Abstract
Messenger RNA (mRNA) is a type of RNA that carries genetic information from DNA to the ribosome, where it is translated into proteins. mRNA has emerged as a powerful platform for development of new types of medicine, especially after the clinical approval of COVID-19 mRNA vaccines. Chemical modification and nanoparticle delivery have contributed to this success significantly by improving mRNA stability, reducing its immunogenicity, protecting it from enzymatic degradation, and enhancing cellular uptake and endosomal escape. Recently, substantial progresses have been made in new modification chemistries, sequence design, and structural engineering to generate more stable and efficient next-generation mRNAs. These innovations could further facilitate the clinical translation of mRNA therapies and vaccines. Given that numerous review articles have been published on mRNA nanoparticle delivery and biomedical applications over the last few years, we herein focus on overviewing recent advances in mRNA chemical modification, mRNA sequence optimization, and mRNA engineering (e.g., circular RNA and multitailed mRNA), with the aim of providing new perspectives on the development of more effective and safer mRNA medicines.
Keywords: mRNA, self-amplifying RNA, circular RNA, multitailed mRNA, chemical modification, machine learning
1. Introduction
Messenger RNA (mRNA) is a single-stranded RNA that carries genetic information from DNA to ribosomes for translation into functional proteins [1]. It was discovered in the 1960s as an intermediate molecule between DNA and protein [2]. In the late 1980s, it was demonstrated that by introducing mRNA into mice via intramuscular injection, the local expression of the encoded protein was achievable [3]. Therefore, it is believed that mRNA could be used as a therapeutic agent or an antigen carrier for generation of desired proteins in target cells or tissues. Compared to viral gene therapy that has been clinically successful, mRNA therapy has unique advantages, such as direct translation into target proteins in the cytoplasm without the need of entering the nucleus, thus also avoiding the potential risk of insertional mutations [4]. mRNA could also largely avoid other challenges associated with viral gene therapy, such as re-dosing and packaging size.
The most exemplary application of mRNA technology is the development of vaccines against COVID-19, the disease caused by the novel coronavirus SARS-CoV-2. In December 2020, two mRNA vaccines, BNT162b2 from Pfizer-BioNTech and mRNA-1273 from Moderna, received emergency use authorization from the US Food and Drug Administration (FDA) and were widely administered worldwide [5, 6]. More recently, another mRNA vaccine was approved by the FDA for respiratory syncytial virus [7, 8]. mRNA-based neoantigen vaccines have also shown promising clinical results for cancer treatment [9–11]. The promise of mRNA vaccines against infectious diseases and cancer has promoted tremendous interest and investment in mRNA technology for other biomedical fields, such as protein replacement, immunotherapy, gene editing, and cell engineering [12–14].
When native mRNA is injected into the human body, it could trigger a series of heterologous immune responses and be degraded by the immune system [15, 16], similar to how the human body resists viral invasion, thus severely limiting the applicability of mRNA therapy. To overcome this challenge, various chemical modifications have been incorporated into mRNA molecules, thereby changing their physical, chemical, and biological characteristics. One prominent change is the substitution of uridine with pseudouridine (Ψ) or its methylated derivative, N1-methyl pseudouridine (m1Ψ), which could significantly reduce the immune stimulation [17–19]. Notably, the FDA-approved mRNA vaccines adopted the m1Ψ modification. Besides, chemical modification could also improve the stability and translation efficiency of mRNA molecules. In parallel, nanoparticle technologies (such as lipid nanoparticles) have contributed significantly to the success of mRNA delivery, by protecting mRNA from enzymatic degradation and increasing its cellular uptake and endosomal escape. Nanoparticles could also improve systemic mRNA delivery by prolonging the pharmacokinetics and enhancing accumulation in target organs or diseased tissues [20–26]. Many impactful and comprehensive review articles have been published on nanoparticle delivery and biomedical applications of mRNA [27–32], which will not be overviewed in this work.
Herein, we focus on recent progresses in three aspects of mRNA therapy: modification chemistry, sequence design and optimization, and structure engineering, which have shown the possibility for fine-tuning mRNA characteristics and providing new functions. Chemical modifications of nucleosides have demonstrated to be essential for development of mRNA vaccines and therapies [33]; yet, a recent finding hints that some modifications like m1Ψ might have unintended effects [34]. New modifications on the 5′ cap and poly(A) tail have recently been reported to improve mRNA stability, translation efficiency, and immunogenicity [35, 36]. In addition to chemical modification, mRNA sequence and structure are also important factors that affect the efficacy of mRNA therapy [37]. With the advancement of artificial intelligence, mRNA sequence optimization is now entering a new stage [38]. Different from traditional linear mRNA, novel mRNA structure, including circular RNA (circRNA) [39], self-amplifying RNA (saRNA) [40], trans-amplifying RNA (taRNA) [41], and multitailed mRNA [36], have also been developed to enhance protein production and its duration, which could be demanding for broader therapeutic uses beyond vaccination. These new technologies are expected to create more stable, efficient, and durable next-generation mRNAs, thus expanding the application scope of mRNAs in the biomedical field.
This review first introduces the basic principles and methods of mRNA synthesis, and then describes various modification methods and optimization strategies on mRNAs. We also highlight the design of new types of mRNAs, including saRNA, circRNA, and multitailed mRNA, followed by the broad application prospects of mRNA therapy.
2. Over view of mRNA synthesis
mRNA therapy is emerging as a new type of treatment by guiding the expression of desired proteins in the body. To do that, high-quality mRNA needs to be synthesized. At present, there are two commonly used methods for in vitro RNA synthesis: chemical synthesis and enzymatic transcription synthesis [42–44]. Phosphoramidite chemistry and solid-phase synthesis enable the preparation of oligonucleotides with the correct 3′–5′ phosphodiester bond [42, 44]. To prevent unwanted by-products from reactive substituents in natural nucleoside triphosphates, inert substituents are used during synthesis. Afterward, protective groups are removed, resulting in oligonucleotides structurally similar to cellular RNA [44]. However, this method is generally limited to synthesis of RNAs up to 80 nucleotides.
Alternatively, mRNA drugs are commonly synthesized by in vitro transcription (IVT), which is a process to produce mRNA from a linear DNA template by using RNA polymerases (such as T3, T7, or SP6) in a cell-free system in the presence of cap precursors and free triphosphate nucleotides [27, 45]. The purity, quantity and culture time of the DNA template are the main factors affecting the yield of RNA synthesis. Compared with chemical synthesis methods, IVT can synthesize longer RNAs at a lower cost, but the characteristics of phage RNA polymerase may cause RNA to be incomplete or inconsistent. For example, IVT reactions using T7, SP6 and T3 polymerases require the first nucleotide of the transcript to be guanosine to ensure the highest transcription efficiency [43, 44]. Therefore, some studies have explored mutated phage RNA polymerase to improve transcription quality and reduce side reactions [46, 47].
The structure of IVT mRNA is similar to that of mature mRNA in eukaryotic cells and consists of five main components: 5′ cap, 5′ and 3′ untranslated regions (UTRs), open reading frame (ORF), and 3′ poly(A) tail (Fig. 1(a)) [48, 49]. These components affect the stability, immunogenicity, translation efficiency, and expression time of mRNAs [4, 50]. IVT mRNA can mimic native mRNA in eukaryotic cells and can be translated into specific proteins in the body; thus, IVT mRNA can be used to treat various diseases [51, 52]. With the advancement and maturity of IVT technology, large amounts of mRNAs can be easily synthesized [53, 54]. This process is relatively inexpensive and robustly scalable, as compared to the manufacturing of viruses used in gene therapy and recombinant proteins. To improve the performance and safety of mRNA drugs, chemical modifications and sequence design of each component of mRNA are discussed below.
Figure 1.

Major structural components and modification strategies of IVT mRNA. (a) Key structural elements of IVT mRNA (a 5′ cap of 7-methylguanosine, which is connected to the rest of the mRNA via a 5′–5′ triphosphate bridge; an ORF flanked by 5′ and 3′ UTRs and a 3′ poly(A) tail) and their main functions. (b) Different cap variants, such as methylations at the N7 position of the guanosine cap (ARCA) and 2′-O methylation of the first (Cap 1) or second nucleotide ribose (Cap 2). (c) Commonly used strategies for the modification of native nucleosides in IVT mRNA. (d) Modification methods and chemical structures of some ARCA analogues.
3. mRNA chemical modifications
3.1. Nucleoside
The innate immune system recognizes IVT mRNA as a foreign RNA, activating immune responses through pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) [55–57]. However, this could significantly hinder mRNA translation efficiency and potentially induce severe side effects. For potent and safe biomedical applications, it is crucial to reduce mRNA immunogenicity. One effective approach is to use chemically modified nucleotides to replace natural ones, thereby lowering mRNA affinity for PRRs, minimizing immune activation and improving translation efficiency [37, 50]. Notably, chemical modification of nucleosides has been crucial to the advancement of mRNA technology, enabling the rapid clinical development of effective mRNA vaccines during the COVID-19 pandemic. Two scientists won the Nobel Prize in Physiology or Medicine in 2023 due to their groundbreaking achievements in this field [58].
Reducing mRNA immunogenicity often involves replacing uridine (U) with Ψ [18], because U-rich RNA sequences strongly activate TLRs, triggering immune responses against mRNAs [59, 60]. Ψ-modified mRNA reduces PRR activation, dampening immune reactions and enhancing protein expression [61, 62]. Additionally, Ψ modification improves mRNA translation efficiency, with m1Ψ being particularly effective [63]. Notably, the two clinically approved COVID-19 mRNA vaccines adopted m1Ψ-modified mRNA encoding the SARS-CoV-2 spike protein [33]. In addition, there are several other nucleotide modifications, such as N1-methyladenosine (m1A), N6-methyladenosine (m6A), 5-methylcytidine (m5C), 5-methoxycytidine (mo5C), 5-methyluridine (m5U), 2-thiouridine (s2U) and 5-methoxyuridine (5moU), which can also partially replace the natural nucleotides in mRNA and lower its immunogenicity (Fig. 1(c)) [17, 64].
Although many studies have shown that chemical modifications of nucleotides play a positive role in mRNA translation, some findings have also shown that chemical modifications of nucleotides are not always beneficial [65]. It was recently discovered that nucleotides modified with m1Ψ in mRNA may cause +1 ribosomal frameshifting (Figs. 2(a)–2(c)) [34], resulting in some variant proteins, but without affecting the immune response to mRNA vaccines. This work found that m1Ψ caused ribosome stalling and +1 ribosome shifting during translation of IVT mRNA. To evaluate the effect of this ribosomal shifting code, the SARS-CoV-2 mRNA vaccine BNT162b2 was tested in mice and results showed that the altered proteins could cause an off-target immune response (Figs. 2(d) and 2(e)). Likewise, such off-target proteins from ribosomal shifts were detected in vaccinated humans, leading to unwanted immune reactions [34]. Another study evaluated unmodified and Ψ-modified mRNA in C12–200 lipid nanoparticles and found that Ψ modification did not provide significant benefits for mRNA delivery to the liver. It further noted that Ψ modification might not be necessary for liver therapy with mRNA [65]. In addition, a recent work show that tumor antigen mRNA vaccines without m1Ψ modification activated the interferon-I (IFN-I) signaling pathway more effectively than those with m1Ψ modification and inhibited the growth and metastasis of various tumor models [66]. Therefore, chemical modifications of nucleotides need to be rationally selected and designed according to different application scenarios.
Figure 2.

m1Ψ modification in mRNA may cause +1 ribosomal frameshifting and off-target immunity. (a) Design of Fluc+1FS mRNA containing a sequence encoding the amino-terminal fragment of firefly luciferase (NFluc) located within the in-frame region (green segment) and a complementary carboxy-terminal fragment (CFluc) encoding Fluc located directly downstream outside the in-frame region (orange segment). Following the normal translation process, Fluc+1FS mRNA produces catalytically inactive NFluc; however, if the ribosome deviates from the frame during translation, it produces both in-frame NFluc-containing and out-of-frame CFluc-containing residues, which increase catalytic activity [34]. (b) Comparison of the activity of luciferase translated by Fluc+1FS mRNA with different modifications and unmodified controls. The incorporation of m1Ψ into Fluc+1FS mRNA significantly increased the frameshift of ribosome +1, which was not observed for other modified or unmodified mRNAs [34]. (c) Western blot image of peptides produced during IVT mRNA translation. It revealed that m1Ψ-modified mRNA produced the expected in-frame product but also two additional bands of higher molecular weight that were +1 frameshift peptides [34]. (d) The response of T cells in mice stimulated with different treatments was quantified by the IFNγ ELISpot assay, in which vaccinated mice showed a significant increase in response to the +1 frameshifted spike peptide [34]. (e) IFNγ ELISpot responses of mouse T cells under different treatments. Among them, vaccination with both BNT162b2 and ChAdOx1 nCoV-19 vaccines produced ELISpot responses against in-frame SARS-CoV-2 spike virus [34]. The results of (d) and (e) indicate that the +1 frameshift product encoded in the BNT162b2 spike mRNA is T cell antigen in mice, and off-target immunity to it is detected after vaccination. Reproduced with permission from Ref. [34], © Mulroney, T. E. et al. 2023.
3.2. 5′ cap
The 5′ end of mRNA features a specialized structure known as the 5′ cap, composed of 7-methylguanosine (m7G) linked to the mRNA’s first nucleotide via a 5′–5′-triphosphate bridge (ppp) [67, 68]. This 5′ m7G cap, often referred to as “Cap 0”, plays a crucial role in mRNA translation and stability. It binds to translation initiation factor 4E (eIF4E), facilitating mRNA translation initiation [69–71]. Additionally, the cap resists Dcp1/2 decapping enzymes, prolonging mRNA lifespan [72]. In humans and other higher eukaryotes, Cap 0 can be further modified to a Cap 1 or Cap 2 structure by 2′-O methylation of the ribose of the first or second nucleotide (Fig. 1(b)) [48, 73, 74]. Moreover, Cap 1 or Cap 2 structures are less immunogenic than Cap 0 structures [75]. It was recently reported that as mRNA in the cytoplasm senesces, Cap 1 is converted to Cap 2, which is enriched on mRNAs with longer half-lives. In addition, elevated levels of Cap 1 trigger an immune response that is inhibited by Cap 2 [35].
Two primary methods cap mRNA synthesized by IVT. The first method employs recombinant viral capping enzymes, like vaccinia virus capping enzyme (VCE), post-transcription. This yields the standard m7GpppG structure found in eukaryotic mRNA [76, 77]. While this approach ensures correct cap orientation, it produces only a single standard cap structure [78, 79]. The second method is co-transcriptional capping, where synthetic cap dinucleotides are added during transcription [76, 80–82]. This approach allows diverse cap structures but may lead to partial capping due to competition with GTP [68]. Recognition by the cap-binding protein can also be hindered if the cap dinucleotides are reverse-oriented [83].
Anti-reverse analogues (ARCAs) have been developed to address these challenges (Fig. 1(d)). ARCAs reduce the likelihood of reverse orientation, enhance eIF4E affinity, and improve resistance to decapping enzymes. These modifications enhance mRNA translation efficiency and stability [76, 83, 84]. Additional optimization strategies include modification of the first nucleotide or the triphosphate bridge. For instance, one study synthesized and evaluated a group of 15 chemical cap analogs containing a 5′-PSL moiety and revealed that the 5′-PSL molecule stabilizes the mRNA cap’s interaction with eIF4E, promoting mRNA translation and reducing cap sensitivity. These findings offer new structural insights for screening novel cap analogs with excellent biological properties [80]. Another recent work synthesized m7GpppBn6AmpG and incorporated it into mRNA using T7 polymerase. This modification exhibits significant resistance to fat mass and obesity-associated protein (FTO) demethylation and is easily purified via reversed-phase high-performance liquid chromatography (RP-HPLC). Additionally, the m7GpppBn6AmpG cap enhance protein translation efficiency [85]. Interestingly, the modification strategies used in the mRNA cap structure can sometimes be applied to the mRNA backbone. For instance, replacing the phosphoric acid in nucleic acids with phosphorothioate, where a sulfur atom substitutes for a non-bridging oxygen atom, creates a nucleic acid analogue with improved biological stability and cellular membrane permeability [86].
Unlike improving mRNA expression by modifying the 5′ cap, a new modification idea is to achieve controlled mRNA expression by modifying m7G in the 5′ cap. Specifically, a photoactivatable 5′ cap analog called “FlashCaps” was developed to block binding to eIF4E and resist decapping enzymes [87]. When exposed to laser irradiation, FlashCap rapidly and efficiently converts to the natural Cap 0 structure without leaving scars, thus restoring efficient translation. These findings demonstrate that FlashCaps offer a versatile approach for spatiotemporal control of mRNA expression. In summary, chemical modification of the 5′ cap, which is attributed to the critical role of the 5′ cap structure in mRNA translation initiation, provides an important means of regulating mRNAs. Not only can novel cap analogues and structural optimization improve mRNA expression, but photoactivated cap analogues also offer exciting possibilities for precise spatiotemporal control of gene expression.
3.3. poly(A) tail
Almost all mRNAs encoding cellular proteins have a poly(A) tail, which is a non-template addition of adenosine residues to the 3′ end of mRNA in eukaryotes. In mammalian cells, most actively translated mRNAs have a poly(A) tail of approximately 100–250 adenosine residues [88]. For the IVT mRNA used for mRNA therapy, the poly(A) tail is either encoded in the DNA template or added to the mRNA as a separate step by an enzymatic method after the IVT process [64, 89]. The advantage of DNA template-encoded poly(A) is that it can produce clear and repeatable poly(A) lengths. In contrast, the enzymatic posttranscriptional polyadenylation of mRNAs can produce transcripts with different poly(A) lengths, which may not meet quality control requirements [52].
The poly(A) tail significantly impacts mRNA translation and stability. It interacts with poly(A)-binding proteins (PABPs) [90], recruits eukaryotic initiation factor 4G (eIF4G) [91], and enhances cap affinity, forming a “closed loop structure” [92]. This structure facilitates binding of the 40S translation initiation complex, promoting translation initiation and regulating efficiency. Additionally, the poly(A) tail resists exonuclease degradation, prolonging mRNA lifespan. The poly(A) tail length also matters. Generally, translation efficiency correlates with the number of adenosines in the tail. However, different cells and conditions require varying tail lengths. Traditionally, a minimum of 20 nt was thought necessary for sufficient mRNA translation. Yet, stable β-actin has a shorter tail, and in human primary T cells, 425 and 525 nt tails improve transfection efficiency [50, 93].
In addition to the length of the poly(A) tail, a recent work made a new discovery that m6A modification occurs on the poly(A) tail of Trypanosoma brucei’s variable surface glycoprotein (VSG) mRNA [94]. Unlike in humans and mice, where m6A is enriched near the 3′ UTR and stop codon, in Trypanosoma brucei, it predominantly localizes to the poly(A) tail. Remarkably, m6A acts as a protective factor, enhancing VSG mRNA stability. Future research identifying the enzymes and proteins involved in m6A addition, reading, or removal could lead to even better mRNA poly(A) modifications.
4. mRNA sequence optimization
The design of UTRs and ORF sequences for mRNAs has garnered increased attention. Optimized sequences not only enhance protein expression and stability but also reduce immunogenicity. Traditional methods involve optimizing codon usage, adjusting GC content, and incorporating efficient sequences. However, sequence optimization is a complex process, and traditional approaches fall short in fully exploring the design space for highly stable mRNAs. In contrast, novel technologies leverage machine learning, high-throughput screening, and advanced algorithms to efficiently and comprehensively optimize mRNA sequences. These innovative methods hold promise for developing mRNA vaccines and drugs, addressing the limitations of traditional approaches.
4.1. UTRs
UTRs are noncoding regions located on both sides of the coding sequence; these regions do not encode proteins but play key roles in regulating mRNA stability and protein translation [64]. mRNA UTRs can be divided into 5′ and 3′ sequences, which affect the translation process through their sequence, secondary structure, and length. The 5′ UTR is an important element for ribosome recruitment and start codon selection [95], as it is the binding site for the preinitiation complex to initiate protein translation. The binding of eukaryotic initiation factor 4A (eIF4A) to the 5′ UTR is essential for unwinding the mRNA secondary structure before protein translation occurs [95]. It was demonstrated that the thermodynamic stability, hairpin loop position and GC percentage of the 5′ UTR directly affect mRNA translation [96]. It has been reported that shorter 5′ UTRs without complex secondary structures and start codons (such as AUG and CUG) are favorable for initiating mRNA translation [37]. In addition, internal ribosome entry sites (IRES) in the 5′ UTR can also recruit ribosomes and initiate translation in a cap- and eIF4E-independent manner [97]. The 3′ UTR is usually a regulatory element that also affects the stability and half-life of the mRNA [98]. Most eukaryotic mRNA 3′ UTRs contain mRNA degradation signals that regulate mRNA stability.
Adding regulatory sequences to the 5′ and 3′ UTRs is a strategy for improving mRNA stability and translation. The UTR sequences of highly expressed genes (such as α- and β-globins) are widely used for mRNA synthesis as mRNAs containing these UTRs usually have high levels of translation and stability. The use of an artificial 5′ UTR containing a strong Kozak translation signal and an α-globin 3′ UTR has been shown to increase protein yield [99]. The addition of β-globin 5′ and 3′ UTRs increases translation efficiency [50, 100]. In addition, transcriptional efficiency can be improved by adding the 3′ UTR sequence twice in tandem, but it is important to note that this boost varies across cell types [50]. Accelerated mRNA degradation can be achieved by introducing an adenylate-uridylic acid-rich element into the 3′ UTR of the mRNA for specific situations where a shorter protein expression time is required [101].
4.2. ORF
The ORF is a region of genetic information encoding proteins, and whether the codon information in the ORF can be deciphered smoothly is closely related to the protein expression efficiency of the mRNA [37, 102]. Because the same amino acid can be translated from a set of different codons, optimizing the ORF using synonymous codons is a common and effective method for improving the efficiency and stability of mRNA expression [103]. The specific reasons are as follows: first, different synonymous codons can affect the binding speed and efficiency of ribosomes and mRNAs; by choosing to use more common synonymous codons, the binding abilities of ribosomes and mRNAs can increase, thus accelerating the translation process and improving the rate of protein synthesis [104]. Second, synonymous codons encode the same amino acids, but the corresponding tRNA types and abundances may differ [105]. The tRNAs corresponding to certain synonymous codons may be more abundant and more easily recognized and utilized by the tRNA pool in the cell. By choosing to use synonymous codons corresponding to enriched tRNAs, translation efficiency can be improved, and protein expression levels can be increased. In addition, by selecting synonymous codons with greater stability, it is also possible to extend the lifetime of mRNA and increase the stability of protein expression [106]. It is worth noting that, as aforementioned, m1Ψ modification may lead to ribosomal +1 shifting phenomenon, which can produce unintended proteins during protein translation. However, by rationally designing the slippery shift site, the occurrence of +1 shift can be effectively reduced without affecting the translation of proteins, thus improving the safety and efficacy of mRNA therapeutics [34]. Avoiding uridine-rich regions in the ORF is another optimization method because they may be recognized and activated by RIG-I, leading to premature termination of mRNA translation [107, 108]; moreover, uridine removal is not necessarily limited to the ORF of the mRNA but can also be applied to the entire mRNA molecule.
However, optimizing the code is not an easy task. The use of synonymous codes in place of unusual ones sometimes affects the structure and function of proteins and even leads to the development of new biologically active peptides that have not yet been discovered [109, 110]. In some cases, high translation rates of mRNA are not always beneficial, and some proteins require slower translation rates to fold and remain stable [77, 111]. By increasing the amount of GC in the ORF, the resistance of mRNA to degradation by ribonucleases could be increased, which can help to improve the stability and persistence of mRNA and thus the expression of mRNA in vivo [112, 113]. In addition, the concentration of GC should not be too high because it may cause the secondary structure of RNA molecules to be too stable, making it difficult to unravel during the translation process and thus affecting translation efficiency [104, 114]. Therefore, the use of synonymous codons may differ in different species, tissues and conditions, and a specific optimization design is needed for practical applications.
4.3. Artificial intelligence assisted sequence optimization
The rapid development of artificial intelligence (AI) technology has provided new opportunities for mRNA design. Recently, using classical concepts from computational linguistics, the LinearDesign algorithm was used to simultaneously optimize mRNA stability and codon optimality and identified the optimal mRNA sequence from hundreds of millions of codon combinations in approximately ten minutes, which is difficult to achieve by traditional algorithms or humans (Fig. 3(a)). In particular, the mRNA sequence designed by the LinearDesign algorithm is up to 5-fold more stable (mRNA molecular half-life), up to 3-fold more protein-expressed, and up to 128-fold more antibody-responsive than the benchmark COVID-19 mRNA vaccine optimized for codon optimization in the current clinical trial (Figs. 3(b) and 3(c)). Moreover, the Varicella–Zoster virus (VZV) mRNA vaccine sequences designed by the LinearDesign algorithm also significantly outperform the conventional VZV mRNA vaccine in terms of stability and protein expression level. These results demonstrate the great potential of AI in mRNA sequence optimization, which is likely to play a key role in future pandemics. Moreover, this algorithm is not limited to mRNA vaccine design but also provides a promising tool for mRNA drug design for protein replacement therapy [38].
Figure 3.
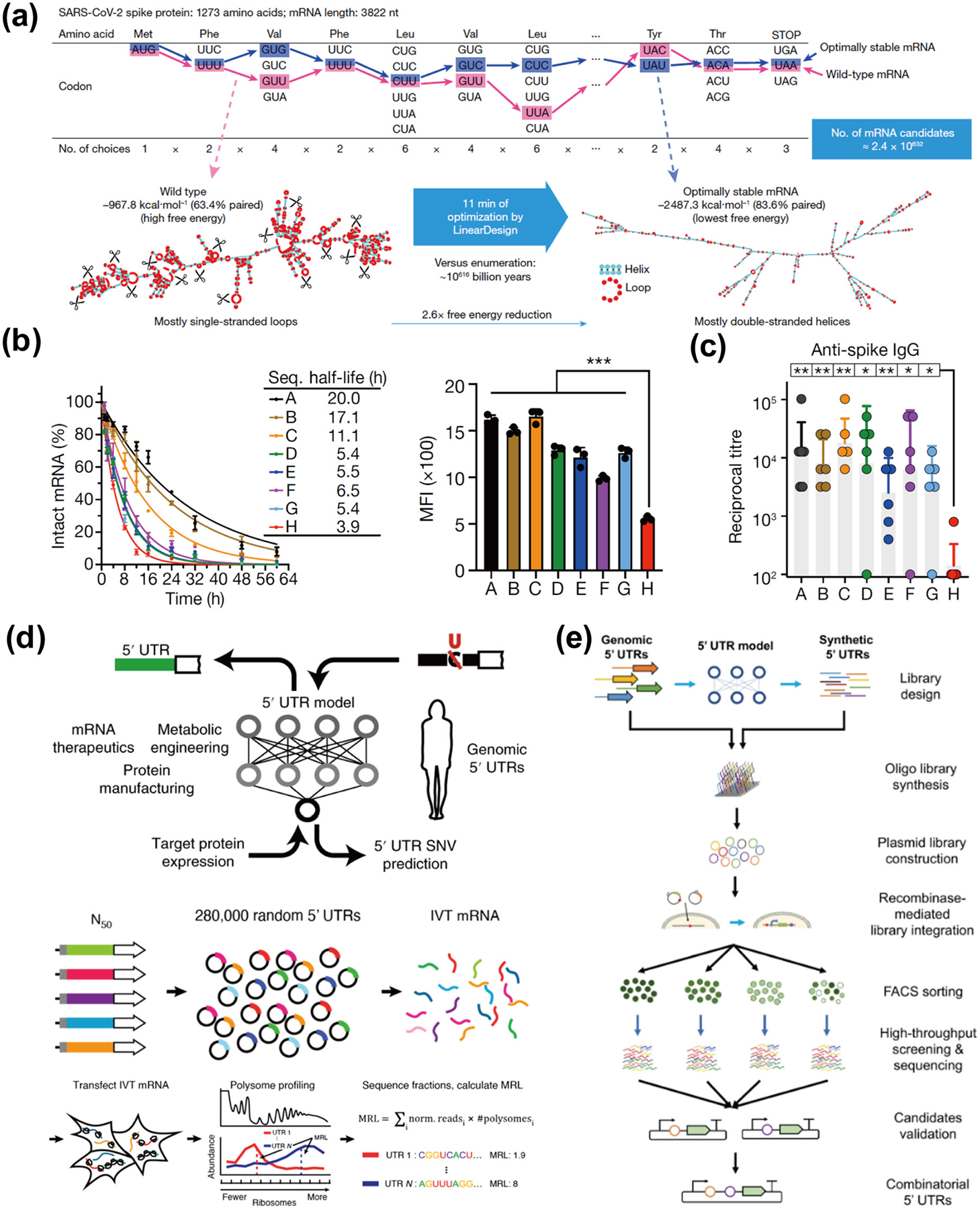
Application of artificial intelligence in mRNA sequence design. (a) Each amino acid is encoded by a triplet codon, and together with the fact that most amino acids have multiple codons, this results in a SARS-CoV-2 spike protein with 1273 amino acids that can be encoded by approximately 2.4 × 10632 mRNA sequences. It would take approximately 1 × 10616 billion years to cite each possible sequence. However, optimization using LinearDesign requires only approximately 11 min to obtain the most stable mRNA sequence [38]. Reproduced with permission from Ref. [38], © Zhang, H. et al. 2023. (b) Comparison of the chemical stability and protein expression levels of seven sequences (sequences A–G) designed using the LinearDesign algorithm and sequence H designed with the widely used codon optimization tool OptimumGene as a benchmark. All seven mRNAs generated by LinearDesign are significantly more chemically stable and have significantly better protein expression levels than the benchmark sequence H [38]. Reproduced with permission from Ref. [38], © Zhang, H. et al. 2023. (c) The linear design of mRNA sequences A–D induced 57- to 128-fold increases in the anti-spiking IgG antibody titer [38]. Reproduced with permission from Ref. [38], © Zhang, H. et al. 2023. (d) A workflow combining high-throughput assays and machine learning for characterizing mRNA regulation and high-performance engineering of UTR sequences. Specifically, a library of 280,000 gene sequences was first created, consisting of random 5′ UTRs and a constant region containing the enhanced green fluorescent protein (eGFP) CDS, as well as a 3′ UTR. The IVT mRNA library was generated by in vitro transcription from linearized DNA templates obtained by PCR from a plasmid library. Cells transfected with IVT library mRNA were then grown for 12 h prior to multimerization analysis. The read counts for each fraction were used to calculate the mean ribosome load (MRL) for each UTR, and the resulting data were used to train a convolutional neural network (CNN) [115]. Reproduced with permission from Ref. [115], © Sample, P. J. et al. under exclusive licence to Springer Nature America, Inc. 2019. (e) A platform for the systematic screening and design of 5′ UTRs that enhance protein expression in mammalian cells. Natural 5′ UTRs with different translational activities in multiple human cell types were first identified, and then genetic algorithms were applied to obtain synthetic 5′ UTRs. Twelve thousand different 5′ UTRs were tested in high throughput by a recombinase-based library screening strategy, and candidate sequences of 5′ UTRs that enhance GFP expression were selected and experimentally validated. Finally, the top-ranked validated 5′ UTRs were combined to test for increased gene expression [116]. Reproduced with permission from Ref. [116], © Cao, J. et al. 2021.
Machine learning and deep learning networks are emerging as powerful design tools for DNA and protein design [117, 118], which encourages researchers to explore the promising future of machine learning as well as deep learning in mRNA design. A recent work used a library containing 280,000 random 5′ UTRs to construct a model called Optimus 5-Prime capable of predicting the ribosome loading of human 5′ UTR variants and designing new 5′ UTRs for targeted expression (Fig. 3(d)) [115]. Results demonstrate that the model predicts the ribosome loading of these sequences, with 3577 naturally occurring variations and 35,212 truncated human 5′ UTRs evaluated. The researchers used this model to create new 5′ UTRs for tailored ribosome loading using Optimus 5-Prime and genetic algorithms. Future studies could expand the application of this strategy to include mRNA 5′ ends, 5′ cap structures, and even 3′ UTR regions for ribosome loading [115].
In another example, a high-throughput method for designing, screening, and optimizing 5′ UTRs was created by a team who first identified natural 5′ UTRs with high translational efficiency and then used this information and in silico genetic algorithms to generate synthetic 5′ UTRs (Fig. 3(e)). The next step involves screening approximately 12,000 5′ UTRs via recombinase-mediated integration. Ultimately, they discovered three artificial 5′ UTRs that performed better at expressing protein payloads than widely utilized nonviral gene therapy plasmids [116]. With the accumulation of data and the continuous improvement of algorithms, we can expect more breakthroughs and innovations in these methods in the field of genetic engineering.
5. mRNA engineering
Beyond chemical modification and sequence optimization, mRNA engineering is another strategy to enhance its stability and translational efficiency, and could play a pivotal role in advancing development of better mRNA vaccines and therapies. Below we discuss several new types of engineered mRNAs: saRNA, circRNA, and multitailed mRNA.
5.1. saRNA
5.1.1. Major differences between saRNA and traditional mRNA
saRNA shares many structural similarities with conventional mRNA: it is a linear single-stranded RNA molecule synthesized with a 5′ cap, 3′ poly(A) tail, and 5′ UTR and 3′ UTR [119]. However, compared to that of conventional mRNAs, the main structural difference in saRNAs lies in their ORF, which is much larger. This is because the saRNA ORF includes not only the relevant protein or vaccine antigen but also four nonstructural proteins (nsP1–4) and a subgenomic promoter (sgPr) (Fig. 4(a)) [120–122]. These nonstructural proteins (nsPs) typically originate from alphaviruses such as Venezuelan equine encephalitis virus (VEEV), Sindbis virus (SIN), or Semliki forest virus (SFV) [123]. saRNAs leverage the self-replicating ability of these alphaviruses, preserving their nonstructural proteins with replicative capacity [124]. Simultaneously, it replaces viral structural proteins with the gene of interest (GOI), preventing the mRNA from generating infectious viral particles [81]. In addition, conserved sequence elements (CSEs) can function as the 5′ and 3′ UTR in saRNA. The presence of these 5′ and 3′ CSEs ensures that the alphavirus replicase specifically amplifies the RNA. Consequently, similar to the replication mechanism of alphaviruses, the replicase can amplify saRNA and transcribe subgenomic RNA [125, 126]. Although saRNA is structurally distinct from traditional mRNA, it can be produced in the same way in the IVT reaction as described above [119, 122].
Figure 4.

Schematic representation of the structure and amplification mechanism of saRNA and taRNA. (a) In contrast to conventional mRNAs, saRNA structures include 5′ and 3′ conserved sequence elements (CSE) sequences, viral nonstructural protein (nsP1–4) sequences and subgenomic promoters (sgPr). When saRNAs reach the cytoplasm of host cells, nsP1–4 are translated and form an early replication complex. nsP123 + nsP4 replicate the (−) sense RNA using the (+) sense RNA as a template. At a later stage, the early replication complex forms a cleaved replicase, which produces a new copy of the original genomic RNA using the (−) sense RNA strand as a template and at the same time recognizes sgPr and triggers the production of a large amount of subgenomic RNA, ultimately leading to generation of more proteins than conventional mRNA. (b) taRNA is a split vector system consisting of a trans-replicon encoding only the GOI and another separate RNA providing the alphavirus replicase.
Compared to conventional nonreplicating mRNAs, saRNAs offer unique advantages. First, due to their self-replicating ability, saRNA maintain relatively high levels of protein expression and significantly extend the duration of protein expression [123, 127]. Second, saRNA requires a lower vaccine dose, reducing side effects, and the approach itself is cost-effective [121]. It was shown that subcutaneous injection of a saRNA vaccine encoding the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein prolonged antigen expression and neutralizing antibody response, and reduced SARS-CoV-2 infection and lung inflammation in mice [128]. However, saRNA also has some drawbacks. Compared with conventional mRNA, saRNA is a more complex and larger molecule, making its delivery more complex. Additionally, saRNA encodes not only the protein of interest but also viral nsPs, potentially limiting the repeated use of saRNA-based therapies [4, 119, 120, 123]. Unlike conventional mRNA, which benefit from nucleotide chemical modifications to evade innate immune responses, saRNA impose limitations on such modifications. Replication-responsive conserved sequence elements in saRNA are incompatible with m1Ψ, resulting in uncapped and immunogenic replicons [129]. Furthermore, during the self-amplification process, the inevitable formation of new double-stranded RNA intermediates may trigger the body’s immune response [120].
5.1.2. Clinical stage saRNA vaccines
During the COVID-19 pandemic, mRNA vaccines have been shown to be effective [130], but there are still some shortcomings in terms of persistence and side effects [31]. To address these issues, the development of saRNA vaccines has become a focus of much research [131]. The saRNA vaccine (ARCT-154) developed by Arcturus Therapeutics and CSL for combating COVID-19 recently received full regulatory approval in Japan, becoming the world’s first saRNA vaccine [132]. Clinical trials have shown that ARCT-154, used as a booster shot, produces higher and longer-lasting virus antibodies than standard mRNA COVID-19 vaccines [133]. This finding indicates that saRNA technology could have broad prospects for the prevention and treatment of infectious diseases [134].
5.1.3. Improvements in saRNA
As saRNA is a larger and more complex molecule than conventional mRNA, its applications may somewhat be limited. A new derivative strategy called taRNA has recently been developed to reduce the size of saRNA, which employ two different forms of RNA: a trans-replicon (TR) RNA that encodes the corresponding antigen and is regulated by sgPr, and an IVT mRNA that encodes the alphavirus replicase and can be translated directly in situ (Fig. 4(b)) [41, 135]. This provides more freedom in choosing the type of mRNA to be amplified because the mRNA encoding the replicase is distinct from the target mRNA. Furthermore, taRNAs are easier to scale up for production because they are shorter than saRNAs. Notably, the mRNA translation efficiency of this trans-amplified RNA vaccine is similar to that of saRNA [41]. A chikungunya virus (CHIKV)-based taRNA candidate vaccination elicited robust humoral and cellular immune responses and shielded mice from CHIKV challenge infection [136]. A unique bivalent taRNA vaccination candidate is also available; it consists of two TR-RNAs encoding the antigen and one RNA encoding the replicase [137].
In addition to improvements in the molecular size of saRNAs, several other strategies have been used to increase the efficiency of saRNA expression. For example, Trilink Biotechnologies designed CleanCap Reagent AU specifically for the generation of Cap 1 structures for saRNA based on the positive-sense alphavirus genome, where the AU cap preserves the true 5′ end of the alphavirus compared to the AG cap, resulting in efficient capping and high yields [120]. Conserved viral sequence elements were used as the 5′ and 3′ UTRs in the saRNA vaccine [81]; optimizing the 5′ and 3′ UTRs based on evolutionary optimization of naturally occurring alphaviruses is another viable approach to improve the translation of saRNA vaccines [138].
5.2. CircRNA
5.2.1. Biological functions of circRNA
CircRNA is a type of single-stranded RNA with a closed-loop structure, which is produced by back-splicing [44, 139]. Previously, circRNA was considered to be a by-product of splicing, without biological significance, and was generated only by chance [140]. However, in the past decade, with the development of RNA sequencing (RNA-seq) technology, our understanding of circRNA has undergone a fundamental change [141–143]. Studies have shown that circRNA is not just a splicing by-product but has the following characteristics: wide range of sources, stability, conservation, strong tissue specificity, and multiple biological functions (e.g., acting as an miRNA sponge, regulating transcription, transporting proteins, and promoting protein‒protein interactions) [144]. Due to the lack of 5′ end, most circRNA are classified as non-coding RNA, which cannot be translated into proteins. But recent studies have reported that circRNA containing IRES elements can initiate ribosome translation without a free 5′ end (Fig. 5(a)) [139, 145]. In addition, m6A can promote the widespread translation of circRNAs [139, 146], revealing the protein-coding functions of some circRNAs and providing new hope for protein translation applications.
Figure 5.

The advantages of circRNA over linear modified mRNA. (a) Schematic representation of the structure of circRNAs and their advantages over conventional mRNAs. (b) Schematic representation of the construction of mRNA encoding the SARS-CoV-2 RBD antigen into a circRNA; the circularized RNA is called circRNARBD [147]. (c) Experiments with HEK293T cells transfected with Lipofectamine MessengerMax showed that circRNA produced much higher levels of RBD antigen at all time points and maintained it for a longer period than m1Ψ-mRNA and unmodified mRNA [147]. (d) Different types of mRNAs were encapsulated in lipid nanoparticles and stored at 4 °C (left) or 25 °C (right), and the antigenic levels of the circRNAs were greater than those of the other two groups of mRNAs [147]. (e) Three circRNA vaccines were tested for their ability to neutralize Omicron variants, and of the three, circRNARBD-Delta maintained sufficient neutralizing activity against Omicron [147]. Reproduced with permission from Ref. [147], © Qu, L. et al. Published by Elsevier Inc. All rights reserved 2021.
Based on these works, the application of circRNAs synthesized in vitro for disease treatment has attracted increasing attention. Compared with linear RNA, circRNA can avoid degradation by exonucleases, which is the main reason for the limited biological stability of linear RNA. Therefore, circRNAs usually have greater stability and longer half-lives [148]. Moreover, circRNAs cause a much less adverse immune response than unmodified linear mRNAs because they do not activate RNA sensor [149]. A circRNA vaccine was reported to elicit potent neutralizing antibodies and T-cell responses by expressing the trimeric RBD of the spike protein, which provides strong protection against SARS-CoV-2 in mice and macaques. The circRNA vaccine is protective against both Delta and Omicron variants, and produces higher and more durable antigen levels than the m1Ψ-modified mRNA vaccine (Figs. 5(b)–5(e)) [147]. Synthetic circRNAs not only encode proteins and peptides for use as therapeutic agents or vaccines but also function to regulate gene and protein expression. For example, synthetic circRNAs that do not encode any proteins/peptides can act as miRNA/protein sponges or as siRNAs, thus serving as potential therapeutic drugs [148, 150–153].
5.2.2. In vitro circRNA synthesis
To optimize the therapeutic effect and reduce adverse side effects, various methods have been developed to synthesize circRNAs [44]. The process of generating circRNAs in vitro mainly includes two steps: First, a linear RNA precursor is synthesized, and then the two ends are connected to form a covalently closed loop. This can be achieved by chemical or enzymatic methods. The chemical method involves the use of condensing agents such as bromine cyanide (BrCN) or 1-ethyl-3-(3′-dimethylaminopropyl) carbodiimide (EDC) to condense the 5′ end phosphate and 3′ end hydroxyl of the linear RNA, forming a circular RNA (Fig. 6(a)) [154]. Chemical approaches face two challenges: First, circular RNA competes with linear RNA, and low input concentrations of linear RNA favor circRNA generation but reduce overall yield [44, 155]; second, side reactions, such as 2′–5′ phosphodiester bond formation, lead to circRNA containing 2′ or 3′ hydroxyls, resulting in lower efficiency [156].
Figure 6.

Several strategies for in vitro synthesis of circRNA. (a) Schematic representation of in vitro chemical ligation of circRNA by conjugating the 5′-end phosphate to the 3′-end hydroxyl group using EDC or BrCN. (b) Schematic representation of different enzyme ligation strategies. With the help of a DNA splint, T4 DNA ligase allows for accurate RNA ligation but requires full complementarity at the ligation junction. T4 RNA ligase 1 accepts both DNA and RNA fragments as substrates and can also react with less specific single-stranded substrates, with the retention of 2–3 unpaired nucleotides at the ligated junction to improve efficiency. T4 RNA ligase 2 is compatible with both double- and single-stranded substrates but ligates double-stranded RNA with the highest notch efficiency, typically using an RNA or DNA splice to join the ends of the RNA together. (c) The PIE system based on group I and group II introns. The group I intron splicing system replaces native group I introns and inserts custom sequences into the exon regions (E1 and E2), which are spontaneously linked by two esterifications with the addition of GTP and Mg2+ as cofactors, forming a loop and releasing two half-intron fragments. The group II intron splicing system involves the attachment of a 5′ splice site at the end of an exon to a 3′ splice site at the start of the same exon, which then automatically joins and excises the long-chain intermediate.
Compared with chemical cyclization, a more common method for RNA cyclization is enzymatic ligation using several enzymes from the bacteriophage T4. Among them, T4 DNA ligase (T4 Dnl) can join double-stranded duplexes, such as DNA/RNA hybrids, but it requires complementary DNA templates or bridges (Fig. 6(b)) [149, 157–159]. A short DNA splint that hybridizes to single-stranded RNA ends and forms a double-stranded region is required for DNA ligase to cyclize RNA in vitro. The advantage of this method is the high accuracy of the ligation sites, but the disadvantage is the low ligation efficiency; therefore, only a few studies have used this method [149, 156, 158]. T4 RNA ligase 1 (T4 Rnl 1) and T4 RNA ligase 2 (T4 Rnl 2) can catalyze the ligation of the 5′-terminal monophosphate on the “donor” RNA to the 3′-hydroxyl on the “acceptor” RNA [160]. T4 Rnl 1 can only bind to single-stranded RNA and prefers cytosine and adenine at ligation sites, and it is also affected by the RNA secondary structure [161]. To facilitate intramolecular RNA cyclization, linear RNA can be oriented to bring its ends together, and auxiliary oligonucleotides can be added to the reaction to improve the cyclization efficiency. For example, an RNA splint can bind to precursor ends and leave 2–3 unpaired nucleotides at the junction (Fig. 6(b)) [44, 161, 162]. T4 Rnl 2 can bind to both double- and single-stranded RNA and has the greatest ability to ligate gaps in double-stranded RNA [162, 163]. T4 Rnl 2 can form intramolecular junctions on double-stranded RNA; this process is facilitated by the use of complementary DNA or RNA splints (Fig. 6(b)) [164].
CircRNAs can also be synthesized using ribozymes, with the Permuted Introns and Exons (PIE) method, which utilizes RNA cyclase ribozyme [165–167] and has increasingly gained attention due to its efficiency [168, 169]. The principle is based on the self-splicing function of group I or II introns, which causes the intron to circularize and the intermediate sequence to connect in the presence of magnesium ions and free GTP, thus producing circRNA [156, 170, 171]. Group I or II intron ribozymes use different mechanisms for the similar PIE strategy (Fig. 6(c)). In contrast to chemical and proteinase ligations, this method can also be used for the cyclization of larger RNA precursors, and the reaction conditions and purification methods are relatively simple. Recently, an improved PIE method was used to circularize various RNAs up to 5 kb in length in vitro [172]. In addition, circRNAs prepared by the PIE method with an IRES and optimized accessory sequences showed strong and stable protein expression in eukaryotic cells [172]. However, the group I PIE method also has several limitations, such as the need to retain the terminal part of the original phage or Anabaena exon and the influence of the RNA secondary structure and exogenous exon sequence [44]. In contrast to group I intron encapsulation, group II intron splicing uses group II introns in the yeast mitochondrial genome, which can produce circRNAs without the need for native exons. This method can achieve more accurate linear RNA precursor connections and generate perfect sequence analogues. However, this method also produces nonnatural 2′,5′-phosphodiester bonds, and the mechanism of its in vitro implementation is still unclear [162, 173].
5.2.3. Limitations of circRNA
Whereas circRNA has shown great therapeutic potential, its translation also faces significant challenges. First, current circRNA synthesis techniques may be limited, particularly for large-scale production, where high costs, low yield, purification difficulties, and immature manufacturing equipment present considerable obstacles [156]. Second, introducing chemical modifications or non-natural nucleotides into circRNA synthesis is another challenge [156]. Due to the lack of terminal structures in circRNA, the modification is more difficult compared to linear RNA. Furthermore, while circRNAs are known to function as miRNA sponges, transcriptional regulators, and even protein-coding elements, their full biological roles remain unclear [174]. Similar to linear mRNA, efficient delivery of circRNA to target tissues remains elusive, despite that evolving delivery systems such as extracellular vesicles and lipid nanoparticles have shown promise in RNA delivery [139]. Therefore, overcoming challenges in synthesis, purification, and delivery could be essential for future clinical application of circRNAs in disease prevention and treatment.
5.3. Multitailed mRNA
As mentioned in the section 3.3, the poly(A) tail plays a significant role in mRNA translation and stability. It interacts with PABPs, recruits eIF4G, and enhances cap affinity, forming a closed-loop structure that promotes translation initiation. Moreover, PABPs also extend mRNA stability by cloaking the poly(A) tail to prevent deadenylation. Engineering the poly(A) tail of mRNAs has thus been proposed to improve mRNA stability and translation efficiency. A recent work has successfully designed and synthesized mRNA with multiple poly(A) tails, by combining chemical and enzymatic approaches [36]. By introducing non-natural chemical modifications and branched topological structures at the mRNA end, this approach significantly improves translation efficiency and expression durability. In vitro experiments revealed that multitailed luciferase mRNA exhibited 4.7 to 19.5 times higher luminescent signals than mRNA control. Furthermore, the signal duration in vivo extended from less than 7 days in the control group to 14 days. Decay kinetics indicated that the half-life of multitailed mRNA increased from an estimated 7.0 ± 0.2 to 16.8 ± 1.2 h. Subsequently, the use of multitailed mRNAs in gene editing was explored. Co-injection of single-guide RNAs (sgRNAs) targeting Pcsk9 and Angptl3 genes with multitailed Cas9 mRNA achieved efficient gene editing in mouse liver. These results indicate that the strategy of topologically branched multitailed mRNA has potential applications, as well as the compatibility of the RNA translation mechanism to unnatural linkage structures, which provides new ideas for mRNA drug design. Prior to this work, a messenger-oligonucleotide conjugated RNA (mocRNA) was developed by the same team [175]. Using T4 RNA ligase, they introduced chemically synthesized oligonucleotides with nuclease resistance at site-specific poly(A) tails. This protective strategy preserves poly(A) tail integrity without compromising translation ability, significantly improving mRNA stability. These groundbreaking studies validate the feasibility of selectively modifying mRNA ends and demonstrate the compatibility of RNA translation mechanisms with non-natural linkage structures.
6. Conclusions
Over the last few years, we have witnessed tremendous scientific advances in mRNA-based medicines. Beyond applications in infectious diseases, many mRNA-based clinical trials are being conducted for cancer immunotherapy, protein replacement therapy, gene editing, and others. Meanwhile, we have also increasingly recognized some challenges mRNA therapies face. The first one is how to improve mRNA stability for higher and more durable protein expression. mRNA generally has a short life cycle and can be easily degraded, which poses an obstacle for long-lasting therapies. Many efforts have been made to address this issue, such as chemical modification, sequence optimization, and structural engineering, as overviewed in this article. In addition, next-generation mRNA therapies may also need to act on specific target tissues or cells. Therefore, how to achieve selective tissue/cell delivery of mRNA is another challenge. Recent advances in lipid nanoparticles have demonstrated the potential for selective mRNA delivery to certain extrahepatic organs, such as the lung and spleen, after intravenous administration [20, 22, 176, 177]. Intraperitoneal administration of lipid nanoparticles has also been shown to result in a different organ distribution than intravenous administration [178]. There is also a link between the structure of ionizable lipids and organ distribution; however, the differences in biodistribution of different structures and the general relationship between structure and activity remain to be fully understood. Ligand-mediated active targeting is another viable strategy to improve cell-selective delivery of mRNAs, e.g., surface modification with small-molecule ligands or antibodies to deliver mRNAs to specific types of cells [179]. Interestingly, programmable design of mRNA sequences could also be used to increase cell-specific translation by exploiting reactive 3D structures in the 5′ UTR of the mRNA, unique tRNA expression patterns between cell types, and degradation rates in different cell populations [180–182]. Another design involves incorporating specific microRNA targets into mRNA, allowing the endogenous microRNA pool to selectively degrade synthetic mRNA. This approach enables mRNA encoding therapeutic proteins to induce apoptosis in tumor cells while sparing healthy liver cells from damage [183]. In addition, the modification of photocaged 5′ cap provides a novel strategy for targeted, spatio-temporal expression of mRNAs [87]. In summary, mRNA medicine holds immense promise in biomedical applications, while addressing challenges related to mRNA stability and selective delivery will be critical for their future successful integration into clinical practice. We expect mRNA medicine to bring new hope for the treatment of many intractable diseases.
Acknowledgements
Jinjun Shi acknowledges the support from the U.S. National Institutes of Health grants (Nos. R01CA200900, R01HL159012, and R33HL168751), and the Innovation Discovery Grants award from the Mass General Brigham. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Mascola JR; Fauci AS Novel vaccine technologies for the 21st century. Nat. Rev. Immunol 2020, 20, 87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brenner S; Jacob F; Meselson M An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 1961, 190, 576–581. [DOI] [PubMed] [Google Scholar]
- [3].Wolff JA; Malone RW; Williams P; Chong W; Acsadi G; Jani A; Felgner PL Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [DOI] [PubMed] [Google Scholar]
- [4].Pardi N; Hogan MJ; Porter FW; Weissman D mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov 2018, 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Polack FP; Thomas SJ; Kitchin N; Absalon J; Gurtman A; Lockhart S; Perez JL; Pérez Marc G; Moreira ED; Zerbini C et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med 2020, 383, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baden LR; El Sahly HM; Essink B; Kotloff K; Frey S; Novak R; Diemert D; Spector SA; Rouphael N; Creech CB et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med 2021, 384, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Goswami J; Baqui AH; Doreski PA; Perez Marc G; Jimenez G; Ahmed S; Zaman K; Duncan CJA; Ujiie M; Rämet M et al. Humoral immunogenicity of mRNA-1345 RSV vaccine in older adults. J. Infect. Dis, in press, DOI: 10.1093/infdis/jiae316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilson E; Goswami J; Baqui AH; Doreski PA; Perez-Marc G; Zaman K; Monroy J; Duncan CJA; Ujiie M; Rämet M et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N. Engl. J. Med 2023, 389, 2233–2244. [DOI] [PubMed] [Google Scholar]
- [9].Sabnis S; Kumarasinghe ES; Salerno T; Mihai C; Ketova T; Senn JJ; Lynn A; Bulychev A; McFadyen I; Chan J et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther 2018, 26, 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weber JS; Carlino MS; Khattak A; Meniawy T; Ansstas G; Taylor MH; Kim KB; McKean M; Long GV; Sullivan RJ et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [DOI] [PubMed] [Google Scholar]
- [11].Mackensen A; Haanen JBAG; Koenecke C; Alsdorf W; Wagner-Drouet E; Borchmann P; Heudobler D; Ferstl B; Klobuch S; Bokemeyer C et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: The phase 1 BNT211–01 trial. Nat. Med 2023, 29, 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xiao YF; Tang ZM; Huang XG; Chen W; Zhou J; Liu HJ; Liu C; Kong N; Tao W Emerging mRNA technologies: Delivery strategies and biomedical applications. Chem. Soc. Rev 2022, 51, 3828–3845. [DOI] [PubMed] [Google Scholar]
- [13].Xiao YL; Chen J; Zhou H; Zeng XD; Ruan ZP; Pu ZY; Jiang XY; Matsui A; Zhu LL; Amoozgar Z et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun 2022, 13, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yi JZ; Lei XL; Guo FT; Chen QB; Chen XY; Zhao KT; Zhu CL; Cheng XM; Lin JW; Yin H et al. Co-delivery of Cas9 mRNA and guide RNAs edits hepatitis B virus episomal and integration DNA in mouse and tree shrew models. Antiviral Res. 2023, 215, 105618. [DOI] [PubMed] [Google Scholar]
- [15].Karikó K; Ni HP; Capodici J; Lamphier M; Weissman D mRNA is an endogenous ligand for toll-like receptor 3. J. Biol. Chem 2004, 279, 12542–12550. [DOI] [PubMed] [Google Scholar]
- [16].Heil F; Hemmi H; Hochrein H; Ampenberger F; Kirschning C; Akira S; Lipford G; Wagner H; Bauer S Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [DOI] [PubMed] [Google Scholar]
- [17].Xiong QQ; Lee GY; Ding JX; Li WL; Shi JJ Biomedical applications of mRNA nanomedicine. Nano Res. 2018, 11, 5281–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karikó K; Muramatsu H; Welsh FA; Ludwig J; Kato H; Akira S; Weissman D Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther 2008, 16, 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Warminski M; Mamot A; Depaix A; Kowalska J; Jemielity J Chemical modifications of mRNA ends for therapeutic applications. Acc. Chem. Res 2023, 56, 2814–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fu LY; Zhang Y; Farokhzad RA; Mendes BB; Conde J; Shi JJ ‘Passive’ nanoparticles for organ-selective systemic delivery: Design, mechanism and perspective. Chem. Soc. Rev 2023, 52, 7579–7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Breda L; Papp TE; Triebwasser MP; Yadegari A; Fedorky MT; Tanaka N; Abdulmalik O; Pavani G; Wang YP; Grupp SA et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 2023, 381, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cheng Q; Wei T; Farbiak L; Johnson LT; Dilliard SA; Siegwart DJ Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol 2020, 15, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun YH; Chatterjee S; Lian XZ; Traylor Z; Sattiraju SR; Xiao YF; Dilliard SA; Sung YC; Kim M; Lee SM et al. In vivo editing of lung stem cells for durable gene correction in mice. Science 2024, 384, 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu S; Cheng Q; Wei T; Yu XL; Johnson LT; Farbiak L; Siegwart DJ Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater 2021, 20, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin YX; Wang Y; Ding JX; Jiang AP; Wang J; Yu M; Blake S; Liu SS; Bieberich CJ; Farokhzad OC et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med 2021, 13, eaba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Islam MA; Xu YJ; Tao W; Ubellacker JM; Lim M; Aum D; Lee GY; Zhou K; Zope H; Yu M et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng 2018, 2, 850–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sahin U; Karikó K; Türeci Ö mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov 2014, 13, 759–780. [DOI] [PubMed] [Google Scholar]
- [28].Chaudhary N; Weissman D; Whitehead KA mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov 2021, 20, 817–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hou XC; Zaks T; Langer R; Dong YZ Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater 2021, 6, 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barbier AJ; Jiang AY; Zhang P; Wooster R; Anderson DG The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol 2022, 40, 840–854. [DOI] [PubMed] [Google Scholar]
- [31].Rohner E; Yang R; Foo KS; Goedel A; Chien KR Unlocking the promise of mRNA therapeutics. Nat. Biotechnol 2022, 40, 1586–1600. [DOI] [PubMed] [Google Scholar]
- [32].Conde J; Langer R; Rueff J mRNA therapy at the convergence of genetics and nanomedicine. Nat. Nanotechnol 2023, 18, 537–540. [DOI] [PubMed] [Google Scholar]
- [33].Nance KD; Meier JL Modifications in an emergency: The role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci 2021, 7, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mulroney TE; Pöyry T; Yam-Puc JC; Rust M; Harvey RF; Kalmar L; Horner E; Booth L; Ferreira AP; Stoneley M et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024, 625, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Despic V; Jaffrey SR mRNA ageing shapes the Cap2 methylome in mammalian mRNA. Nature 2023, 614, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen HY; Liu DL; Guo JT; Aditham A; Zhou YM; Tian JK; Luo SC; Ren JY; Hsu A; Huang JH et al. Branched chemically modified poly(A) tails enhance the translation capacity of mRNA. Nat. Biotechnol, in press, DOI: 10.1038/s41587-024-02174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhou F; Huang LJ; Li SQ; Yang WF; Chen FM; Cai ZX; Liu XL; Xu WJ; Lehto VP; Lächelt U et al. From structural design to delivery: mRNA therapeutics for cancer immunotherapy. Exploration 2024, 4, 20210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang H; Zhang L; Lin A; Xu CC; Li ZY; Liu KB; Liu BX; Ma XP; Zhao FF; Jiang HL et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Santer L; Bär C; Thum T Circular RNAs: A novel class of functional RNA molecules with a therapeutic perspective. Mol. Ther 2019, 27, 1350–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim DY; Atasheva S; McAuley AJ; Plante JA; Frolova EI; Beasley DWC; Frolov I Enhancement of protein expression by alphavirus replicons by designing self-replicating subgenomic RNAs. Proc. Natl. Acad. Sci. USA 2014, 111, 10708–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Beissert T; Perkovic M; Vogel A; Erbar S; Walzer KC; Hempel T; Brill S; Haefner E; Becker R; Türeci Ö et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol. Ther 2020, 28, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Usman N; Cedergren R Exploiting the chemical synthesis of RNA. Trends Biochem. Sci 1992, 17, 334–339. [DOI] [PubMed] [Google Scholar]
- [43].Rong MQ; He B; McAllister WT; Durbin RK Promoter specificity determinants of T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 1998, 95, 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Obi P; Chen YG The design and synthesis of circular RNAs. Methods 2021, 196, 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Magadum A; Kaur K; Zangi L mRNA-based protein replacement therapy for the heart. Mol. Ther 2019, 27, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].E C; Dai LQ; Yu J Switching promotor recognition of phage RNA polymerase in silico along lab-directed evolution path. Biophys. J 2022, 121, 582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lu XL; Wu H; Xia H; Huang FT; Yan Y; Yu BB; Cheng R; Drulis-Kawa Z; Zhu B Klebsiella phage KP34 RNA polymerase and its use in RNA synthesis. Front. Microbiol 2019, 10, 2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang XG; Kong N; Zhang XC; Cao YH; Langer R; Tao W The landscape of mRNA nanomedicine. Nat. Med 2022, 28, 2273–2287. [DOI] [PubMed] [Google Scholar]
- [49].To KKW; Cho WCS An overview of rational design of mRNA-based therapeutics and vaccines. Expert Opin. Drug Discov 2021, 16, 1307–1317. [DOI] [PubMed] [Google Scholar]
- [50].Qin SG; Tang XS; Chen YT; Chen KP; Fan N; Xiao W; Zheng Q; Li GH; Teng YQ; Wu M et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther 2022, 7, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Van Hoecke L; Roose K How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med 2019, 17, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wadhwa A; Aljabbari A; Lokras A; Foged C; Thakur A Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].de Mey W; De Schrijver P; Autaers D; Pfitzer L; Fant B; Locy H; Esprit A; Lybaert L; Bogaert C; Verdonck M et al. A synthetic DNA template for fast manufacturing of versatile single epitope mRNA. Mol. Ther. Nucl. Acids 2022, 29, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kis Z; Shattock R; Shah N; Kontoravdi C Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol. J 2019, 14, 1–2. [DOI] [PubMed] [Google Scholar]
- [55].Chow KT; Gale M Jr.; Loo YM RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol 2018, 36, 667–694. [DOI] [PubMed] [Google Scholar]
- [56].Loo YM; Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hornung V; Ellegast J; Kim S; Brzózka K; Jung A; Kato H; Poeck H; Akira S; Conzelmann KK; Schlee M et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 2006, 314, 994–997. [DOI] [PubMed] [Google Scholar]
- [58].Buggert M; Höglund P The prize of prizes: mRNA research paving the way for COVID-19 vaccine success wins the Nobel Prize in Physiology or Medicine 2023. Scand. J. Immunol 2023, 98, e13340. [DOI] [PubMed] [Google Scholar]
- [59].Diebold SS; Massacrier C; Akira S; Paturel C; Morel Y; Sousa CRE Nucleic acid agonists for toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur. J. Immunol 2006, 36, 3256–3267. [DOI] [PubMed] [Google Scholar]
- [60].Hornung V; Barchet W; Schlee M; Hartmann G RNA recognition via TLR7 and TLR8. In Toll-Like Receptors (TLRs) and Innate Immunity. Bauer S; Hartmann G, Eds.; Springer: Berlin, Heidelberg, 2008; pp 71–86. [DOI] [PubMed] [Google Scholar]
- [61].Karikó K; Buckstein M; Ni HP; Weissman D Suppression of RNA recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [DOI] [PubMed] [Google Scholar]
- [62].Anderson BR; Muramatsu H; Nallagatla SR; Bevilacqua PC; Sansing LH; Weissman D; Karikó K Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Andries O; Cafferty SM; De Smedt SC; Weiss R; Sanders NN; Kitada T N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [DOI] [PubMed] [Google Scholar]
- [64].Weng YH; Li CH; Yang TR; Hu B; Zhang MJ; Guo S; Xiao HH; Liang XJ; Huang YY The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv 2020, 40, 107534. [DOI] [PubMed] [Google Scholar]
- [65].Kauffman KJ; Mir FF; Jhunjhunwala S; Kaczmarek JC; Hurtado JE; Yang JH; Webber MJ; Kowalski PS; Heartlein MW; DeRosa F et al. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials 2016, 109, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sittplangkoon C; Alameh MG; Weissman D; Lin PJC; Tam YK; Prompetchara E; Palaga T mRNA vaccine with unmodified uridine induces robust type I interferon-dependent antitumor immunity in a melanoma model. Front. Immunol 2022, 13, 983000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shanmugasundaram M; Senthilvelan A; Kore AR Recent advances in modified cap analogs: Synthesis, biochemical properties, and mRNA based vaccines. Chem. Rec 2022, 22, e202200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ramanathan A; Robb GB; Chan SH mRNA capping: Biological functions and applications. Nucleic Acids Res. 2016, 44, 7511–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ziemniak M; Strenkowska M; Kowalska J; Jemielity J Potential therapeutic applications of RNA cap analogs. Future Med. Chem 2013, 5, 1141–1172. [DOI] [PubMed] [Google Scholar]
- [70].Sonenberg N; Gingras AC The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr. Opin. Cell Biol 1998, 10, 268–275. [DOI] [PubMed] [Google Scholar]
- [71].Grudzien E; Stepinski J; Jankowska-Anyszka M; Stolarski R; Darzynkiewicz E; Rhoads RE Novel cap analogs for in vitro synthesis of mRNAs with high translational efficiency. RNA 2004, 10, 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Li Y; Kiledjian M Regulation of mRNA decapping. Wiley Interdiscip. Rev. RNA 2010, 1, 253–265. [DOI] [PubMed] [Google Scholar]
- [73].Devarkar SC; Wang C; Miller MT; Ramanathan A; Jiang FG; Khan AG; Patel SS; Marcotrigiano J Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl. Acad. Sci. USA 2016, 113, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schlake T; Thess A; Thran M; Jordan I mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci 2019, 76, 301–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sikorski PJ; Warminski M; Kubacka D; Ratajczak T; Nowis D; Kowalska J; Jemielity J The identity and methylation status of the first transcribed nucleotide in eukaryotic mRNA 5′ cap modulates protein expression in living cells. Nucleic Acids Res. 2020, 48, 1607–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Muttach F; Muthmann N; Rentmeister A Synthetic mRNA capping. Beilstein J. Org. Chem 2017, 13, 2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Linares-Fernández S; Lacroix C; Exposito JY; Verrier B Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med 2020, 26, 311–323. [DOI] [PubMed] [Google Scholar]
- [78].Fuchs AL; Neu A; Sprangers R A general method for rapid and cost-efficient large-scale production of 5′ capped RNA. RNA 2016, 22, 1454–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Miao L; Zhang Y; Huang L mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wojtczak BA; Sikorski PJ; Fac-Dabrowska K; Nowicka A; Warminski M; Kubacka D; Nowak E; Nowotny M; Kowalska J; Jemielity J 5′-Phosphorothiolate dinucleotide cap analogues: Reagents for messenger RNA modification and potent small-molecular inhibitors of decapping enzymes. J. Am. Chem. Soc 2018, 140, 5987–5999. [DOI] [PubMed] [Google Scholar]
- [81].Blakney AK; Ip S; Geall AJ An update on self-amplifying mRNA vaccine development. Vaccines 2021, 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Peng ZH; Sharma V; Singleton SF; Gershon PD Synthesis and application of a chain-terminating dinucleotide mRNA cap analog. Org. Lett 2002, 4, 161–164. [DOI] [PubMed] [Google Scholar]
- [83].Stepinski J; Waddell C; Stolarski R; Darzynkiewicz E; Rhoads RE Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA 2001, 7, 1486–1495. [PMC free article] [PubMed] [Google Scholar]
- [84].Strenkowska M; Kowalska J; Lukaszewicz M; Zuberek J; Su W; Rhoads RE; Darzynkiewicz E; Jemielity J Towards mRNA with superior translational activity: Synthesis and properties of ARCA tetraphosphates with single phosphorothioate modifications. New J. Chem 2010, 34, 993–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Warminski M; Trepkowska E; Smietanski M; Sikorski PJ; Baranowski MR; Bednarczyk M; Kedzierska H; Majewski B; Mamot A; Papiernik D et al. Trinucleotide mRNA cap analogue N6-benzylated at the site of posttranscriptional m6Am mark facilitates mRNA purification and confers superior translational properties in vitro and in vivo. J. Am. Chem. Soc 2024, 146, 8149–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kawaguchi D; Kodama A; Abe N; Takebuchi K; Hashiya F; Tomoike F; Nakamoto K; Kimura Y; Shimizu Y; Abe H Phosphorothioate modification of mRNA accelerates the rate of translation initiation to provide more efficient protein synthesis. Angew. Chem., Int. Ed 2020, 59, 17403–17407. [DOI] [PubMed] [Google Scholar]
- [87].Klöcker N; Weissenboeck FP; van Dülmen M; Špaček P; Hüwel S; Rentmeister A Photocaged 5′ cap analogues for optical control of mRNA translation in cells. Nat. Chem 2022, 14, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tang TTL; Passmore LA Recognition of poly(A) RNA through its intrinsic helical structure. Cold Spring Harb. Symp. Quant. Biol 2019, 84, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Körner CG; Wahle E Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. J. Biol. Chem 1997, 272, 10448–10456. [DOI] [PubMed] [Google Scholar]
- [90].Goss DJ; Kleiman FE Poly(A) binding proteins: Are they all created equal. Wiley Interdiscip. Rev. RNA 2013, 4, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hershey JWB Regulation of protein synthesis and the role of eIF3 in cancer. Braz. J. Med. Biol. Res 2010, 43, 920–930. [DOI] [PubMed] [Google Scholar]
- [92].Pelletier J; Sonenberg N The organizing principles of eukaryotic ribosome recruitment. Annu. Rev. Biochem 2019, 88, 307–335. [DOI] [PubMed] [Google Scholar]
- [93].Meijer HA; Bushell M; Hill K; Gant TW; Willis AE; Jones P; de Moor CH A novel method for poly(A) fractionation reveals a large population of mRNAs with a short poly(A) tail in mammalian cells. Nucleic Acids Res. 2007, 35, e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Viegas IJ; de Macedo JP; Serra L; De Niz M; Temporão A; Pereira SS; Mirza AH; Bergstrom E; Rodrigues JA; Aresta-Branco F et al. N6-methyladenosine in poly(A) tails stabilize VSG transcripts. Nature 2022, 604, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hinnebusch AG; Ivanov IP; Sonenberg N Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Babendure JR; Babendure JL; Ding JH; Tsien RY Control of mammalian translation by mRNA structure near caps. RNA 2006, 12, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gómez-Aguado I; Rodríguez-Castejón J; Vicente-Pascual M; Rodríguez-Gascón A; Solinís MÁ; del Pozo-rodríguez A Nanomedicines to deliver mRNA: State of the art and future perspectives. Nanomaterials 2020, 10, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Matoulkova E; Michalova E; Vojtesek B; Hrstka R The role of the 3′ untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol. 2012, 9, 563–576. [DOI] [PubMed] [Google Scholar]
- [99].Kozak M At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol 1987, 196, 947–950. [DOI] [PubMed] [Google Scholar]
- [100].Adibzadeh S; Fardaei M; Takhshid MA; Miri MR; Rafiei Dehbidi G; Farhadi A; Ranjbaran R; Alavi P; Nikouyan N; Seyyedi N et al. Enhancing stability of destabilized green fluorescent protein using chimeric mRNA containing human beta-globin 5′ and 3′ untranslated regions. Avicenna J. Med. Biotechnol 2019, 11, 112–117. [PMC free article] [PubMed] [Google Scholar]
- [101].Fotin-Mleczek M; Duchardt KM; Lorenz C; Pfeiffer R; Ojkić-Zrna S; Probst J; Kallen KJ Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother 2011, 34, 1–15. [DOI] [PubMed] [Google Scholar]
- [102].Truong B; Allegri G; Liu XB; Burke KE; Zhu XL; Cederbaum SD; Häberle J; Martini PGV; Lipshutz GS Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 21150–21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gustafsson C; Govindarajan S; Minshull J Codon bias and heterologous protein expression. Trends Biotechnol. 2004, 22, 346–353. [DOI] [PubMed] [Google Scholar]
- [104].Novoa EM; de Pouplana LR Speeding with control: Codon usage, tRNAs, and ribosomes. Trends Genet. 2012, 28, 574–581. [DOI] [PubMed] [Google Scholar]
- [105].Cannarozzi G; Schraudolph NN; Faty M; von Rohr P; Friberg MT; Roth AC; Gonnet P; Gonnet G; Barral Y A role for codon order in translation dynamics. Cell 2010, 141, 355–367. [DOI] [PubMed] [Google Scholar]
- [106].Presnyak V; Alhusaini N; Chen YH; Martin S; Morris N; Kline N; Olson S; Weinberg D; Baker KE; Graveley BR et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vaidyanathan S; Azizian KT; Haque AKM; Henderson JM; Hendel A; Shore S; Antony JS; Hogrefe RI; Kormann MSD; Porteus MH et al. Uridine depletion and chemical modification increase cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther. Nucl. Acids 2018, 12, 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhang JJ; Liu YH; Li C; Xiao Q; Zhang DD; Chen Y; Rosenecker J; Ding XY; Guan S Recent advances and innovations in the preparation and purification of in vitro-transcribed-mRNA-based molecules. Pharmaceutics 2023, 15, 2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mauro VP; Chappell SA A critical analysis of codon optimization in human therapeutics. Trends Mol. Med 2014, 20, 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Weissman D mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [DOI] [PubMed] [Google Scholar]
- [111].Spencer PS; Siller E; Anderson JF; Barral JM Silent substitutions predictably alter translation elongation rates and protein folding efficiencies. J. Mol. Biol 2012, 422, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mauger DM; Cabral BJ; Presnyak V; Su SV; Reid DW; Goodman B; Link K; Khatwani N; Reynders J; Moore MJ et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kudla G; Lipinski L; Caffin F; Helwak A; Zylicz M High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006, 4, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Konu Ö; Li MD Correlations between mRNA expression levels and GC contents of coding and untranslated regions of genes in rodents. J. Mol. Evol 2002, 54, 35–41. [DOI] [PubMed] [Google Scholar]
- [115].Sample PJ; Wang B; Reid DW; Presnyak V; McFadyen IJ; Morris DR; Seelig G Human 5′ UTR design and variant effect prediction from a massively parallel translation assay. Nat. Biotechnol 2019, 37, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cao JC; Novoa EM; Zhang ZZ; Chen WCW; Liu DB; Choi GCG; Wong ASL; Wehrspaun C; Kellis M; Lu TK High-throughput 5′ UTR engineering for enhanced protein production in non-viral gene therapies. Nat. Commun 2021, 12, 4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Linder J; Seelig G Fast activation maximization for molecular sequence design. BMC Bioinformatics 2021, 22, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Linder J; Bogard N; Rosenberg AB; Seelig G A generative neural network for maximizing fitness and diversity of synthetic DNA and protein sequences. Cell Syst. 2020, 11, 49–62.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Brito LA; Kommareddy S; Maione D; Uematsu Y; Giovani C; Berlanda Scorza F; Otten GR; Yu D; Mandl CW; Mason PW et al. Self-amplifying mRNA vaccines. Adv. Genet 2015, 89, 179–233. [DOI] [PubMed] [Google Scholar]
- [120].Minnaert AK; Vanluchene H; Verbeke R; Lentacker I; De Smedt SC; Raemdonck K; Sanders NN; Remaut K Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across. Adv. Drug Deliv. Rev 2021, 176, 113900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Papukashvili D; Rcheulishvili N; Liu C; Ji Y; He YJ; Wang PG Self-amplifying RNA approach for protein replacement therapy. Int. J. Mol. Sci 2022, 23, 12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Geall AJ; Mandl CW; Ulmer JB RNA: The new revolution in nucleic acid vaccines. Semin. Immunol 2013, 25, 152–159 [DOI] [PubMed] [Google Scholar]
- [123].Bloom K; van den Berg F; Arbuthnot P Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Blakney AK; McKay PF; Shattock RJ Structural components for amplification of positive and negative strand VEEV splitzicons. Front. Mol. Biosci 2018, 5, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ljungberg K; Liljeström P Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines 2015, 14, 177–194. [DOI] [PubMed] [Google Scholar]
- [126].Schmidt C; Schnierle BS Self-amplifying RNA vaccine candidates: Alternative platforms for mRNA vaccine development. Pathogens 2023, 12, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Maruggi G; Zhang CL; Li JW; Ulmer JB; Yu D mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther 2019, 27, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lin GB; Yan H; Sun J; Zhao JC; Zhang Y Self-replicating RNA nanoparticle vaccine elicits protective immune responses against SARS-CoV-2. Mol. Ther. Nucl. Acids 2023, 32, 650–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Blakney AK; McKay PF; Bouton CR; Hu K; Samnuan K; Shattock RJ Innate inhibiting proteins enhance expression and immunogenicity of self-amplifying RNA. Mol. Ther 2021, 29, 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Meo SA; Bukhari IA; Akram J; Meo AS; Klonoff DC COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci 2021, 25, 1663–1669. [DOI] [PubMed] [Google Scholar]
- [131].Vogel AB; Lambert L; Kinnear E; Busse D; Erbar S; Reuter KC; Wicke L; Perkovic M; Beissert T; Haas H et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther 2018, 26, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Dolgin E Self-copying RNA vaccine wins first full approval: What’s next. Nature 2023, 624, 236–237. [DOI] [PubMed] [Google Scholar]
- [133].Oda Y; Kumagai Y; Kanai M; Iwama Y; Okura I; Minamida T; Yagi Y; Kurosawa T; Greener B; Zhang Y et al. Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: A double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Infect. Dis 2024, 24, 351–360. [DOI] [PubMed] [Google Scholar]
- [134].Geall AJ; Verma A; Otten GR; Shaw CA; Hekele A; Banerjee K; Cu Y; Beard CW; Brito LA; Krucker T et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Spuul P; Balistreri G; Hellström K; Golubtsov AV; Jokitalo E; Ahola T Assembly of alphavirus replication complexes from RNA and protein components in a novel trans-replication system in mammalian cells. J. Virol 2011, 85, 4739–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Schmidt C; Haefner E; Gerbeth J; Beissert T; Sahin U; Perkovic M; Schnierle BS A taRNA vaccine candidate induces a specific immune response that protects mice against Chikungunya virus infections. Mol. Ther. Nucl. Acids 2022, 28, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Schmidt C; Hastert FD; Gerbeth J; Beissert T; Sahin U; Perkovic M; Schnierle BS A bivalent trans-amplifying RNA vaccine candidate induces potent Chikungunya and ross river virus specific immune responses. Vaccines 2022, 10, 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hyde JL; Chen RB; Trobaugh DW; Diamond MS; Weaver SC; Klimstra WB; Wilusz J The 5′ and 3′ ends of alphavirus RNAs-Non-coding is not non-functional. Virus Res. 2015, 206, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Liu X; Zhang Y; Zhou SR; Dain L; Mei L; Zhu GZ Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J. Control. Release 2022, 348, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nigro JM; Cho KR; Fearon ER; Kern SE; Ruppert JM; Oliner JD; Kinzler KW; Vogelstein B Scrambled exons. Cell 1991, 64, 607–613. [DOI] [PubMed] [Google Scholar]
- [141].Jeck WR; Sharpless NE Detecting and characterizing circular RNAs. Nat. Biotechnol 2014, 32, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wilusz JEA 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip. Rev. RNA 2018, 9, e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Westholm JO; Miura P; Olson S; Shenker S; Joseph B; Sanfilippo P; Celniker SE; Graveley BR; Lai EC Genome-wide analysis of Drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 2014, 9, 1966–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chen LL The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol 2020, 21, 475–490. [DOI] [PubMed] [Google Scholar]
- [145].Filbin ME; Kieft JS Toward a structural understanding of IRES RNA function. Curr. Opin. Struct. Biol 2009, 19, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yang Y; Fan XJ; Mao MW; Song XW; Wu P; Zhang Y; Jin YF; Yang Y; Chen LL; Wang Y et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017, 27, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Qu L; Yi ZY; Shen Y; Lin LR; Chen F; Xu YY; Wu ZG; Tang HX; Zhang XX; Tian F et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Liu X; Abraham JM; Cheng YL; Wang ZX; Wang Z; Zhang GJ; Ashktorab H; Smoot DT; Cole RN; Boronina TN et al. Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Mol. Ther. Nucl. Acids 2018, 13, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chen YG; Kim MV; Chen XQ; Batista PJ; Aoyama S; Wilusz JE; Iwasaki A; Chang HY Sensing self and foreign circular RNAs by intron identity. Mol. Cell 2017, 67, 228–238.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wang Z; Ma K; Cheng YL; Abraham JM; Liu X; Ke XQ; Wang ZR; Meltzer SJ Synthetic circular multi-miR sponge simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma. Lab. Invest 2019, 99, 1442–1453. [DOI] [PubMed] [Google Scholar]
- [151].Schreiner S; Didio A; Hung LH; Bindereif A Design and application of circular RNAs with protein-sponge function. Nucleic Acids Res. 2020, 48, 12326–12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang LL; Liang DW; Chen CM; Wang Y; Amu G; Yang JL; Yu LJ; Dmochowski IJ; Tang XJ Circular siRNAs for reducing off-target effects and enhancing long-term gene silencing in cells and mice. Mol. Ther. Nucl. Acids 2018, 10, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Zhang LL; Liang DW; Wang Y; Li D; Zhang JH; Wu L; Feng MK; Yi F; Xu LZ; Lei LD et al. Caged circular siRNAs for photomodulation of gene expression in cells and mice. Chem. Sci 2018, 9, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Dolinnaya NG; Sokolova NI; Ashirbekova DT; Shabarova ZA The use of BrCN for assembling modified DNA duplexes and DNA-RNA hybrids; comparison with water-soluble carbodiimide. Nucleic Acids Res. 1991, 19, 3067–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Petkovic S; Müller S Synthesis and engineering of circular RNAs. In Circular RNAs. Dieterich C; Papantonis A, Eds.; Humana Press: New York, 2018; pp 167–180. [DOI] [PubMed] [Google Scholar]
- [156].Chen XJ; Lu Y Circular RNA: Biosynthesis in vitro. Front. Bioeng. Biotechnol 2021, 9, 787881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Chen CY; Sarnow P Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 1995, 268, 415–417. [DOI] [PubMed] [Google Scholar]
- [158].Moore MJ Joining RNA molecules with T4 DNA ligase. In RNA’Protein Interaction Protocols. Haynes SR, Ed.; Humana Press: Totowa, 1999; pp 11–19. [DOI] [PubMed] [Google Scholar]
- [159].Kershaw CJ; O’Keefe RT Splint ligation of RNA with T4 DNA ligase. In Recombinant and In Vitro RNA Synthesis. Conn GL, Ed.; Humana Press, Totowa, 2013; pp 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Gholamalipour Y; Karunanayake Mudiyanselage A; Martin CT 3′ end additions by T7 RNA polymerase are RNA self-templated, distributive and diverse in character-RNA-Seq analyses. Nucleic Acids Res. 2018, 46, 9253–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lang K; Micura R The preparation of site-specifically modified riboswitch domains as an example for enzymatic ligation of chemically synthesized RNA fragments. Nat. Protoc 2008, 3, 1457–1466. [DOI] [PubMed] [Google Scholar]
- [162].Petkovic S; Müller S RNA circularization strategies in vivo and in vitro. Nucleic Acids Res. 2015, 43, 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Nandakumar J; Ho CK; Lima CD; Shuman S RNA substrate specificity and structure-guided mutational analysis of bacteriophage T4 RNA ligase 2. J. Biol. Chem 2004, 279, 31337–31347. [DOI] [PubMed] [Google Scholar]
- [164].Chen H; Cheng K; Liu XL; An R; Komiyama M; Liang XG Preferential production of RNA rings by T4 RNA ligase 2 without any splint through rational design of precursor strand. Nucleic Acids Res. 2020, 48, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Puttaraju M; Been M Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 1992, 20, 5357–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ford E; Ares M Jr. Synthesis of circular RNA in bacteria and yeast using RNA cyclase ribozymes derived from a group I intron of phage T4. Proc. Natl. Acad. Sci. USA 1994, 91, 3117–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Rausch JW; Heinz WF; Payea MJ; Sherpa C; Gorospe M; Le Grice SFJ Characterizing and circumventing sequence restrictions for synthesis of circular RNA in vitro. Nucleic Acids Res. 2021, 49, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Xu SF; Xu Y; Solek NC; Chen JG; Gong FL; Varley AJ; Golubovic A; Pan AN; Dong ST; Zheng G et al. Tumor-tailored ionizable lipid nanoparticles facilitate IL-12 circular RNA delivery for enhanced lung cancer immunotherapy. Adv. Mater 2024, 36, 2400307. [DOI] [PubMed] [Google Scholar]
- [169].Li HJ; Peng K; Yang K; Ma WB; Qi SL; Yu XY; He J; Lin X; Yu GC Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics 2022, 12, 6422–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Jarrell KA Inverse splicing of a group II intron. Proc. Natl. Acad. Sci. USA 1993, 90, 8624–8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mikheeva S; Hakim-Zargar M; Carlson D; Jarrell K Use of an engineered ribozyme to produce a circular human exon. Nucleic Acids Res. 1997, 25, 5085–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wesselhoeft RA; Kowalski PS; Anderson DG Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun 2018, 9, 2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Müller S; Appel B In vitro circularization of RNA. RNA Biol. 2017, 14, 1018–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhang J; Luo Z; Zheng Y; Duan MY; Qiu ZJ; Huang C CircRNA as an Achilles heel of cancer: Characterization, biomarker and therapeutic modalities. J. Transl. Med 2024, 22, 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Aditham A; Shi HL; Guo JT; Zeng H; Zhou YM; Wade SD; Huang JH; Liu J; Wang X Chemically modified mocRNAs for highly efficient protein expression in mammalian cells. ACS Chem. Biol 2022, 17, 3352–3366. [DOI] [PubMed] [Google Scholar]
- [176].Qiu M; Tang Y; Chen JJ; Muriph R; Ye ZF; Huang CF; Evans J; Henske EP; Xu QB Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Zhao XW; Chen JJ; Qiu M; Li YM; Glass Z; Xu QB Imidazole-based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem., Int. Ed 2020, 59, 20083–20089. [DOI] [PubMed] [Google Scholar]
- [178].Melamed JR; Yerneni SS; Arral ML; LoPresti ST; Chaudhary N; Sehrawat A; Muramatsu H; Alameh MG; Pardi N; Weissman D et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci. Adv 2023, 9, eade1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rurik JG; Tombácz I; Yadegari A; Méndez Fernández P; Shewale SV; Li L; Kimura T; Soliman OY; Papp TE; Tam YK et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Green AA; Silver PA; Collins JJ; Yin P Toehold switches: De-novo-designed regulators of gene expression. Cell 2014, 159, 925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zhao EM; Mao AS; de Puig H; Zhang KH; Tippens ND; Tan X; Ran FA; Han I; Nguyen PQ; Chory EJ et al. RNA-responsive elements for eukaryotic translational control. Nat. Biotechnol 2022, 40, 539–545. [DOI] [PubMed] [Google Scholar]
- [182].Plotkin JB; Robins H; Levine AJ Tissue-specific codon usage and the expression of human genes. Proc. Natl. Acad. Sci. USA 2004, 101, 12588–12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Jain R; Frederick JP; Huang EY; Burke KE; Mauger DM; Andrianova EA; Farlow SJ; Siddiqui S; Pimentel J; Cheung-Ong K et al. MicroRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct. Nucl. Acid Ther 2018, 28, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]


