteria warmed to 37°C for 5 min prior to fixation and processing for TEM (24). For monolayers of THP-1 cells, the supernate was replaced with prewarmed RPMI 1640 containing fresh 10% AB serum, and the plates were incubated for 5 min at 37°C prior to fixation and processing.
We scored ultrastructural aspects of the interaction between bacteria and macrophages after 5 min of incubation at 37°C by the following criteria. We scored a bacterium as being engulfed within an “asymmetric pseudopod loop” (or “looping phagocytosis”) when the bacterium was embraced by a single, loosely fitting, pseudopod extension. (“Loosely fitting” was defined as a distance greater than 100 nm between the pseudopod and the bacterium.) When an additional pseudopod extension was present on the other side of the bacterium, this extension was less than one-third the length of the main pseudopod extension. Conversely, we scored a bacterium as being internalized within “symmetric ruffles” when it resided within loosely fitting, symmetrically arranged pseudopod pairs. If the pseudopod pairs were of disparate lengths, then the difference was of a factor of 3 or less. We scored bacteria as being internalized by “conventional phagocytosis” when they resided within symmetrical pseudopod extensions that were tightly adherent (within 100 nm) to the surface of the bacterium. We defined “spacious vacuoles” as those with cross-sectional area at least threefold greater than the cross-sectional area of the enclosed bacterium. Conversely, we defined “tight vacuoles” as those with a cross-sectional area less than threefold greater than the cross-sectional area of the enclosed bacterium. In most cases, the symmetry or asymmetry of uptake and the spaciousness or tightness of vacuoles was immediately obvious (well within the above-stated criteria) so that formal measurement was rarely required.
For negative staining of bacteria, F. tularensis LVS and RCI and a recent clinical isolate of N. gonorrhea were grown overnight on chocolate agar enriched with hemoglobin and IsoVitale X (Becton Dickinson Microbiology Systems), scraped into normal saline, mixed 1:2 with 2.7% glutaraldehyde in 0.1 M PIPES, pH 7.4, containing 6% sucrose, adhered to formvar-coated grids, rinsed in 0.9% saline solution, negatively stained with 2% uranyl acetate, and viewed by TEM.
RESULTS
F. tularensis enters macrophages via spacious pseudopod loops.
We examined the ultrastructural features of uptake of live or formalin-killed F. tularensis by human peripheral blood monocyte-derived macrophages (MDM) and macrophage-like THP-1 cells that were fixed and prepared for transmission electron microscopy (TEM) at 5 min after incubation with the bacteria. We have studied a virulent recent clinical isolate (RCI) of F. tularensis subspecies tularensis and an attenuated live vaccine strain (LVS) of subspecies holarctica. We found that live F. tularensis RCI and LVS were both initially enclosed within loosely fitting, exuberant loops of pseudopodia (Fig. 1A to C). We found this to be true for uptake of F. tularensis both by human MDM in suspension (Fig. 1A and B) and by adherent THP-1 cells (Fig. 1C). The bacteria at the periphery of the macrophage were usually enclosed within spacious compartments that appeared to result from fusion of the pseudopod loops with the plasma membrane (Fig. 1D and E). Occasionally the plane of the EM section did not include the macrophage cell body, yielding the appearance of a bacterium within an isolated loop of pseudopodium (Fig. 1F).
FIG. 1.
Human MDM engulf F. tularensis within spacious pseudopod loops. Live F. tularensis RCI were preoposonized with 10% fresh human serum, pelleted onto human peripheral blood MDM (A, B, and D to F) or differentiated THP-1 cells (C), fixed, and processed for TEM after 5 min of incubation at 37°C. Similar results were also obtained with F. tularensis LVS. Size bars indicate 1 μm in all panels.
In six separate experiments examining over 200 uptake profiles of F. tularensis RCI (at least 25 uptake profiles per experiment), we found that in 95% of the cases the ultrastructural profiles were consistent with uptake of F. tularensis RCI within loosely fitting, asymmetric loops of pseudopodia (Figs. 1A to C and Table 1). In 5% of the cases, the bacteria were found within loosely fitting, symmetrical pairs of pseudopodia, resembling the ruffling/triggered macropinocytosis (“splash structure”) described for S. enterica serovar Typhimurium (1, 11, 17). In the case of F. tularensis LVS, we have observed a total of 50 uptake profiles in two separate experiments and found that 47 (94%) of the uptake profiles were consistent with uptake within loosely fitting loops of pseudopodia and that 3 (6%) of the bacteria were within loosely fitting symmetrical pairs of pseudopodia (Table 1). We have not observed any instances of uptake of live F. tularensis RCI or LVS by conventional phagocytosis or within tightly fitting pseudopod coils characteristic of coiling phagocytosis (24).
TABLE 1.
Ultrastructure of uptake of F. tularensis, S. enterica, and S. flexneri by human MDMa
| Bacterium | % of bacteria with indicated uptake morphology (no. of bacteria)
|
% of bacteria within:
|
|||||
|---|---|---|---|---|---|---|---|
| Vacuoles at the periphery of the macrophage
|
Vacuoles within the main macrophage cell body
|
||||||
| Asymmetric pseudopod loopsb | Symmetric rufflesc | Conventional phagocytosisd | Spaciouse | Tight | Spacious | Tight | |
| F. tularensis RCI | 95 ± 4 (276) | 5 ± 4 (14) | 0 ± 0 (0) | 80 ± 5 (74) | 20 ± 5 (18) | 8 ± 3 (20) | 92 ± 3 (223) |
| F. tularensis LVS | 94 ± 0 (47) | 6 ± 1 (3) | 0 ± 0 (0) | NDf | ND | ND | ND |
| Formalin-killed RCI | 95 ± 1 (95) | 1 ± 1 (1) | 4 ± 0 (4) | ND | ND | ND | ND |
| Periodate-treated RCI | 12 ± 2 (19) | 11 ± 4 (17) | 76 ± 7 (117) | ND | ND | ND | ND |
| S. enterica | 6 ± 3 (9) | 76 ± 8 (104) | 18 ± 11 (24) | 73 ± 16 (11) | 27 ± 16 (4) | 3 ± 5 (2) | 97 ± 5 (76) |
| S. flexneri | 4 ± 6 (7) | 72 ± 8 (117) | 23 ± 7 (38) | 39 ± 20 (9) | 61 ± 20 (14) | 6 ± 6 (30) | 94 ± 6 (2) |
Results represent the means±SE of at least two experiments. Numbers in parentheses indicate the total number of bacteria counted in the indicated category.
Bacteria were scored as being within “asymmetric pseudopod loops” if they were enclosed within a single loosely fitting pseudopod loop. If a second pseudopod extension was present on the other side of the bacterium, then that extension was less than one-third the length of the main pseudopod extension. “Loosely fitting” was defined as distances of greater than 100 nm between the pseudopod loop and the bacterium.
Bacteria were scored as being within “symmetric ruffles” if they were found within loosely fitting symmetrical pairs of pseudopod extensions whose lengths differed by less than a factor of 3.
Bacteria were scored as being internalized by conventional phagocytosis if found within symmetrical pseudopod extensions tightly adherent (within 100 nm) to the surface of the bacteria.
Vacuoles were scored as “spacious” if their cross-sectional area was at least 3 times that of the enclosed bacterium. They were scored as being “tight” if the cross-sectional area was less than 3 times that of the enclosed bacterium.
ND, not determined.
Whereas F. tularensis bacteria at the periphery of the cell were enclosed within spacious compartments, the bacteria located within the main cell body of the macrophage were usually within more tightly fitting vacuoles (Fig. 2A to D), consistent with the morphology that we (8) and others (20) have documented at later infection time points. This suggests that the initial spacious vacuoles at the cell periphery (shown in Fig. 1D and E) undergo rapid remodeling to form tighter vacuoles as the nascent bacterial vacuoles mature and move centripetally. In an examination of 92 consecutive F. tularensis RCI vacuoles in which the bacteria were located outside the main body of the macrophage (examining from 4 to 22 peripherally located vacuoles in each of six separate experiments), 74 (80%) of the “vacuoles” were spacious, as exemplified in Fig. 1D and E. In contrast, in an examination of 243 consecutive F. tularensis RCI vacuoles located within the main macrophage cell body (at least 24 vacuoles in each of the same six experiments) only 20 (8%) were spacious, and the remaining 92% were tight fitting (Fig. 2 and Table 1). Interestingly, in approximately 1 out of every 100 F. tularensis phagosomes examined, the bacteria appeared to have pili or cell wall protrusions that attached to the host cell phagosomal membrane (Fig. 2B to D). It is possible that these intimate contacts between the bacteria and the phagosomal membrane play a role in pathogenesis by mediating delivery of bacterial molecules into the phagosomal membrane or the cytoplasm of the host cell.
FIG. 2.
TEM demonstrates that within 5 min of uptake, the majority (90%) of F. tularensis RCI (A to C) and LVS (D) that are located within the main cell body of the MDM reside within tightly fitting vacuoles. In approximately 1% of F. tularensis RCI (B and C) and LVS (D) vacuoles, pili or protrusions of the bacterial cell wall appear to be in direct contact with the phagosomal walls. Size bars indicate 0.5 μm in all panels.
The majority of formalin-killed F. tularensis RCI enter peripheral blood MDM and adherent THP-1 cells by a process that appears ultrastructurally similar to that of live F. tularensis, i.e., the bacteria are engulfed within a loose loop of pseudopodium (Fig. 3A and B). As with live bacteria, the pseudopodia are often very exuberant (Fig. 3A and B). Of 50 consecutive uptake profiles viewed in each of two separate experiments (100 uptake profiles total), 95 of the uptake profiles were consistent with uptake within loosely fitting pseudopod loops (Fig. 3A and B), 4 uptake profiles were consistent with conventional phagocytosis (Fig. 3C), and 1 profile resembled that described for ruffling/triggered macropinocytosis, with the bacterium residing within a pair of symmetrical pseudopodia (Table 1). We have also examined the uptake of F. tularensis RCI that have been killed either by boiling for 30 min or by treatment with 2% glutaraldehyde and found that these bacteria also enter human macrophages via spacious pseudopod loops. Extensive treatment of the F. tularensis with protease K prior to formalin killing also did not alter the morphology of uptake (not shown). However, we found that treatment of the bacteria with 1% periodic acid (to oxidize the carbohydrates of the bacterial capsule and lipopolysaccharide) and cross-linking of the resultant aldehyde groups with lysine led to uptake of the bacteria predominantly by conventional phagocytosis (Fig. 3D and E). In examinations of 75 and 78 uptake profiles of periodate-treated bacteria in each of two separate experiments (153 uptake profiles in total) (Table 1), 117 (76%) of the uptake profiles were consistent with conventional phagocytosis, 17 (11%) of the bacteria appeared to be internalized within symmetrical pairs of pseudopodia (consistent with ruffling/triggered macropinocytosis) and 19 (12%) were internalized within spacious pseudopod loops, similar to those shown in Fig. 1A and C and 3A and B.
FIG. 3.
Interaction of formalin-killed and periodate-treated F. tularensis with human MDM. (A and B) The majority of formalin-killed F. tularensis bacteria are internalized by human MDM within exuberant pseudopod loops that resemble those of live bacteria. (C) A low percentage (4%) of the bacteria are internalized by conventional phagocytosis. (D and E) The majority (76%) of periodate-treated F. tularensis are internalized by conventional phagocytosis. Size bars indicate 1 μm in all panels.
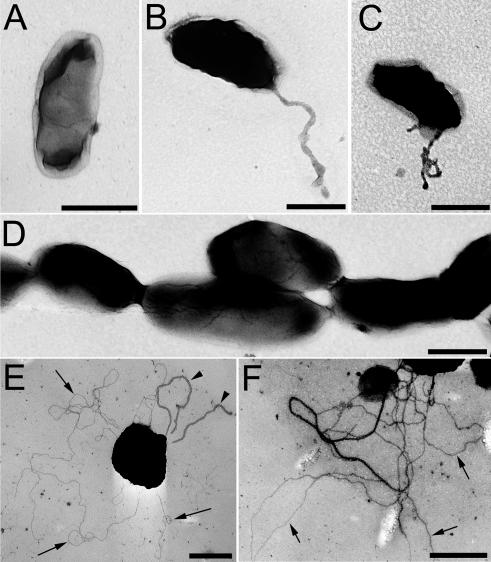
Our observation that a relatively tight phagosome is formed within seconds or minutes of entry (Fig. 2A to D) argues against the possibility that an enormous electron lucent capsule fills the space between the bacterium and the pseudopod loops. Moreover, negative staining of the bacteria revealed bacterial widths of 0.5 μm and lengths of 1 μm (Fig. 4A), considerably smaller than the dimensions of the spacious loops and peripherally located phagosomal compartments. Long pili or filaments on the surface of the bacterium might also explain the apparently spacious phagocytic loops. However, consistent with the report by Gil et al. (19), we have observed that more than 75% of the F. tularensis RCI and LVS grown on enriched solid media (used in the uptake experiments) do not express pili (Fig. 4A). Those that do usually have only a single thick pilus (Fig. 4B), though rarely additional pili are observed (Fig. 4C). These pili appear to be sex pili, as mating pairs are occasionally observed (data not shown). In examinations of at least 200 negatively stained bacteria from each of three separate experiments, the percentage of bacteria expressing the thick sex pili was variable, but in all cases it was less than 25%. The size, distribution, and relatively low frequency of the sex pili on the F. tularensis make it unlikely that they can account for the uniform uptake of F. tularensis within loosely fitting pseudopod loops. We believe that it is unlikely that there are large capsules that are missed by our negative staining, because we frequently observe the bacteria to be closely juxtaposed (Fig. 4D). We did not observe any fine, type IV pili on any of our plate-grown F. tularensis RCI or LVS, again consistent with the report of Gil et al. (19). As a positive control for the capacity of our negative staining technique to visualize the thin type IV pili, we stained a recently obtained clinical isolate of Neisseria gonorrhea in two separate staining experiments using the same negative staining procedure and observed thin, type IV pili on 68 of 100 and 59 of 100 consecutive negatively stained N. gonorrhea in the first and second experiment, respectively (Fig. 4E and F).
FIG. 4.
TEM of negatively stained preparations of F. tularensis RCI (A to D) and a clinical isolate of N. gonorrhea (E and F) after overnight growth on agar plates. (A and D) The majority of F. tularensis RCI have no apparent pili, and the dimensions of the bacteria observed by negative staining of whole bacteria are similar to the dimensions of the bacteria observed in thin sections. (B and C) A low percentage of F. tularensis RCI possess pili, but the dimensions and distribution of these pili do not account for the spacious pseudopod loops observed during uptake by macrophages. (D) F. tularensis bacteria are frequently observed to be closely juxtaposed, arguing against the presence of large capsules that might be invisible in the negative staining. (E and F) In contrast to plate-grown F. tularensis RCI, the majority of organisms of a recent clinical isolate of N. gonorrhea exhibit fine type IV pili (arrows) and some also have thicker sex pili similar to those of F. tularensis (E) (arrowheads). Size bars indicate 0.5 μm in all panels.
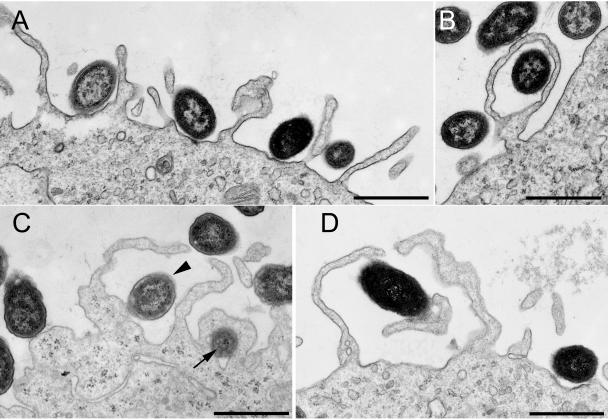
The ultrastructure of uptake of live F. tularensis by human MDM differs from the ruffling/triggered macropinocytotic uptake of S. enterica serovar Typhimurium and S. flexneri (Fig. 5 A to D). In two separate experiments examining the uptake of S. enterica serovar Typhimurium by human MDM (examining a total of 137 uptake profiles) (Table 1), we observed no uptake profiles that were similar to the exuberant, spacious loops of F. tularensis shown in Fig. 1A to C. Instead, 76% of the S. enterica bacteria were within relatively short, loosely fitting, symmetrical pairs of pseudopodia (Fig. 5A and C, arrowhead, and Table 1). While 6% of the S. enterica serovar Typhimurium uptake profiles were asymmetrical (i.e., the bacteria were embraced by a single pseudopod rather than a pair of pseudopodia), the pseudopod loops were shorter and less spacious than those observed for F. tularensis. In contrast to live F. tularensis, for which we have never observed any uptake profiles consistent with conventional phagocytosis, 18% of the S. enterica serovar Typhimurium bacteria appeared to be internalized by conventional phagocytosis (Table 1). Whereas F. tularensis bacteria at the macrophage periphery were found within remarkably spacious loops and vacuoles, under our opsonization and uptake conditions, S. enterica serovar Typhimurium bacteria at the cell periphery were found within more tightly fitting loops or vacuoles (e.g., Fig. 5B and C, arrow). We obtained similar results in two separate experiments examining a total of over 150 consecutive uptake profiles of S. flexneri by human MDM (Table 1). Of the uptake profiles examined, 72% showed internalization of S. flexneri within relatively short, symmetrical pairs of pseudopodia (Fig. 5D). While approximately 4% of the S. flexneri bacteria were found within single, asymmetric pseudopod loops, these loops were not as elaborate as those observed for F. tularensis. Uptake profiles of 23% of the S. flexneri bacteria were consistent with conventional phagocytosis (tightly adherent pseudopodia moving circumferentially around the bacteria).
FIG. 5.
S. enterica serovar Typhimurium (A to C) and S. flexneri (D) are internalized by human MDM by an ultrastructural process that differs from that of F. tularensis. S. enterica serovar Typhimurium bacteria (A and C) (arrowhead) are internalized within symmetric pairs of pseudopodia that are shorter than the pseudopod loops that engulf F. tularensis. Pseudopod loops (B) and vacuoles located at the periphery of the macrophage (C) (arrow) are less spacious than those observed for F. tularensis. (D) Uptake of S. flexneri within a symmetric pair of pseudopodia. Size bars indicate 1 μm in all panels.
Under the conditions of our uptake assay, we find that within 5 min of uptake the majority of S. enterica serovar Typhimurium and S. flexneri bacteria that are within the main macrophage cell body (i.e., the cell excluding its periphery) are in relatively tight nascent phagosomes. Of 78 consecutive S. enterica serovar Typhimurium vacuoles located within the main macrophage cell body within 5 min of uptake, we observed only 2 to be spacious. Similarly, of 32 consecutive S. flexneri vacuoles located within the macrophage cell body within 5 min of uptake, we also observed only 2 to be spacious. For S. enterica serovar Typhimurium, these results are consistent with the electron micrograph image published by Francis et al. (17) showing tight nascent phagosomes simultaneous with triggered macropinocytotic uptake by Hep2 cells. For S. flexneri, this is consistent with the tight nascent phagosomes in electron micrographs published by Clerc et al. (9) and Sansonetti et al. (33).
Uptake of F. tularensis is inhibited by cytochalasin B.
Engulfment of F. tularensis by macrophage pseudopodia presumably requires the participation of actin microfilaments. However, it has been stated in review articles that uptake of F. tularensis by macrophages occurs by a cytochalasin B-insensitive mechanism (14, 16). To assess the role of microfilaments in uptake of F. tularensis, we quantitated numbers of adherent and internalized F. tularensis after incubation with human MDM and THP-1 cells in the presence or absence of cytochalasin B. We found that cytochalasin B decreased the number of internalized bacteria and increased the number of adherent, noninternalized, bacteria in a dose-dependent fashion (Fig. 6). We observed this dose-dependent inhibition of internalization for formalin-killed F. tularensis RCI, for live F. tularensis RCI and LVS, and for both THP-1 cells and MDM. We used transmission electron microscopy to examine the ultrastructure of the interaction of F. tularensis RCI with THP-1 macrophages with and without treatment with cytochalasin B and confirmed that macrophages treated with cytochalasin B exhibited a dramatic loss of pseudopod formation (data not shown).
FIG. 6.
Cytochalasin B inhibits the internalization of F. tularensis by human THP-1 cells. THP-1 cells were pretreated for 15 min with the indicated concentration of cytochalasin B prior to incubation for 60 min with F. tularensis RCI at a MOI of 35:1 (bacteria:macrophage) in the same concentration of cytochalasin B and 10% serum in RPMI 1640. Monolayers were washed, fixed, stained by immunofluorescence for extracellular bacteria, permeabilized, and stained for intracellular bacteria with a different fluorescent antibody. Bacteria that were stained by fluorescent antibody only after permeabilization were counted as internalized (▪); bacteria stained by fluorescent antibody prior to permeabilization were counted as external (□). Data shown are mean values (± standard errors [SE]) of triplicate counts of more than 300 macrophages each. We obtained similar results with MDM and with F. tularensis LVS and with formalin-killed F. tularensis.
Uptake of F. tularensis by human macrophages requires complement and complement receptors.
At the relatively low multiplicity of infection (MOI) that we employ in our fluorescence microscopy experiments (35:1), we observe that efficient internalization of F. tularensis by human macrophages is critically dependent on preopsonization of the bacteria with serum or on the presence of serum in the incubation medium. We examined the relationship between the serum concentration and the efficiency of uptake and observed that adherence and/or uptake of both F. tularensis LVS and RCI increased as the percentage of serum increased from 0% to 10% (Fig. 7A). We have observed good uptake of the bacteria by THP-1 cells or by MDM only in the presence of freshly prepared human AB serum that was handled carefully so as to preserve complement activity. When we used heat-inactivated fetal bovine serum or heat-inactivated human AB serum, we observed only low levels of bacteria associated with the macrophage monolayers (data not shown).
FIG.7.
Adherence/uptake of F. tularensis by human macrophages requires serum, complement factor C3, and complement receptors. (A) Monolayers of human MDM on glass coverslips were incubated with F. tularensis live vaccine strain (LVS) or a virulent recent clinical isolate (RCI) in culture medium containing 10% HI-FBS and 0 to 10% autologous serum at an MOI of 35:1 for 1 h at 37°C. Monolayers were washed to remove nonadherent bacteria, fixed, and stained by immunofluorescence for F. tularensis. The number of adherent or internalized bacteria per MDM (top panel) and the percentage of MDM with adherent or internalized bacteria (bottom panel) were determined by immunofluorescence microscopy. Values represent the means (± SE) of triplicate determinations of at least 100 MDM examined for each data point. (B) Monolayers of human MDM on glass coverslips were incubated with F. tularensis live vaccine strain (LVS) or a virulent recent clinical isolate (RCI) at an MOI of 35:1 in culture medium containing 10% C3-depleted human AB serum supplemented with 0, 32.5, or 65 μg/ml of purified C3, and the numbers of bacteria per macrophage were determined as described above. (C) Monolayers of human MDM were treated with or without mouse anti-human complement receptor antibodies (anti-CD11b and/or anti-CD18) or an isotypic control antibody (anti-HLA-DR) for 30 min before incubation with F. tularensis LVS or RCI and with the same monoclonal antibody (all antibodies at 10 μg/ml) in the culture medium with 10% AB serum for 60 min at 37°C. Monolayers were washed, fixed, stained, and viewed by immunofluorescence microscopy to determine the number of bacteria per macrophage as described above. Values shown represent means (± SE) of triplicate determinations of at least 100 macrophages each.
We also examined the interaction of F. tularensis LVS and RCI with monolayers of HeLa cells in the presence of either fresh or heat-inactivated AB serum. In marked contrast to our observations with MDM and THP-1 cells, incubation of the bacteria with HeLa cells at an MOI of approximately 50:1 for 60 min resulted in only low levels of adherence or uptake of the bacteria; less than 0.5% of the HeLa cells exhibited adherent or internalized F. tularensis LVS or RCI bacteria. The level of adherence/uptake of bacteria by the HeLa cells was not altered by the use of heat-inactivated or fresh serum (data not shown).
HeLa cells lack receptors present on professional phagocytes, including the mannose receptor, the Fc receptor, and complement receptors. The inefficient uptake of F. tularensis by HeLa cells and the requirement for fresh serum for optimal adherence and uptake of F. tularensis by macrophages might be explained if complement and complement receptors played an important role in mediating internalization of F. tularensis. To determine whether complement factor C3 was required for uptake and internalization, we incubated the bacteria with macrophages in the presence of serum depleted of complement factor C3 or in this serum replenished with purified C3. We found that adherence and/or uptake of F. tularensis RCI and LVS required complement factor C3 (Fig. 7B). However, complement factor C3 alone (in the absence of C3-depleted serum) did not promote adherence or uptake of the bacteria (data not shown). To examine the role of complement receptors in adherence and uptake of F. tularensis, we treated MDM with antibody to CD11b or CD18 (or both) prior to and during infection with F. tularensis RCI and LVS. (CD11b forms part of complement receptor CR3; CD18 forms part of both complement receptors CR3 and CR4.) This treatment significantly inhibited the adherence and/or uptake of F. tularensis by the macrophages (Fig. 5C). As a control, incubation of the monolayers with an isotypic control anti-HLA-DR antibody had no effect on the adherence/uptake of F. tularensis RCI or LVS by the macrophages (Fig. 5C). We obtained similar results using THP-1 cells (data not shown).
DISCUSSION
Our data show that macrophages internalize F. tularensis by a cytochalasin-sensitive, morphologically unique process of engulfment within spacious, asymmetric pseudopod loops, that efficient uptake of F. tularensis by human macrophages requires serum with intact complement activity, and that complement component C3 is essential for uptake. Uptake of the bacteria is promoted by complement receptor CR3 and likely other complement receptors interacting with complement fixed on the surface of the bacteria. Internalization of the bacteria via complement and complement receptors may be an important aspect of pathogenesis, because uptake by this process does not trigger an oxidative burst by human macrophages (44).
McElree and Downs (27) observed that rat mononuclear cells phagocytosed F. tularensis in vitro and that nonimmune heat-treated guinea pig or calf serum enhanced uptake markedly (10-fold). Paradoxically, rat, horse, and rabbit serum did not promote uptake. The authors noted that the sera that promoted uptake also supported viability of the monolayer, whereas sera that did not support uptake were less supportive of viability. The period of incubation of the bacteria with the monolayers was lengthy in these studies, and this confounding factor makes it unclear whether the serum played an opsonic role or instead was needed for the health of the macrophages. In addition to containing opsonic factors, serum can stimulate pinocytosis (10). However, our studies have demonstrated that complement factor C3 is essential for uptake and that serum depleted of C3 does not promote uptake of F. tularensis, indicating a primary opsonic role for serum complement.
Vaccination of humans with the LVS strain has been reported to induce a humoral immune response and complement-fixing antibodies that can be detected by deposition of C3c on bacterial sonicates in a complement-fixing enzyme-linked immunosorbent assay (25). However, it should be noted that this assay employed sonicated, rather than intact, encapsulated bacteria. The capacity of encapsulated F. tularensis bacteria to act as acceptors of complement deposition has not been reported.
The molecular targets of complement fixation and opsonization on intact F. tularensis have not been elucidated, but components of the bacterial capsule, including LPS, are likely candidates. LPS purified from F. tularensis LVS has been shown to activate complement by the classical pathway (18). It has been reported that normal human serum is bactericidal for a capsule-deficient mutant strain of F. tularensis and that this is mediated largely by complement activated by natural IgM antibody directed against the bacterial LPS (32). Encapsulated F. tularensis bacteria are resistant to the bactericidal action of serum, and this appears to be due to low accessibility of the antigenic targets because of the O-side chains of the capsular LPS (40). Virulent strains of F. tularensis and the attenuated LVS strain are reported to be encapsulated. While we do not observe extremely large capsules by negative staining, it is likely that our bacteria also have the previously described O-linked polysaccharide capsular surfaces. While encapsulated F. tularensis are less efficiently bound by natural anti-LPS antibody and are less efficient in activating complement, we believe it likely that they do fix some complement and that this is an important opsonic factor mediating uptake.
Complement and/or complement receptors have been shown to play a role in the internalization of many other intracellular pathogens, including L. pneumophila (30), M. tuberculosis (39), M. leprae (36, 37), M. avium (4), Leishmania donovani (5), Leishmania major (28), Listeria monocytogenes (13), Histoplasma capsulatum (6), and Trypansoma cruz (31). Although all of these pathogens are internalized via complement and complement receptors, the ultrastructural process of their internalization and their subsequent intracellular compartments show great variability. For example, whereas M. tuberculosis is internalized via “conventional phagocytosis,” with the bacterium sinking into the macrophage between tightly juxtaposed pseudopodia, L. pneumophila is internalized via coiling phagocytosis, with the bacterium being engulfed within a tightly fitting pseudopod that coils repeatedly around the bacterium.
Conventional phagocytosis, a process that has been demonstrated for a large variety of microorganisms and inert particles, represents a sequential interaction between ligands on the surface of the particle and phagocytic receptors, leading to engulfment of the particle within tightly fitting pseudopodia that move circumferentially and symmetrically around the particle (21-23). In coiling phagocytosis, exemplified by L. pneumophila (24), the interaction between the phagocyte pseudopod and the bacterium is also very tight, but the bacterium becomes wrapped within multiple layers of an asymmetrical coil. In this report, we show that both live and formalin-killed F. tularensis enter human macrophages by a process that is ultrastructurally distinct from both conventional phagocytosis and coiling phagocytosis. We have used identical procedures to examine the uptake of other pathogenic and nonpathogenic bacteria, including L. pneumophila, M. tuberculosis, M. leprae, Escherichia coli, and pneumococcus, and have not observed this phenomenon of uptake within spacious pseudopod loops (7, 23, 24, 38, 39). We hypothesize that uptake within spacious pseudopod loops represents a form of triggered macropinocytosis requiring participation of complement factor C3 and complement receptors. The complement-complement receptor interaction may be needed to bring the bacterium into intimate contact with the macrophage or to initiate intracellular signaling events or both. In either case, it is clear that both live and formalin-killed F. tularensis possess molecules that can trigger a dramatic protrusion of macrophage pseudopodia. The fact that formalin-killed bacteria enter via a similar morphological process indicates that the bacterial molecules are preformed and do not require metabolic activity on the part of the bacteria to trigger pseudopod extension by the host macrophage. The fact that heat treatment and protease treatment of the bacteria do not alter the morphology of uptake indicates that the bacterial molecules that trigger the uptake mechanism are heat stable and protease resistant; perhaps they are lipopolysaccharides. Consistent with this hypothesis, we have found that oxidation and cross-linking of bacterial carbohydrates by sequential treatment with periodate and lysine leads to uptake of F. tularensis by conventional phagocytosis.
S. enterica serovar Typhimurium and S. flexneri bacteria enter macrophages by a process of triggered ruffling and macropinocytosis (1, 17) (Fig. 5). However, the phagocytosis of F. tularensis is ultrastructurally distinct from that of S. enterica serovar Typhimurium and S. flexneri in that it involves uptake within an asymmetric, spacious pseudopod loop rather than within the more closely juxtaposed, symmetrical pairs of pseudopod extensions (ruffles) induced by S. enterica serovar Typhimurium and S. flexneri. Whereas S. enterica serovar Typhimurium bacteria enter and persist in a spacious phagosome subsequent to phagocytosis (2), the F. tularensis phagosome rapidly becomes tight following uptake (Fig. 2) and remains relatively tight until escape of the bacteria into the host cell cytoplasm (8, 20).
In the case of L. pneumophila, as with F. tularensis, formalin-killed bacteria are also taken up by a process morphologically similar to that of live bacteria. While there are morphological differences between the uptake of F. tularensis and L. pneumophila, it is intriguing that both are highly dependent on complement (30), that both involve dramatic pseudopod extensions, and that these two intracellular pathogens have genetic similarities (14). In addition, both L. pneumophila and F. tularensis have lipopolysaccharides with atypical structures and biological activities, including low endotoxicity (29, 41, 43).
F. tularensis is well adapted to the intracellular environment of the macrophage, and an efficient uptake process by these cells is of central importance to the pathogenicity of F. tularensis. Further elucidation of the molecular interactions that trigger its uptake by host cells will be important in understanding its pathogenicity and remarkably high infectivity.
Acknowledgments
We thank Birgitta Sjostrand and Matthew Schibler for expert technical assistance.
This work was supported by grants AI053403 and HL077000 from the National Institutes of Health and grant DAMD17-03-1-0052 from the U.S. Army Medical Research and Materiel Command.
Editor: D. L. Burns
REFERENCES
- 1.Adam, T., M. Arpin, M.-C. Prévost, P. Gounon, and P. J. Sansonetti. 1995. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J. Cell Biol. 129:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpuche-Aranda, C. M., E. L. Racoosin, J. A. Swanson, and S. I. Miller. 1994. Samonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 179:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony, L., R. Burke, and F. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., L. S. Young, and H. Enkel. 1991. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect. Immun. 59:1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell, J. M., R. A. B. Ezekowitz, M. B. Roberts, J. Y. Channon, R. B. Sim, and S. Gordon. 1985. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J. Exp. Med. 162:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullock, W. E., and S. D. Wright. 1987. Role of the adherence-promoting receptors CR3, LFA-1, and p150,95 in binding of Histoplasma capsulatum by human macrophages. J. Exp. Med. 165:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens, D. L., and M. A. Horwitz. 1992. Membrane sorting during phagocytosis: selective exclusion of MHC molecules but not complement receptor CR3 during conventional and coiling phagocytosis. J. Exp. Med. 175:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens, D. L., B. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerc, P. L., A. Ryter, J. Mounier, and P. J. Sansonetti. 1987. Plasmid-mediated killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect. Immun. 55:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn, Z. A., and B. Benson. 1965. The in vitro differentiation of mononuclear phagocytes. II. The influence of serum on granule formation, hydrolase production, and pinocytosis. J. Exp. Med. 121:835-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehio, C., M. C. Prevost, and P. J. Sansonetti. 1995. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src mediated signalling pathway. EMBO J. 14:2471-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis, D., T. Inglesby, D. Henderson, J. Bartlett, M. S. Ascher, E. Eitzen, A. Fine, A. Friedlander, J. Hauer, M. Layton, S. Lillibridge, J. McDade, M. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russel, K. Tonat, and the Working Group on Civilian Biodefense. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 13.Dreverts, D. A., and P. A. Campbell. 1991. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect. Immun. 59:2645-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis, J., P. C. F. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortier, A., D. Leiby, R. Narayanan, E. Asafoadjel, R. Crawford, C. Nacy, and M. Meltzer. 1995. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect. Immun. 63:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortier, A. H., S. J. Green, T. Polsinelli, T. R. Jones, R. M. Crawford, D. A. Leiby, K. L. Elkins, M. S. Meltzer, and C. A. Nacy. 1994. Life and death of an intracellular pathogen: Francisella tularensis and the macrophage. Immunol. Ser. 60:349-361. [PubMed] [Google Scholar]
- 17.Francis, C., T. Ryan, B. Jones, S. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 18.Fulop, M., T. Webber, and R. Manchee. 1993. Activation of the complement system by Francisella tularensis lipopolysaccharide. New Microbiol. 16:141-147. [PubMed] [Google Scholar]
- 19.Gil, H., J. Benach, and D. Thanassi. 2004. Presence of pili on the surface of Francisella tularensis. Infect. Immun. 72:3042-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjöstedt. 2003. An attenuated strain of the facultative intracellular bacterium F. tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin, F. M., Jr., J. A. Griffin, J. E. Leider, and S. C. Silverstein. 1975. Studies of the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 142:1263-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin, F. M., Jr., J. A. Griffin, J. E. Leider, and S. C. Silverstein. 1975. Studies on the mechanisms of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG coated bone marrow derived lymphocytes. J. Exp. Med. 144:788-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz, M. A. 1982. Phagocytosis of microorganisms. Rev. Infect. Dis. 4:104-123. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz, M. A. 1984. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell 36:27-33. [DOI] [PubMed] [Google Scholar]
- 25.Koskela, P. 1985. Humoral immunity induced by a live Francisella tularensis vaccine. Complement fixing antibodies determined by an enzyme-linked immunosorbent assay (CF-ELISA). Vaccine 3:389-391. [DOI] [PubMed] [Google Scholar]
- 26.Long, G., J. Oprandy, R. Narayanan, A. Fortier, K. Porter, and C. Nacy. 1993. Detection of Francisella tularensis in blood by polymerase chain reaction. J. Clin. Micro. 31:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McElree, H., and C. Downs. 1961. The phagocytosis of Pasteurella tularensis by rat mononuclear cells as influenced by normal serums and various irritants. J. Infect. Dis. 109:98-106. [DOI] [PubMed] [Google Scholar]
- 28.Mosser, D. M., and P. J. Edelson. 1985. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135:2785-2789. [PubMed] [Google Scholar]
- 29.Otten, S., S. Iyer, W. Johnson, and R. Montgomery. 1986. Serospecific antigens of Legionella pneumophila. J. Bacteriol. 167:893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne, N. R., and M. A. Horwitz. 1987. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J. Exp. Med. 166:1377-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rimoldi, M. T., A. J. Tenner, D. A. Bobak, and K. A. Joiner. 1989. Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi trypomastigotes. J. Clin. Investig. 84:1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandstrom, G., S. Lofgren, and A. Tarnvik. 1988. A capsule-deficient mutant of Francisella tularensis LVS exhibits enhanced sensitivity to killing by serum but diminished sensitivity to killing by polymorphonuclear leukocytes. Infect. Immun. 56:1194-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saslaw, S., H. T. Eigelsbach, J. Prior, H. Wilson, and S. Carhart. 1961. Tularemia vaccine study. I: intracutaneous challenge. Arch. Intern. Med. 107:121-133. [DOI] [PubMed] [Google Scholar]
- 35.Saslaw, S., H. T. Eigelsbach, J. Prior, H. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702-714. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger, L. S., and M. A. Horwitz. 1991. Phagocytosis of M. leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and interferon gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147:1983-1994. [PubMed] [Google Scholar]
- 37.Schlesinger, L. S., and M. A. Horwitz. 1990. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J. Clin. Investig. 85:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlesinger, L. S., and M. A. Horwitz. 1990. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J. Clin. Investig. 85:1304-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlesinger, L. S., C. G. Bellinger-Kawahara, N. R. Payne, and M. A. Horwitz. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771-2780. [PubMed] [Google Scholar]
- 40.Sorokin, V. M., N. V. Pavlovich, and L. A. Prozorova. 1996. Francisella tularensis resistance to bactericidal action of normal human serum. FEMS Immunol. Med. Microbiol. 13:249-252. [DOI] [PubMed] [Google Scholar]
- 41.Vinogradov, E., M. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 42.White, J. D., J. R. Rooney, P. A. Prickett, E. B. Derrenbacher, C. W. Beard, and W. R. Griffith. 1964. Pathogenesis of experimental respiratory tularemia in monkeys. J. Infect. Dis. 114:277-283. [DOI] [PubMed] [Google Scholar]
- 43.Wong, K., C. W. Moss, D. H. Hochstein, R. J. Arko, and W. O. Schalla. 1979. “Endotoxicity” of the Legionnaires' disease bacterium. Ann. Intern. Med. 90:624-627. [DOI] [PubMed] [Google Scholar]
- 44.Wright, S., and S. Silverstein. 1983. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158:2016-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]