Abstract
Nuchal humps are morphological traits that occur among vertebrate species and have multiple functions. The emergence of nuchal humps in Xiphophorus multilineatus males maintained in the laboratory, a species that does not develop humps in the wild, provided a unique opportunity to explore the development and function of this trait. The current study investigated the tissue composition of the hump and analyzed the influence of rearing temperature and diet restriction on hump development. Through histological examination and gene expression profiling, our findings show that the hump is composed primarily of adipose tissue, which suggests a role in energy storage via fat deposition. Nuchal humps grew to a larger size in the cold environment (20 °C) than in the warm (25 °C), and the differential gene expression pattern between temperature treatments suggests increased fat deposition in the cold versus the warm environment. For example, the G0S2 gene, which inhibits lipid catabolism, is upregulated in the cold environment, and the WARS-1 gene, which leads to increased fat stores when under-expressed, is upregulated in the warm environment. The hypothesis that temperature influences hump development by stimulating shifts in fat metabolism is further supported by the finding that males from the warm environment used fat stores in the nuchal hump at a faster rate when placed on a restricted diet than males from the cold. The influence of temperature, diet, and potentially activity levels on fat deposition suggests X. multilineatus as an evolutionary animal model for gaining insights into the mechanisms involved in human obesity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10695-025-01539-2.
Keywords: Nuchal hump, Lipid storage, Temperature, Xiphophorus multilineatus
Introduction
Nuchal humps, broadly defined as a tissue mass located dorsal posterior to the head, are present across multiple vertebrate taxa. The tissue composition of nuchal humps varies among vertebrate species, as does the development and function of this structure (Alexander and Player 1965; Cave and Allbrook 1958; Manamendra-Arachchi et al. 2006; Susuki et al. 2014; Ward and Ward 2020). In sexually monomorphic fish species where the hump is persistent, such as Gila cypha, an anti-predatory function is likely. Both sexes would benefit from the presence of a nuchal hump due to increased body depth, which can limit the ability of predators to capture individuals once the hump develops (Douglas 1993; Portz and Tyus 2004); Ward and Ward 2020). Among sexually dimorphic species, nuchal humps are often sexually selected traits, and proposed functions include species recognition, sex recognition, and indication of mate quality (Barlow and Siri 1997; Takahashi 2018; Rometsch et al. 2021). There is also evidence to suggest that the nuchal hump may play a role in energy storage, based on increased expression of genes known to promote adipogenesis and cell proliferation within nuchal hump tissue of the African cichlid Cyrtocara moorii (Lecaudey et al. 2019), and enlargement of the hump in another African cichlid, Cyphotilapia gibberosa, commonly known as Mpimbwe Blue Frontosa, was due to hypertrophy of the hypodermal layer and increased fat storage (Takahashi 2018).
The nuchal hump is a diagnostic trait for the swordtail species Xiphophorus birchmanni (Rauchenberger et al. 1990) and may play a role in mate choice (Rosenthal et al. 2003) as well as influence swimming endurance in relation to the sword (Johnson et al. 2014). Nuchal humps are not known to occur in the wild for any of the other Xiphophorus fishes. The development of this trait in the laboratory occurred in X. multilineatus adult males that were sampled from a wild population and brought to the laboratory as well as in males that were reared from birth in the laboratory (Tompkins et al. 2021). Females prefer males with humps versus males without (Tompkins et al. 2021), suggesting a potential benefit for the hump via sexual selection if they were to develop in the wild. One aspect of the laboratory environment that promotes the hump is diet, as X. multilineatus males reared on high quality diets (i.e., higher fat content in addition to protein) were more likely to develop larger humps than males reared on low quality diets (Tompkins et al. 2021).
Temperature influences fat utilization in fish by stimulating shifts between catabolizing fat as an energy source at warmer acclimation temperatures to storing fat in liver and muscle tissues at colder acclimation temperatures (Stone and Sidell 1981); Egginton and Sidell 1989). Accordingly, we hypothesize that temperature influences the process of hump development. Therefore, to further examine the possible function of the nuchal hump in X. multilineatus, we used a split-brood design to examine the development of the hump in the laboratory under two different temperature regimes. We first addressed the following questions: (1) What is the tissue composition of the nuchal hump in X. multilineatus? While the presence of lipid droplets has been detected in the nuchal hump of X. multilineatus (Tompkins et al. 2021), it is not known if the hump is composed primarily of adipose tissue. (2) Are there differences in the genes expressed in hump tissue in males reared in a cold environment versus warm? (3) How does rearing temperature influence the allometric growth of the nuchal hump? Second, we reduced the diet of adult males for approximately 1 month to ask (4) is the size of the hump influenced by a reduction in diet? (5) Is the influence of diet reduction consistent across temperature treatments? And finally, we examined activity levels to ask (6) is the size of the hump influenced by activity? (7) Is the influence of activity consistent across temperature treatments?
Methods
Animals and sample collection
X. multilineatus males used to test the effects of rearing temperature on the development of nuchal humps, through both RNA sequencing (RNA-Seq) analyses and multivariate analysis of hump size and growth rate, were obtained by isolating 50 pregnant females from an existing community tank with only courter male X. multilineatus located in the Morris Lab. Courter males are one of two alternative reproductive tactics (ARTs) found in this species. ARTs in X. multilineatus are genetically influenced by variation in copy number of mc4r B alleles on the Y chromosome (Lampert et al. 2010). The ARTs are dimorphic for several traits including body size (courter males are larger than sneakers) and reproductive behavior, where courter males use only courtship displays to attract females and sneaker males use either courtship or forced copulation to gain access to females (Liotta et al. 2021). Courter males in the lab were observed to have larger humps relative to their body size than sneaker males, so only males with a courter lineage were used in this study due to the higher propensity of humps forming on courter males. Females were allowed to drop fry, and the fry were split between two environmental chambers. Environmental chambers were set at 25 °C (warm treatment) and 20 °C (cold treatment). Fry were transferred to individual 2.5-L tanks in respective environmental chambers at 30 days of age: 72 fry in warm and 72 fry in cold. All fry were fed a high-quality diet of Ken’s Spirulina flake food in the morning daily and brine shrimp in the afternoon 5 days a week. The flake food was the same high-quality diet used by Tompkins et al. (2021) that promoted growth of larger humps. Each tank was equipped with a Whisper® Powerfilter that circulated the water and agitated the water surface. Measures of dissolved oxygen saturation were similar between treatments ((N = 8 per treatment, cold = 100.8 ± 1.1%; warm = 97.7 ± 1.4%).
Histology
Males used for histology were obtained from a community tank with only X. multilineatus located in the Morris Lab. Fish in the community tank were fed a high-quality diet of Ken’s Spirulina® flake food 7 days per week and Artemia sp. nauplii 5 days per week at a rate that was completely consumed within 5 min. The community tank was maintained at room temperature between 21 and 22 °C. Males were euthanized with MS-222, and transverse tissue sections were taken from the nuchal region (Fig. 1) and preserved in 10% neutral buffered formalin. Tissue samples were processed and stained at the Ohio University Histology Core Facility using standard eosin and hematoxylin techniques. Stained slides were then digitally scanned, and the depth of the hump was measured and denoted using Aperio ImageScope software. Image J software was used to measure standard length (mm) and nuchal hump area (mm2) of the two sections for each fish.
Fig. 1.
Histology of X. multilineatus nuchal hump: A transverse section through nuchal hump of male X. multilineatus. Bar represents depth of the hump as determined by measuring the fish from a 2D image taken before tissue sampling; B adipocytes within nuchal hump (N, nucleus; PM, plasma membrane; H&E); C a male from the warm treatment with a relatively small nuchal hump for its size (0.099 mm2/mm); and D a male from cold treatment with a relatively large hump (0.275 mm2/mm). The white dashed lines (C, D) circumscribe the nuchal regions specifically dissected for nuchal hump tissues
Gene expression profiling
Three sexually mature males from each temperature treatment were selected for gene expression profiling using RNA sequencing (RNA-Seq) and euthanized with MS-222. Each male had a corresponding full sibling in the opposing dietary treatment. Image J software was used to measure standard length (mm) and nuchal hump area (mm2) of each fish. Hump tissue, belly tissues, and livers were dissected from each male (see Fig. 1) and preserved in RNAlater®. RNA was isolated using a method described in an earlier study. Briefly, total RNA from these tissue samples was isolated using TRI-Reagent (Sigma Inc., St. Louis, MO, USA). Tissue samples were homogenized in TRI-Reagent followed by the addition of 200 µl/ml chloroform, and the samples were vigorously shaken and subjected to centrifugation at 12,000 g for 5 min at 4 °C. Total RNA was further purified using the RNeasy mini RNA isolation kit (Qiagen, Valencia, CA, USA). Residual DNA was eliminated by performing column DNase digestion at 25 °C for 15 min. Total RNA concentration was determined using a Qubit 2.0 fluorometer (Life Technologies, Grand Island, NY, USA). RNA quality was verified on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) to confirm that RIN scores were above 8.0 prior to sequencing.
Differentially expressed gene analyses
RNA-Seq was performed upon libraries constructed using the Illumina TruSeq library preparation system (Illumina, Inc., San Diego, CA, USA). RNA libraries were sequenced as 150-bp pair-end fragments using the Illumina Novaseq system (Illumina, Inc., San Diego, CA, USA).
Short sequencing reads were mapped to a reference X. multilineatus genome using Tophat2 (Reference PMID: 23,618,408). Gene expression profiling was conducted by quantifying sequencing reads that were mapped to exons using the Subread package featureCount function (reference PMID: 24,227,677, 23,558,742). Differentially expressed genes were identified using the R-Bioconductor package edgeR with a statistical cutoff as |Log2Fold Change|> 1, false discovery rate < 0.05. Principal component analyses (PCA) were performed using the R prcomp function, using normalized and centered gene expression counts (22287627). Gene Ontology analyses were conducted using g:Profiler (https://biit.cs.ut.ee/gprofiler/gost), using default statistical thresholds.
Comparison between X. multilineatus hump to human adipocyte cell atlas
Three hump tissue samples collected from the cold rearing temperature group were used to compare to genes hallmarking cell types within the human white adipose tissue (PMID: 35,296,864). We used count per million (cpm) > 50 for each sample to determine genes expressed. For each human cell type, we kept genes with false discovery rate (FDR) adjusted P-value < 0.05 and compared to hump expressed genes. A hypergeometric test was performed with the null hypothesis that observing a cell type marker in the hump tissue is random. A hypergeometric test P-value < 0.05 was used to determine statistical significance.
Growth and size of the nuchal hump
To determine the effects of temperature on the growth and size of the nuchal hump, fish from temperature treatments were photographed at 30, 100, 210, and 330 days of age to capture nuchal hump developmental rates. The area of the nuchal hump was measured at each age interval using Image J software (Fig. 1). A period of diet restriction was imposed for 1 month, which consisted of only a single daily feeding of flake food. All males placed on a restricted diet were over 400 days old and sexually mature at the time of the restriction. Only full sibling males were used in the analyses to control for genetic effects.
All statistics regarding hump growth and size were calculated using R software. In addition to treatment, we examined whether adult size and early growth rate influenced the development of humps. To determine what factors influenced the size of the adult nuchal humps, a generalized least squares model was used with a random effect of dam. Treatment was the fixed effect with male size at maturity and early growth rate as covariates. The intraclass correlation coefficient (ICC) was calculated to determine the proportion of total variability in hump size that was attributable to differences among dams. The models were run with the restricted maximum likelihood estimation method (REML) to correct for degrees of freedom. Results from performing ANOVA on the model and coefficient estimates for each fixed effect are reported.
Growth curves for nuchal hump size were fitted to the observed data for each treatment using the von Bertalanffy growth function (VBGF), Gompertz (Gomp), and a logistic (Log) curve with the R package “fishmethods” and the function growth. The growth function with the lowest residual sum-of-squares was considered the best fit and was used to calculate asymptotic hump size at maturity (length at which male growth stops) for both treatments. Growth curves were compared by treatment using a pairwise permutation test with the R function compareGrowthRates in the package “fishmethods.”
Diet restriction and size of nuchal hump
Changes in nuchal hump size before and after a period of diet restriction were compared for each treatment by using a generalized least squares model with a random effect of dam plus a correlation component accounting for repeated measures among males before and after diet restriction. The intraclass correlation coefficient (ICC) was calculated to assess the proportion of total variability in hump size that was attributable to differences among mothers. The degree of correlation between repeated measures per male was estimated by the compound symmetry parameter (rho). Temperature treatment and diet restriction status (either pre- and post-restriction) were used in all models as independent variables, and an interaction term between treatment and diet restriction status was included to determine whether the magnitude of change in hump size after diet restriction was dependent on treatment. Nuchal hump size was log transformed within the model to meet the assumption of normality of the residuals. Results from R function Anova are reported.
Activity levels and size of the nuchal hump
To test the hypothesis that activity may influence the size of the nuchal hump, we tested the activity levels of 15 males reared in the cold environment and 8 males reared in the warm environment. A test tank was placed in each of the environmental chambers, consisting of a 37.85-l aquarium with gravel on the bottom, marked on the outside with lines dividing it into 12 boxes (Supplemental Fig. S2). After transferring a male to a test tank, he was given 5 min to acclimate. We then measured the number of times an individual crossed into another section of the aquarium over a period of 5 min.
We used a generalized linear model to examine variation in relative nuchal hump size (nuchal hump/SL), considering the factors of temperature treatment, activity levels, and their interaction. The best model was selected based on AIC (difference > 2.0).
Results
Histology and gene expression show the hump is adipose tissue
Histological examination shows the hump is primarily composed of adipose tissue (Fig. 1A and B). Stored lipid droplets within adipocytes are cleared during histological processing, leaving only the plasma membrane and peripherally located nucleus visible (Fig. 1B).
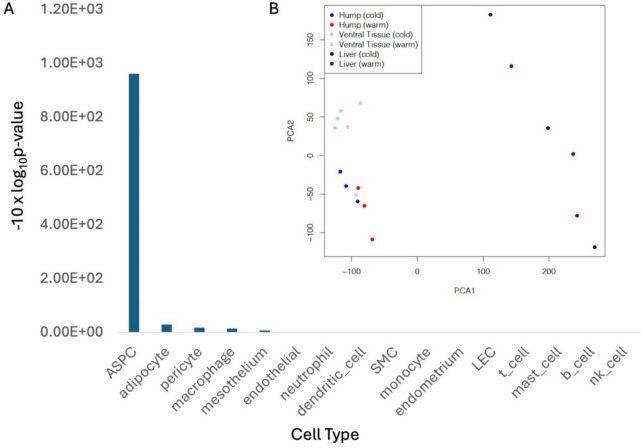
We compared the nuchal hump gene expression profiles to liver and belly tissue. Principal component analyses showed the hump tissue gene expression pattern is more similar to belly tissue gene expression than to either brain or liver. This is evidenced by the proximity of the hump tissue and belly tissue samples on a dimension-reduced x–y grid (Fig. 2B). These observations suggest that not only the tissue histology but also the molecular phenotype of the hump show they are adipocytes and are likely to be energy-storage tissue. In addition, we compared gene expression in the bulk hump tissue to human cell atlas for white adipocyte (PMID: 35296864) to determine if the adipocyte genes are expressed in the hump. For the 16 cell types annotated in human white adipocyte, gene markers for 4 cell types are found to be over-enriched (P-value < 0.05). They are adipose stem and progenitor cells (ASPC), adipocyte, macrophage, and pericyte (Fig. 2A; for gene markers, see Supplemental Table S1 and Fig. S1).
Fig. 2.
Cell type annotation of X. multilineatus hump. A Expressed genes in the bulk hump tissue were compared to human white adipocyte cell atlas. The bar heights represent − 10 × log10 hypergeometric test P-value. P-value < 0.05 is used to determine statistical significance. B A dot plot, showing the principal component analyses of X. multilineatus liver, belly (ventral), and hump
Gene expression within the hump
Males reared in two different temperatures differentially expressed 326 genes in nuchal hump tissue (|Log2FC|> 1; FDR < 0.05). Functional analyses of the differentially expressed genes (DEGs) showed genes involved in development, regulation of cell migration, and regulation of cell communication (Supplemental Table S2). Manual curation of the DEGs showed Xiphophorus orthologs to human adipocyte marker genes hebp2 and lipe, and adipose stem and progenitor cell (ASPC) marker genes cfd, tnfaip2, kifap3, cox6b1, and txn are differentially expressed. In addition, genes that are related to fat deposition and temperature acclimation were identified (Table 1).
Table 1.
Examples of differentially expressed genes in nuchal hump tissue between temperature treatments known to be related to energy homeostasis. Genes had a false detection rate (FDR) of 0.05
| Gene | Related function | Upregulated | Ref |
|---|---|---|---|
| G0S2 | Acts as a molecular brake on triglyceride (TG) catabolism | Cold | Heckmann et al. (2012); Yang et al. (2010) |
| ACP6 | An enzyme that regulates lipid metabolism in the mitochondria | Warm | Hiroyama and Takenawa (1999) |
| HSPA12A | Heat shock protein in the HSP70 family involved in molecular chaperoning and preventing protein denaturing in response to stressors. HSP70 upregulation also inducible by cold shock | Cold | Hu et al. (2022); Reid et al. (2022) |
| PGC-1α | Highly expressed in brown fat in mammals; strongly induced by cold exposure; linking environmental stimulus to adaptive thermogenesis | Cold | Cheng et al. (2018); Liang and Ward (2006) |
| WARS-1 | Reduction of WARS-1 expression in C. elegans increased fat stores and starvation survival | Warm | Webster et al. (2017) |
| IGFBP3 | Insulin-like growth factor binding protein | Cold | Kim (2013) |
| APOD | Atypical apolipoprotein with broad tissue distribution involved in lipid homeostasis | Cold | Perdomo et al. (2010) |
| Col7a1 | Codes for collagen alpha 1 protein, which occurs in the basement membrane beneath stratified squamous epithelia | Cold | Koca et al. (2023) |
| APOA1 | Structural component of high-density lipoprotein (HDL) or “good” cholesterol in plasma | Cold | Bandarian et al. (2013) |
| SFRP2 | Reported in pre-adipocytes, involved in adipocyte differentiation; secreted by adipose tissue | Cold | Crowley et al. (2016) |
Adult hump size
The model that best explained variation in hump size at 330 days of age (all males sexually mature) included treatment, male size (length) at sexual maturity, and early growth rate with dam as a random effect (Table 2). The intraclass correlation coefficient (ICC) revealed that 35.3% of the total variability in hump size was attributable to variability associated with dam. The negative coefficient for temperature treatment (− 1.01) produced by the model indicates that hump size is predicted to be smaller in the warm treatment than the cold when other fixed variables were held constant. Mean hump size at sexual maturity for males from the warm treatment was 4.60 ± 2.02 mm2, while the mean size of humps for males in the cold treatment was 9.62 ± 2.27 mm2. The coefficient for male size at maturity (0.54) reveals a positive relationship between hump size and body size, meaning larger males had larger humps when other variables were held constant. The model produced a negative coefficient (− 22.5) for early growth rate indicating a negative relationship with hump size at maturity, meaning males that grew faster as juveniles developed smaller humps by the time they reached sexual maturity compared with slower growing males.
Table 2.
ANOVA results generated by the best model for explaining variation in nuchal hump size at maturity
| Variable | Numerator DF | Denominator DF | F-value | P-value |
|---|---|---|---|---|
| (Intercept) | 1 | 24 | 488.3557 | < 0.0001 |
| Treatment | 1 | 24 | 126.5105 | < 0.0001 |
| Size at maturity (L) | 1 | 24 | 35.9872 | < 0.0001 |
| Early growth rate | 1 | 24 | 5.0096 | 0.0326 |
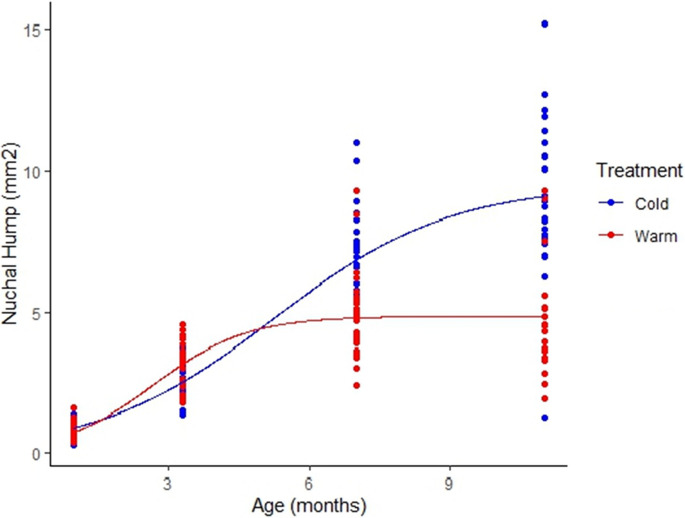
Regarding the growth of the hump, the logistic growth curve had the lowest residual sum of squares and was the best fit compared to the von Bertalanffy and Gompertz for both cold and warm treatments (Table 3). Growth curves for the nuchal hump were significantly different between temperature treatments (P = 0.005) and reflect a pattern whereby males from the warm environment grew humps at a faster rate early in development but had smaller humps by the time they reached maturity than males from the cold environment (Fig. 3).
Table 3.
Nuchal hump growth curve selection data. The logistic (Log) curve had the lowest residual sum of squares (RSS) compared to the von Bertalanffy (VBF) and Gompertz (Gomp) curves in both the warm (A) and cold (B) treatments
| RSS | k ± SE | |
|---|---|---|
| A) Model | ||
| VBF: Linf*(1-exp(-K*(t-t0))) | 217.9 | 0.55 ± 0.15 |
| Gomp: Linf*exp(-exp(-K*(age-t0))) | 214.5 | 0.77 ± 0.17 |
| Log: Linf/(1 + exp(-K*(age-t0)) | 212.5 | 1.12 ± 0.24 |
| B) Model | ||
| VBF: Linf*(1-exp(-K*(t-t0))) | 432.5 | 0.19 ± 0.03 |
| Gomp: Linf*exp(-exp(-K*(age-t0))) | 383.1 | 0.39 ± 0.06 |
| Log: Linf/(1 + exp(-K*(age-t0)) | 376.9 | 0.57 ± 0.07 |
Fig. 3.
Comparison of nuchal hump growth between temperature treatments. Both growth curves are plotted with a logistic function and were significantly different (P = 0.005)
Diet restriction and size of the nuchal hump
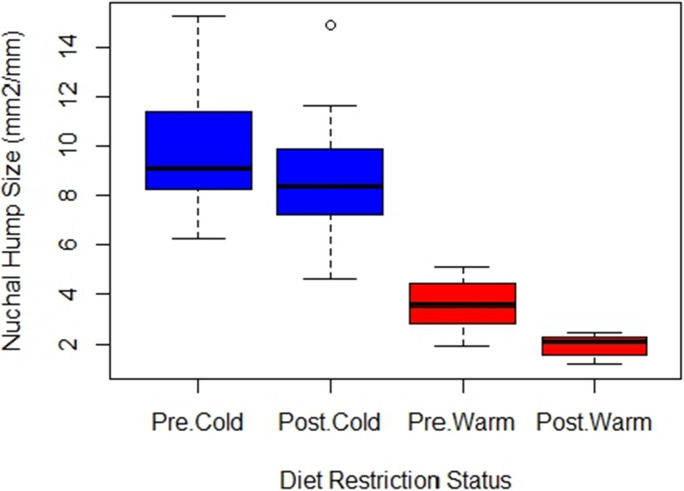
Following a 1-month period of diet restriction, variation in nuchal hump size was explained best by a model that included treatment, diet restriction status (pre- or post-), and an interaction term between them (Table 4). The model which included dam as a random effect and a correlation component to account for repeated measures was the best fit among the three models compared (P < 0.001, Table 4) and variation attributed to dam explained 13.8% of the total variability in hump size in this model as revealed by the intraclass correlation coefficient (ICC). The model produced a high correlation value (rho = 0.805) which suggests that repeated measures for each male are more similar relative to the variability across different males. Negative regression coefficients for treatment (− 1.02) and diet restriction status (− 0.14) indicate that not only were humps estimated to be smaller post-restriction than pre-restriction, but also smaller in the warm treatment versus the cold. More importantly, the coefficient for the interaction term (− 0.45) indicates there is a synergistic effect between treatment and diet restriction, namely that being in the warm environment leads to even smaller post-restriction humps than being in the cold environment. Males from the cold treatment had a mean post-restriction hump size of 8.68 ± 2.58 mm2 versus a pre-restriction size of 9.86 ± 2.46 mm2, representing a reduction in mean hump size of 1.18 mm2 after diet restriction. Males from the warm treatment had a mean post-restriction hump size of 1.97 ± 0.47 mm2 versus a pre-restriction size of 3.62 ± 1.06 mm2, which is a reduction in mean hump size of 1.64 mm2 after diet restriction (Fig. 4).
Table 4.
ANOVA results generated by the best model for explaining variation in nuchal hump size before and after diet restriction
| A | Numerator DF | Denominator DF | F-value | P-value |
|---|---|---|---|---|
| (Intercept) | 1 | 33 | 811.60 | < 0.001 |
| Treatment | 1 | 33 | 127.01 | < 0.001 |
| Restriction status | 1 | 33 | 77.24 | < 0.001 |
| Treatment:restriction status | 1 | 33 | 40.69 | < 0.001 |
Fig. 4.
Mean nuchal hump size per treatment before (pre) and after (post) a 1-month period of diet restriction
Activity levels and size of the nuchal hump
Variation in the relative nuchal hump size (nuchal hump/SL) was best explained by temperature treatment (chi-square = 55.17, df = 1, P < 0.001; reared warm vs cold) and activity levels (chi-square = 5.65, df = 1, P = 0.017), with higher activity levels correlated with relatively smaller hump size in both treatments. The best model did not include an interaction between these two factors. Overall fitted model compared against the intercept only model (chi-square = 30.417, df = 2, P < 0.001) (see Supplemental Fig. S3).
Discussion
Nuchal hump development in Xiphophorus multilineatus males in the laboratory provides a unique opportunity to explore the mechanisms of development and function of this trait. The findings of this study show that the hump consists of adipose tissue, that males reared in a colder environment (20 °C) develop larger humps controlling for overall body size than males reared in a warmer environment (25 °C), and that the differential gene expression pattern in the hump between temperature treatments suggests increased fat deposition in the cold environment versus the warm. Furthermore, changes in the size of the hump due to diet restriction and environmental temperature suggest that the pattern detected is due to increased metabolism in the warm environment stimulating an increased use of fat as an energy source, leading to smaller humps. The implications of these results for understanding the function of the nuchal hump in this system as well as understanding the roles of both diet and activity in human obesity are discussed.
Finding that the nuchal hump is composed primarily of adipocytes suggests an energy storage function of this structure. Certainly, some vertebrates use fat stored in nuchal humps and similar dorsal structures as an energy source when dietary intake is insufficient to maintain metabolic rates, and the amount of fat stored in the hump may reflect seasonal shifts in food quality and quantity (Alexander and Player 1965; Giles et al. 2015; Bengoumi et al. 2005). Within fish taxa, there are examples of nuchal humps composed primarily of adipose tissue, most notably within the species flocks of cichlids that occur in East African rift lakes (Takahashi 2018; Lecaudey et al. 2019). Yet even among this sub-group of fish, energy storage does not appear to be a primary function for humps. Takahashi (2018) found that the nuchal hump of Cyphotilapia gibberosa was formed by a thickening of the hypodermis through fat deposition, but the thickness was not correlated with body fitness in females and only slightly positively correlated in males. In other words, well-conditioned individuals did not store extra lipid in the humps, and poorly conditioned individuals did not consume lipid from their humps. In the current study, however, the size of the nuchal hump was smaller after diet restriction regardless of temperature, suggesting that males were able to utilize stored fat in the nuchal hump as an energy source to make up for reduced caloric intake.
If it is assumed that the function of the nuchal hump is for energy storage in X. multilineatus, how can the pattern of larger humps on the males reared in the cold versus the warm treatments be explained? Fish acclimated to cold temperatures typically increase their storage of lipids in various body tissues including subcutaneous, liver, and muscle. Fish acclimated to warmer temperatures, on the other hand, increase the use of lipids as a direct energy source and store less of it in tissues (Stone and Sidell 1981); Egginton and Sidell 1989). There is evidence that at extremely high temperatures, fish may shift to storing fat as seen in the mummichog (Fundulus heteroclitus, Moerland and Sidell 1986), but fat storage in this case occurred specifically in the liver, not skeletal muscle or hypodermal tissue. The X. multilineatus males in the current study were not subjected to critical maximum or minimum temperatures and followed the typical pattern of temperature-related fat utilization in fish. The species of swordtail that forms the nuchal hump in nature, X. birchmanni, is found at warmer temperatures on average than a closely related species that does not develop nuchal humps, X. malinche (Rauchenberger et al. 1990). These two species often occupy the same stream systems and form hybrid zones (Culumber et al. 2012). When considering the potential influence of temperature alone, hump formation in X. birchmanni seems less likely than in X. malinche. Therefore, the natural occurrence of nuchal humps in X. birchmanni suggests that factors other than temperature are more influential in the development of humps in this species. For example, correlated environmental differences in relation to food resources (higher quality diets) could potentially be more important.
The results of this study suggest that males reared in a warmer environment either utilize more fat than males in the cold or have simply used all the energy available to them with no excess to store in the nuchal humps. The first possibility can be considered the “metabolic difference hypothesis” and is supported by the finding that males from the warm environment used fat stores in the nuchal hump at a faster rate when placed on a restricted diet than males reared in the cold. In addition, seven of the top-tiered, differentially expressed genes in the nuchal hump of X. multilineatus males are linked to lipid metabolism (Table 1) with expression patterns suggesting increased fat deposition in cold males versus warm. Overexpression of the G0S2 switch gene, for example, reduces the rate of lipolysis in adipocytes by acting as a molecular brake on triglyceride catabolism (Yang et al. 2010). A higher expression of G0S2 in X. multilineatus males from the cold treatment makes sense since these males developed larger humps. ACP6 that regulates lipid metabolism was upregulated in the warm environment (Hiroyama and Takenawa 1999). Likewise, males from the cold treatment had lower expression of the WARS-1 gene, which when inactivated in Caenorhabditis elegans leads to increased fat stores and increased starvation survival (Webster et al. 2017). Interestingly, a gene that is highly expressed in brown fat in mammals (PGC-1α) when induced by cold exposure (Cheng et al. 2018) was also overexpressed in X. multilineatus males from the cold treatment. This gene links colder environmental temperature to adaptive thermogenesis by stimulating the catabolic burning of brown fat in mammals; however, fish do not develop brown fat. If the expression pattern of this gene is similar between fish (which are not capable of thermogenesis) and mammals when considering the effect of environmental temperature, its role in thermogenesis among mammals may be a coopted function.
Males from the warm environment grew humps at a faster rate early in development, yet males from the cold developed larger humps by the time they reached sexual maturity (Fig. 3). This is a similar pattern to that of the growth of male body size in general, in that X. multilineatus males from the warm environment grew faster as juveniles but reached a smaller adult size than males from the cold (Tompkins et al. in prep). This similarity is not surprising since hump size and body size had a significant positive relationship in the current study.
Tompkins et al. (2021) suggested that diet could be a primary difference between the laboratory and natural environment for X. multilineatus, inducing development of the nuchal hump in the laboratory. An additional factor to consider, however, is that the environment in which males were reared (isolated, in 2.5 l aquarium) does not allow for activity levels that would be more common in a natural environment. Swordtail males spend a large proportion of their time interacting with other males and courting females in the wild (Rios-Cardenas et al. 2010), and all these energetically costly behaviors were absent in the laboratory setting. The hypothesis that the reduced activity levels in the rearing environment is partially responsible for the development of the nuchal hump is supported by the observation that X. multilineatus males from large mesocosm in our laboratory appear to be much less likely to develop a nuchal hump than males that are reared individually, in addition to the negative relationship we detected between activity levels and relative hump size (Fig. S3). It is also possible that hump size influences activity level due to the hump being a cumbersome feature. However, the shape of X. birchmanni with its increased anterior body depth due to having a nuchal hump (the only species in the clade to form them in the wild) imparts improved endurance swimming performance compared with the dorsoventrally narrower shape of the species it naturally hybridizes with, X. malinche (Johnson et al. 2014). Therefore, it seems more likely that activity influences hump size rather than vice versa.
There are potential benefits of X. multilineatus having the ability to develop a nuchal hump that could be considered. We previously detected female mate preference for males with nuchal humps in X. multilineatus even though the trait does not occur in the wild (Tompkins et al. 2021). We suggest that the preference we detected may be similar to what has been suggested for X. birchmanni (Rosenthal et al. 2003) and reflect a more general preference for larger males. Alternatively, if the hump developed in X. multilineatus in the wild, it could lead to improved swimming performance by increasing anterior body depth, which minimizes drag (Johnson et al. 2014). Compartmentalized fat depots can have dual functionality in other vertebrates. Camels, for example, use the fat in their hump as an energy source, but one hypothesis suggests camels may concentrate body fat in the hump to avoid overheating if the fat was evenly distributed throughout the subcutaneous layer covering the body (Kassa 2016). However, the fact that the humps are not known to form in the natural environments of X. multilineatus suggests that selection for these other functions would be minimal if nonexistent. Future studies of the functions of depositing fat in the nuchal hump in swordtail fishes, in addition to energy storage, should be considered.
While nuchal humps can serve many different functions across fishes, the molecular mechanisms involved in their development have been compared to the development of soft tissues on the face of vertebrates in general (Lecaudey et al. 2019). For example, Lecaudey et al. (2021) identified an upstream transcription factor (foxp1) with reduced expression in the hypertrophic lip region of the vacuum cleaner cichlid (Gnathochromis permaxillaris), and with a function that has been associated with hypertrophy of the upper lip in humans. In addition, transcription factors with increased expression in the flapped snout of several different species of Lake Malawi cichlids were members of the FOX transcription factor family, with two (foxf1 and foxa2) known to be linked to nose morphogenesis in mammals (Duenser et al. 2023). We are also interested in examining the molecular mechanisms involved in the development of the nuchal hump in swordtail fishes. We plan to use omics approaches, collecting samples for single nucleus RNA sequencing assays of different nuchal hump developmental stages to investigate cellular and molecular pathways leading to the hump.
Evidence that swordtail fish use the nuchal hump for energy storage, with the humps composed of adipose tissue, is interesting considering the importance of identifying evolutionary animal models for studying diet-induced obesity. The development of a nuchal hump in X. multilineatus under laboratory conditions, after being fed a high-calorie diet, may be a condition similar to that observed in humans (Schartl and Lu 2024). One of the other aspects of the laboratory environment that appears to promote nuchal hump formation is reduced activity levels, also an important factor in human obesity (Chin et al. 2016). Finally, the influence of environmental temperature on rates of obesity in humans has also been established (Trentinaglia et al. 2021; Moellering and Smith 2012), and cold-induced thermogenesis in mammals is associated with reduced risk of diabetes (Schrauwen et al. 2016; Horino et al. 2022). Considering the influence of temperature, diet, and potentially activity levels on the development of the nuchal hump in X. multilineatus, this model system has the potential to provide future insight into the mechanisms involved in human obesity that other model systems cannot. Variation across species within Xiphophorus in the propensity to develop nuchal humps allows for the consideration of this trait from an evolutionary perspective, including unique opportunities to determine genetic variant site genotypes using hybrids and backcross hybrids of different species, even from the most phylogenetically distant branches of the genus (Shartl and Lu 2024). By examining the development of the nuchal hump in backcross hybrids of X. multilineatus and X. couchianus, where we can determine genetic variant site genotypes and profile their gene expression, we plan to disentangle genetic networks involved in fat deposition.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Grace Vance for collecting the activity level data and Mrs. Korri Weldon for technical assistance.
Author contributions
The first draft of the manuscript was written by K.T., Y. L., and M.M. and all authors commented on previous versions of the manuscript. Photography, histology, and statistical analysis of variability in nuchal hump size was performed by K.T.. RNA-seq data was generated in the Genome Sequencing Facility, which is supported by UT Health San Antonio, NIH-NCI P30 CA054174 (Cancer Center at UT Health San Antonio). RNA-seq data analysis was performed by Y.L. All authors read and approved the final manuscript.
Funding
Funding for this project was provided by a grant from the Graduate Student Ohio University Research Fund, the Department of Biological Sciences Ohio University Rush Elliott Endowed Professorship, a NIH Shared Instrument grant S10OD030311 (S10 grant to NovaSeq 6000 System), and CPRIT Core Facility Award (RP220662).
Data availability
The data that support the findings of this study are available from the authors upon reasonable request.
Declarations
Ethics approval
The protocols followed for handling and care of all fish involved this study have been approved by the Institutional Animal Care and Use Committee of Ohio University in accordance with AAALAC (12-L-042), International. NIH animal use assurance #A3610-01. Collecting Permit: Permiso de Pesca de Fomento No. DGOPA 18317.271113.8982.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexander A, Player IC (1965) A note on the nuchal hump of the square-lipped rhinoceros, Ceratotherium simum simum (Burchell). Lammergeyer 3(2):1–9 [Google Scholar]
- Bandarian F, Hedayati M, Daneshpour MS, Naseri M, Azizi F (2013) Genetic polymorphisms in the APOA1 gene and their relationship with serum HDL cholesterol levels. Lipids 48:1207–1216 [DOI] [PubMed] [Google Scholar]
- Barlow GW, Siri P (1997) Does sexual selection account for the conspicuous head dimorphism in the Midas cichlid? Anim Behav 53:573–584 [Google Scholar]
- Bengoumi M, Faulconnier Y, Tabarani A, Sghiri A, Faye B, Chilliard Y (2005) Effects of feeding level on body weight, hump size, lipid content and adipocyte volume in the dromedary camel. Animal Research 54(5):383–393 [Google Scholar]
- Cave AJ, Allbrook DB (1959) The skin and nuchal eminence of the white rhinoceros. In proceedings of the zoological society of London, Oxford, UK: Blackwell Publishing Ltd 132(1):99–107
- Cheng CF, Ku HC, Lin H (2018) PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci 19:3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SH, Kahathuduwa CN, Binks M (2016) Physical activity and obesity: what we know and what we need to know. Obes Rev 17:1226–1244. 10.1111/obr.12460 [DOI] [PubMed] [Google Scholar]
- Crowley RK, Oreilly MW, Bujalska IJ, Hassan-Smith Z, Hazlehurst JM, Foucault DR, Stewart PM, Tomlinson JW (2016) SFRP2 is associated with increased adiposity and VEGF expression. PLoS ONE 11(9):e0163777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culumber ZW, Shepard DB, Coleman SW, Rosenthal GG (2012) Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: Xiphophorus). J Evol Biol 25(9):1800–1814 [DOI] [PubMed] [Google Scholar]
- Douglas ME (1993) Analysis of sexual dimorphism in an endangered cyprinid fish (Gila cypha) using video image technology. Copeia 1993(2):334–343 [Google Scholar]
- Duenser A, Singh P, Lecaudey LA, Sturmbauer C, Albertson RC, Gessl W, Ahi EP (2023) Conserved molecular players involved in human nose morphogenesis underlie evolution of the exaggerated snout phenotype in cichlids. Genome Biol Evol 15(4):evad045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S, Sidell BD (1989) Thermal acclimation induces adaptive changes in subcellular structure of fish skeletal muscle. Am J Physiol 256(1):R1–R9 [DOI] [PubMed]
- Giles SL, Nicol CJ, Rands SA et al (2015) Assessing the seasonal prevalence and risk factors for nuchal crest adiposity in domestic horses and ponies using the Cresty neck score. BMC Vet Res 11:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann BL, Zhang X, Xie X, Liu J (2012) The G0/G1 Switch Gene 2 (G0S2): regulating metabolism and beyond. Biochim Biophys Acta 1831:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroyama M, Takenawa T (1999) Isolation of a cDNA encoding human lysophosphatidic acid phosphatase that is involved in the regulation of mitochondrial lipid biosynthesis. J Bio Chem 274:29172–29180 [DOI] [PubMed] [Google Scholar]
- Horino M, Ikeda K, Yamada T (2022) The role of thermogenic fat tissue in energy consumption. Curr Issues Mol Biol 44(7):3166–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, Mei H, Liu J, Wang W, Liu Q (2022) Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm 2022:e161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Macedo DC, Passow CN, Rosenthal GG (2014) Sexual ornaments, body morphology, and swimming performance in naturally hybridizing swordtails (Teleostei: Xiphophorus). PLoS ONE 9(10):e109025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa HA (2016) Paper reviewed article on the physiology and adaptive mechanism of camel / dromedary / in semi-desert and arid environment. Int J Curr Res 8:33116–33124 [Google Scholar]
- Kim HS (2013) Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism. Annals Ped Endocr Metab 18:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koca D, Seraudie I, Jardiller R, Cochet C, Filhol O, Guyon L (2023) COL7A1 expression improves prognosis prediction for patients with clear cell renal cell carcinoma atop of stage. Cancers 15:2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert K, Schmidt C, Fischer P, Volff JP, Hoffman C, Muck J, Lohse MJ, Ryan MJ, Schartl M (2010) Determination of onset of sexual maturation and mating behavior by melanocortin receptor 4 polymorphisms. Curr Biol 20:1–6 [DOI] [PubMed] [Google Scholar]
- Lecaudey LA, Singh P, Sturmbauer C, Duenser A, Gessl W, Ahi EP (2021) Transcriptomics unravels molecular players shaping dorsal lip hypertrophy in the vacuum cleaner cichlid, Gnathochromis permaxillaris. BMC Genom 22:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecaudey LA, Sturmbauer C, Singh P, Ahi EP (2019) Molecular mechanisms underlying nuchal hump formation in dolphin cichlid, Cyrtocara Moorii. Scientific Rep 9:20296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF (2006) PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30:131–265 [DOI] [PubMed] [Google Scholar]
- Liotta MN, Abbott JK, Morris MR, Rios-Cardenas O (2021) Antagonistic selection on body size and sword length in a wild population of the swordtail fish, Xiphophorus multilineatus: potential for intralocus tactical conflict. Ecol Evol 11:3941–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manamendra-Arachchi K, de Silva A, Amarasinghe T (2006) Description of a second species of Cohpotis (Reptilia:Agamidae) from the highlands of Sri Lanka. Lyriocephalus 6(1):1–8 [Google Scholar]
- Moellering DR, Smith DL (2012) Ambient temperature and obesity. Curr Obes Rep 1:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerland TS, Sidell BD (1986) Biochemical responses to temperature in the contractile protein complex of striped bass Morone saxatilis. J Exp Zool 238:287–295 [DOI] [PubMed] [Google Scholar]
- Perdomo G, Kim DH, Zhang T, Qu S, Thomas EA, Toledo FGS, Slusher S, Fan Y, Kelley DE, Dong HH (2010) A role of apolipoprotein D in triglyceride metabolism. J Lipid Res 51:1298–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portz DE, Tyus HM (2004) Fish humps in two Colorado River fishes: a morphological response to cyprinid predation? Environ Biol Fishes 71:233–245
- Rauchenberger M, Kallman KD, Morizot DC (1990) Monophyly and geography of the Rio Panuco basin swordtails (genus Xiphophorus) with descriptions of four new species. American Museum Novitates, No. 2975
- Reid CH, Patrick PH, Rytwinski T, Taylor JJ, Willmore WG, Reesor B, Cooke SJ (2022) An updated review of cold shock and cold stress in fish. J Fish Biol 100:1102–1137 [DOI] [PubMed] [Google Scholar]
- Rios-Cardenas O, Darrah A, Morris MR (2010) Female mimicry indirectly enhances a male sexually selected trait; what does it take to fool a male? Behaviour 147(11):1443–1460 [Google Scholar]
- Rometsch SJ, Torres-Dowdall J, Machado-Schiaffino G, Karagic N, Meyer A (2021) Dual function and associated costs of a highly exaggerated trait in a cichlid fish. Ecol Evol 11:17496–17508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal GG, de la Rosa-Reyna XF, Kasianis S, Stephens MJ, Morizot DC, Ryan MJ (2003) Garcia de Leon FJ (2003) Dissolution of sexual signal complexes in a hybrid zone between the swordtails Xiphophorus birchmanni and Xiphophorus malinche (Poeciliidae). Copeia 2:299–307 [Google Scholar]
- Schartl M, Lu Y (2024) Validity of Xiphophorus fish as models for human disease. Dis Model Mech 17(1):050382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, van Marken WD, Lichtenbelt W (2016) Combatting type 2 diabetes by turning up the heat. Diabetologia 59(11):2269–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BB, Sidell BD (1981) Metabolic response of striped bass (Morone saxatilis) to temperature acclimation. J Exp Zool 218:371–379 [DOI] [PubMed]
- Susuki K, Masaki I, Yosuke K, Masahide K, Yasuaki T, Shinji A, Hideaki K (2014) Dorsal hump morphology in pink salmon (Oncorhynchus gorbuscha). J of Morphology 275:514–527 [DOI] [PubMed] [Google Scholar]
- Takahashi T (2018) Function of nuchal humps of a cichlid fish from Lake Tanganyika: inferences from morphological data. Ichthyol Res 65:316–323 [Google Scholar]
- Tompkins KB, Lott MS, Rios-Cardenas O, Jash S, Morris MR (2021) Metabolic growth hypothesis for the evolution of the nuchal hump in swordtail fishes. Environ Biol Fishes 104:1195–1206 [Google Scholar]
- Trentaglia MT, Parolini M, Donzelli F, Olper A (2021) Climate change and obesity: a global analysis. Glob Food Sec 29:100539 [Google Scholar]
- Ward DL, Ward MB (2020) What’s in the hump of the humpback chub? Western North Amer Nat 80:98–104 [Google Scholar]
- Webster CM, Pino EC, Carr CE, Wu L, Zhou B, Ceillo L, Kacergis MC, Curran SP, Soukas AA (2017) Genome-wide RNAi screen for fat regulatory genes in C. elegans identifies a proteostasis-AMPK axis critical for starvation survival. Cell Rep 20:627–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lu X, Lombès M, Rha GB, Chi Y, Guerin TM, Smart EJ, Liu J (2010) The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11:194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.