ABSTRACT
Objective
To examine whether aspirin delays gestational age at delivery (GAD) in pregnancies with placental dysfunction (PD) phenotypes (preeclampsia [PE], small‐for‐gestational‐age [SGA], placental abruption and/or stillbirth).
Design
A secondary analysis of a multicentre stepped‐wedge cluster randomised trial.
Setting
18 maternity/diagnostic units in Asia.
Population
Singleton pregnancies examined at 11–13+6 weeks.
Methods
A model in which the effect of aspirin is to delay the GAD in pregnancies with PD was developed.
Main Outcome Measures
GAD in pregnancies with PD.
Results
Aspirin administration was associated with a significant reduction in PD < 32 weeks (adjusted relative risk 0.543, 95% CI: 0.330–0.864), with a trend for an increase of PD ≥ 32 weeks (test for trend, p‐value = 0.0018). Similar findings were observed individually for PE, SGA and/or placental abruption. At 24 weeks, the aspirin‐induced prolongation of pregnancies with PD was 2.85 weeks (95% CI: 0.44–5.40), and this effect was decreased by −0.19 weeks (95% CI: −0.33 to −0.05) for each week of gestation; therefore, at 28 and 32 weeks' gestation, the aspirin‐induced prolongation was 2.09 and 1.33 weeks, respectively.
Conclusions
In this secondary analysis of a cluster randomised trial, women at high risk of PE who are destined to develop a clinical spectrum of PD may benefit from longer pregnancy duration through aspirin administration in early pregnancy. Aspirin may delay the GAD due to PD, particularly benefiting those deliveries that would occur at earlier gestations without aspirin administration.
Keywords: aspirin, placental dysfunction, pre‐eclampsia
1. Introduction
Impaired placentation can lead to placental dysfunction (PD), a major cause of various pregnancy complications, including preeclampsia (PE), small‐for‐gestational‐age (SGA), placental abruption and/or stillbirth [1, 2, 3]. These complications create a substantial burden on both maternal and perinatal outcomes [1, 2, 3, 4, 5, 6]. PE is a serious, pregnancy‐specific multisystem hypertensive disorder, affecting 2%–8% of pregnant women and one of the leading causes of maternal and perinatal morbidity and mortality [4, 7, 8]. If left untreated or in its severe early form, PE can lead to serious maternal complications, including impairment of hepatorenal and coagulation systems, pulmonary oedema, eclampsia, brain injury and even death [9, 10, 11]. SGA is another placental‐related condition [3], affecting approximately 16% of births globally, ranging from 7% in industrialised countries to 41.5% in South Asia [12]. SGA foetuses/infants face an increased risk of perinatal morbidity and mortality [5, 6, 13, 14], as well as long‐term health problems such as metabolic syndromes, cardiovascular diseases and adverse neurodevelopmental outcomes [15, 16, 17]. PD can also result in placental abruption, a severe obstetric emergency that may lead to iatrogenic preterm birth or stillbirth. Additionally, stillbirth can be attributed to PD and extensive research has demonstrated that a significant majority of preterm stillbirths are associated with PE and/or SGA [5, 6, 18, 19].
Interestingly, PE, SGA, placental abruption and/or stillbirth clinically overlap with one another, sharing common risk factors and biomarkers' profiles [3, 6, 10, 14, 18, 19, 20]. This suggests the notion that PD could be treated as a unified spectrum condition [2, 19, 21]. Moreover, various histopathological studies consistently indicate that these pregnancy complications are associated with impaired placentation [1, 2, 22, 23]. Low‐dose aspirin administered to high‐risk women for preterm‐PE before 16 weeks of gestation has been shown to reduce the rates of preterm‐PE and preterm‐SGA [20, 24, 25]. Wright and Nicolaides suggested a stochastic model that describes an aspirin‐related prolongation of pregnancy in women who eventually developed PE [26]. Data scarcity has hindered a better understanding of the potential role of aspirin in reducing the incidence of placental abruption or stillbirth [27, 28, 29]. Thus, the question remains whether aspirin has a delaying effect on delivery comparable to that previously reported for PE [26], as it may also be applicable to pregnancies that fall within the broader clinical spectrum of PD.
The aim of this secondary analysis of a cluster randomised clinical trial was to examine the hypothesis that aspirin delays the gestational age at delivery (GAD) in pregnancies affected by PD, which may manifest as PE, SGA, placental abruption, stillbirth or any combination of these conditions.
2. Material and Methods
2.1. Study Design and Participants
This study was a secondary analysis of the FORECAST trial, which was a multicentre stepped‐wedge cluster randomised trial [30]. The study protocol is provided in Appendix S1. We followed the Consolidated Standards of Reporting Trials reporting guideline [31]. Approval for the trial was obtained by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (CREC Ref. No. 2018.391) in Hong Kong and the Ethics Committee of each participating hospital in other regions. Inclusion criteria included a singleton pregnancy at 11–13+6 weeks, and age 18 years or older. Exclusion criteria included multiple pregnancies, major fetal defects identified at 11–13+6 weeks of assessment, non‐viable fetus or those under other clinical interventions. Trial recruitment took place in 18 maternity/diagnostic units from 10 regions in Asia between 1 August 2019 and 28 February 2022 [30]. All participants in the FORECAST trial provided written informed consent.
Screening centres were organised into clusters, each containing approximately an equal number of pregnant women. These clusters included maternity/diagnostic units from Hong Kong SAR, Mainland China, Taiwan, Singapore, Thailand, The Philippines, Indonesia, Malaysia, Vietnam and Japan. The formation of clusters was based on the homogeneity of the trial populations. The stepped‐wedge design started with a period where routine antenatal care was provided with no study‐related intervention at all recruiting centres (Figures S1 and S2). Subsequently, at regular 6‐weekly intervals, one cluster was randomised to transition from the non‐intervention phase to the intervention phase. The global pandemic of coronavirus disease 2019 (COVID‐19) significantly impacted recruitment rates. The trial design was adjusted on the assumption that the recruitment rate was halved. By extending the intervention phase by 60 weeks (equivalent to 20‐time steps), the aim was to maintain a trial power of around 65%, while keeping all other assumptions unchanged. The adjusted stepped‐wedge design is illustrated in Figure S3.
During the intervention phase, first‐trimester screening for preterm‐PE using the Bayes theorem‐based Fetal Medicine Foundation (FMF) triple test was performed [32]. For women with an adjusted risk for preterm‐PE ≥ 1 in 100, low‐dose aspirin treatment was initiated from < 16 weeks until 36 weeks, or until delivery or the onset of PE before 36 weeks [33].
2.2. Randomisation and Blinding
A staggered schedule was followed to implement the intervention over a number of time periods. The recruiting centres formed the units of cluster randomisation. An independent statistician conducted randomisation using anonymous cluster codes. All clusters were randomly assigned different starting times for the intervention phase based on computer‐generated random numbers. Each cluster's random start date for the intervention phase was concealed until 3 weeks before the commencement. No masking was used due to the nature of the intervention.
2.3. Intervention Phase of the FORECAST Trial
During each cluster's intervention period, women with an adjusted risk for PE ≥ 1 in 100 were invited to attend a follow‐up visit for further counselling regarding the high‐risk status and the benefits and side effects of aspirin prophylaxis. Those without known bleeding disorders, active peptic ulcer disease or hypersensitivity to aspirin were offered low‐dose aspirin before 16 weeks until 36 weeks, or until delivery or the onset of PE before 36 weeks. The regimen and compliance of aspirin were reported in a previous study [30]. Adherence was considered good, moderate and poor if the reported intake of tablets was ≥ 85%, between 50% and < 85%, and ≤ 50%, respectively [24].
2.4. Outcomes
The outcomes of the study were PE, SGA, placental abruption and stillbirth, which had been pre‐specified in the FORECAST trial. In this secondary analysis, PD‐related complication was defined as any of the following: PE, SGA, placental abruption, stillbirth or any combination of these conditions. SGA neonate was defined as birthweight < 10th percentile for gestational age, adjusted for maternal weight and height, gravida, parity, ethnicity and newborn sex [34]. The study sample included singleton pregnancies that delivered a phenotypically normal livebirth or stillbirth at ≥ 24 weeks' gestation. PE was defined according to the guidelines of the International Society for the Study of Hypertension in Pregnancy [35]. Placental abruption was defined either clinically or according to placental examination [36, 37].
2.5. Statistical Analysis
We designed a secondary analysis of data from the FORECAST; a cluster randomised trial, to investigate the impact of aspirin on the GAD with PD.
2.5.1. Exploratory Analysis
For baseline characteristics, continuous data were presented as mean and standard deviation or median and interquartile range. Categorical data were expressed as numbers and percentages. Residual analysis was carried out aiming to detect outliers ±3 standard deviations (SDs) and to subsequently review such cases to identify any possible lack of clinical plausibility, but no such cases were found.
2.5.2. Between‐Group Comparisons
We calculated the incidence with 95% confidence interval (CI) of PD‐related pregnancy complications, stratified by aspirin administration. The treatment effect of aspirin on the rate of PD‐related complications, stratified for gestational age at deliveries (< 32, 32–36+6 and ≥ 37 weeks), was estimated by computing the relative risks (RR) between aspirin/non‐aspirin groups with their 95% CI. RR (95% CI; aRR) was adjusted using a propensity score (PS) methodology with stabilised inverse probability of treatment weighting (IPTW) to balance the confounding effect of baseline characteristics between the aspirin and non‐aspirin groups [38, 39]. The following covariates were included in the PS model: maternal age, smoking at conception, method of conception, previous history of PE, chronic hypertension, type 1 diabetes, type 2 diabetes, systemic lupus erythematosus and antiphospholipid syndrome. Standardised mean differences (SMDs) were calculated before and after IPTW to assess the balance among the covariates. Balance between the two groups was considered optimal if the SMD for a given covariate was 10% or less. We adjusted for clustering in the data with random effect for cluster. The differences of aRRs among subgroups (deliveries at < 32, 32–36+6 and ≥ 37 weeks) were examined by using Z‐statistic as previously described [40].
2.5.3. Shift Model for the Effect of Aspirin on the Gestational Age at Delivery With Placental Dysfunction‐Related Complications
We designed a model based on the hypothesis that aspirin shifts the delivery of a pregnancy with a placental dysfunction‐related complication to a more advanced gestational age. Specifically, we assumed that D is a random variable with Gaussian distribution that describes the GAD in the no‐aspirin group with a mean that was allowed to depend on the logit transformed risk for preterm‐PE and a constant standard deviation. In the aspirin group, we made the same distributional assumptions, but D was modified by the effect of aspirin d. Therefore, the D in the non‐aspirin group was shifted to D + d under aspirin's effect, which was defined as d = a0 + a1*(D‐24) so that d is dependent on gestational age. The term a0 will provide the effect of aspirin at 24 weeks, according to this parameterisation.
We used Gaussian survival regression analysis for the outcome variable, which was GAD with PD. In this model, deliveries without PD were treated as censored observations. This modelling structure allowed us to derive the parameter's inferences by the PD cases weighing in for the aspirin effect and by assuming Gaussian distributions, we improved the goodness of fit and the simplicity of the clinical interpretation.
The model was fitted within the Bayesian framework using Markov chain Monte Carlo techniques which enabled all parameters to be estimated within a single analysis. We used a non‐informative prior distribution for the unknown parameters and inferences were obtained using a total of 100 000 iterations after a burn‐in period of 5000 iterations. Inferences for model parameters are summarised as posterior means and 95% credibility intervals. Convergence was assessed by autocorrelation plots, trace plots and density plots.
Data analyses were performed using R, version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria) [41].
3. Results
In a cohort of 48 647 women with singleton pregnancies offered the FMF triple test for preterm‐PE screening, 42 897 women (88.2%) opted to undergo screening. The study was divided into two phases: the non‐intervention phase that included 10 294 women and the intervention phase that comprised 27 965 women. In the non‐intervention phase, 1158 (11.25%) women were identified as high‐risk for developing preterm‐PE and were not administered aspirin. In the intervention phase, 3530 (12.62%) women were identified as high‐risk. Among these high‐risk women, 2909 were in the aspirin group and 621 did not take aspirin for various reasons and were included in the non‐aspirin group (Figure S4, Table S1). Consequently, 2909 pregnancies that constituted the aspirin group and 1779 pregnancies that constituted the non‐aspirin group were used for this secondary analysis.
Among these high‐risk women, 92.71% (2697/2909) demonstrated good adherence. Maternal and pregnancy characteristics of high‐risk women with PD‐related complications are given in Table S2. IPTW weighting resulted in no significant SMDs for the baseline characteristics between the aspirin and non‐aspirin groups, demonstrating an optimal balance between groups.
Aspirin administration was associated with a significant reduction of PD‐related complications < 32 weeks, with a significant trend for a simultaneous increase of PD‐related complications ≥ 32 weeks (test for trend, p value = 0.0018, Table 1, Figure S5). Similar findings were observed for PE, SGA and placental abruption (Table 1). In particular, the RRs for each gestational age window and the comparative differences in these RRs are presented in Table 1. The prevalence of each phenotype of PD‐related complications (PE, SGA, placental abruption and stillbirth) when they occur in combination with one another is shown in Table S3.
TABLE 1.
Relative risk (aspirin/non‐aspirin) and 95% confidence interval for the effect of aspirin on placental dysfunction related complications in women at high‐risk for preterm‐preeclampsia, stratified by gestational age at delivery.
| Aspirin | Non‐aspirin | Crude RR (95% CI) | Adjusted RR (95% CI) | |
|---|---|---|---|---|
| N = 2909 | N = 1779 | |||
| Placental dysfunction | ||||
| < 32 weeks | 34 (1.17) | 31 (1.74) | 0.671 (0.408–1.093) | 0.543 (0.33–0.864) a |
| 32–36+6 weeks | 179 (6.15) | 83 (4.67) | 1.319 (1.029–1.758) | 1.228 (0.95–1.625) c |
| ≥ 37 weeks | 540 (18.56) | 305 (17.14) | 1.083 (0.944–1.287) | 1.057 (0.91–1.248) b |
| PE | ||||
| < 32 weeks | 18 (0.62) | 18 (1.01) | 0.612 (0.314–1.181) | 0.496 (0.255–0.939) a |
| 32–36+6 weeks | 88 (3.03) | 56 (3.15) | 0.961 (0.685–1.355) | 0.868 (0.618–1.216) |
| ≥ 37 weeks | 173 (5.95) | 84 (4.72) | 1.260 (0.980–1.674) | 1.181 (0.918–1.558) b |
| SGA | ||||
| < 32 weeks | 33 (1.13) | 25 (1.41) | 0.807 (0.479–1.371) | 0.643 (0.386–1.060) |
| 32–36+6 weeks | 138 (4.74) | 58 (3.26) | 1.455 (1.087–2.034) | 1.368 (1.021–1.901) a , c |
| ≥ 37 weeks | 414 (14.23) | 231 (12.98) | 1.096 (0.936–1.323) | 1.085 (0.925–1.307) |
| Stillbirth | ||||
| < 32 weeks | 3 (0.10) | 1 (0.06) | 1.835 (0.235–37.123) | 1.763 (0.224–34.770) |
| 32–36+6 weeks | 4 (0.14) | 4 (0.22) | 0.612 (0.144–2.587) | 0.585 (0.137–2.446) |
| ≥ 37 weeks | 1 (0.03) | 0 (0.00) | — | — |
| Placental abruption | ||||
| < 32 weeks | 2 (0.07) | 3 (0.17) | 0.408 (0.054–2.460) | 0.214 (0.029–0.985) a |
| 32–36+6 weeks | 15 (0.52) | 1 (0.06) | 9.173 (1.866–166.713) | 9.410 (1.882–182.048) a , c |
| ≥ 37 weeks | 15 (0.52) | 4 (0.22) | 2.293 (0.833–8.079) | 2.473 (0.879–9.133) b |
Note: Values are given as n (%); % of the total non‐aspirin (N = 1779) or aspirin group (N = 2909). The following covariates were included in the propensity score methodology with stabilised inverse probability of treatment weighting: maternal age, smoking at conception, method of conception, previous history of PE, chronic hypertension, type 1 diabetes, type 2 diabetes, systemic lupus erythematosus and antiphospholipid syndrome.
Abbreviations: CI, confidence interval; N, the number of women in the aspirin or non‐aspirin group; PE, preeclampsia; RR, relative risk; SGA, small for gestational age.
Significant adjusted relative risk.
Z‐statistic [40]: significant difference for RR comparison: < 32 versus ≥ 37 weeks.
Z‐statistic [40]: significant difference for RR comparison: < 32 versus 32–37 weeks.
Z‐statistic [40]: significant difference for RR comparison: 32–37 versus ≥ 37 weeks.
3.1. Shift Model for the Effect of Aspirin on the Gestational Age at Delivery With Placental Dysfunction
The model's convergence was optimal. The higher the risk for PE, the greater the shift to earlier GAD with PD (Table 2, Figures 1 and 2). Aspirin had a significant effect in delaying delivery in pregnancies with PD, which was gestational age‐dependent. Specifically, the earlier the gestational age where delivery with PD would have occurred, the greater the aspirin effect. In particular, at 24 weeks, the aspirin‐induced prolongation of a pregnancy affected by PD was 2.85 weeks (95% credibility interval: 0.44–5.40) and the effect of aspirin in delaying the delivery was decreased by −0.19 weeks (95% credibility interval: −0.33 to −0.05) for each week of advancing gestation (Table 2, Figures 1 and 2).
TABLE 2.
Posterior means and 95% credibility interval for the parameters of the aspirin shift model for pregnancies with placental dysfunction.
| Term | Estimate (95% credibility interval) |
|---|---|
| PE | SGA | Placental abruption | Stillbirth | |
| Intercept | 35.79 (34.90 to 36.58) |
| Logit risk for preterm‐PE | −1.71 (−1.97 to −1.48) |
| Aspirin | 2.85 (0.44 to 5.40) |
| AspirinX (gestational age‐24) | −0.19 (−0.33 to −0.05) |
| Standard deviation | 3.39 (3.22 to 3.57) |
FIGURE 1.
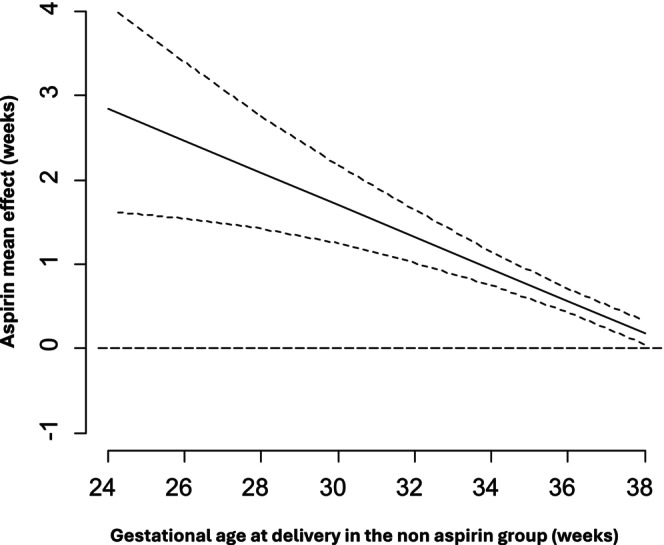
Shift model for the estimated aspirin effect in delaying gestational age at delivery of pregnancies with placental dysfunction‐related complications (black line) with 95% credibility intervals.
FIGURE 2.

Gestational age dependent effect of aspirin in delaying delivery of pregnancies with placental dysfunction‐related complications (PD). The arrows indicate the magnitude of the aspirin‐induced delay in the delivery of pregnancies with PD.
4. Discussion
4.1. Main Findings
This study has demonstrated that aspirin is associated with a 45% reduction in PD‐related complications < 32 weeks of gestation, when administered in women at high risk of PE following first‐trimester screening (Table 1, Figure S5). Interestingly, there is a simultaneous significant trend showing an increasing incidence of PD‐related complications ≥ 32 weeks of gestation (Table 1, Figure S5). These exploratory findings show a progressively lower relative reduction of PD for higher GAD in the aspirin group, supporting the hypothesis that aspirin delays the delivery of pregnancies affected by PD.
We have subsequently developed a survival model that describes the shift effect of aspirin for pregnancies with PD. The aspirin‐related delay in GAD for pregnancies affected by PE, SGA, placental abruption and/or stillbirth is related to the severity of PD as reflected in the GAD. The earlier the delivery would have occurred without aspirin administration, the greater the delaying effect of aspirin (Table 2, Figures 1 and 2).
4.2. Strengths and Limitations
This study has important strengths as it is the first study that has examined the role of aspirin in determining the GAD in pregnancies affected by PD. The large sample size resulted from the first multicentre stepped‐wedge randomised trial that evaluated both the effectiveness and clinical feasibility of a universal screen‐and‐prevent strategy for preterm‐PE [30]. Training was carried out according to standardised protocols and quality assurance measures with regular independent monitoring implemented (Appendix S1). The aspirin shift model effectively captures the effect of aspirin through the application of censoring, and the Gaussian assumption enhances the clinical relevance and validity of the model's inferences.
We applied IPTW knowing the outcomes of the study; however, there was no need for any model selection process; therefore, there was no risk for selection bias. Although this is a secondary analysis of the FORECAST trial, PE was the primary outcome and SGA, placental abruption and stillbirth were the pre‐specified secondary outcomes. A realistic limitation is that this cluster randomised clinical trial was conducted within an Asian population; therefore, the findings may not be generalisable to other populations.
4.3. Interpretation (In Light of Other Evidence)
In this secondary analysis, we have developed a model that supports the hypothesis of an aspirin‐related shift effect, suggesting that the delay in GAD associated with different phenotypes of PD, including PE, SGA, placental abruption and/or stillbirth, is more pronounced at earlier gestational ages than at later ones (Table 2, Figures 1 and 2). Therefore, we expand the previously observed shift effect of aspirin for PE [26], incorporating other phenotypes of PD such as SGA, placental abruption and stillbirth (Table 2, Figures 1 and 2).
Our primary analysis of the cluster randomised trial has demonstrated that aspirin prophylaxis effectively reduces the incidence of early‐onset PE and preterm‐PE by 54% (adjusted odd ratio [aOR] 0.59, 95% CI: 0.37–0.92) and 41% (aOR 0.46; 95% CI: 0.23–0.93) among high‐risk women, respectively, while there is no significant effect on the incidence of term‐PE [42]. This secondary analysis provides additional evidence to support the concept that aspirin reduces the rate of preterm‐PE by delaying the onset or progression of the condition [24, 26].
A meta‐analysis involving 20 randomised controlled trials involving a total of 12 585 participants reported that aspirin at a daily dose of ≥ 100 mg, administered at ≤ 16 weeks of gestation, for the prevention of PE, may reduce the risk of placental abruption [28]. However, the analysis did not evaluate how aspirin affects the timing of delivery in cases of placental abruption. Our study shows that the shift effect of aspirin is evident for different phenotypes of PD and timing of delivery with placental abruption following the same trends (Table 1, Figures 1 and 2). Interestingly, aspirin appears to delay placental abruption, reducing its incidence by 79% < 32 weeks' gestation, while concurrently increasing the incidence by 9.41 times at 32–36+6 weeks' gestation (Table 1).
Previously, Wright and Nicolaides reported an exploratory analysis based on the ASPRE trial in support of the aspirin‐related delay hypothesis [26]. By delaying the onset of PE, more cases of term‐PE arise from pregnancies that were originally expected to develop preterm‐PE, resulting in a significant reduction in preterm‐PE without affecting the incidence of term‐PE. They reported that the aspirin‐related delay in delivery for pregnancies destined to develop PE at 24 weeks of gestation was 4.4 weeks (95% CI: 1.4–7.1 weeks) and this effect decreased by an estimated 0.23 weeks (95% CI: 0.021–0.40 weeks) for each week of gestation [26]. We expanded on this concept by incorporating additional phenotypes of PD, developing a unified model with direct clinical applicability.
A study that utilised untargeted metabolomic analysis of plasma samples from aspirin/placebo‐treated high‐risk pregnant women in the ASPRE trial found that aspirin significantly decreased metabolic gestational age by 1.27 weeks (95% CI: 0.66 to1.88 weeks), and partially reversed one‐fourth of the metabolites that changed with advancing gestational age, lending further support to the aspirin‐related delay hypothesis [42]. The finding that aspirin prolongs pregnancies affected by PD is also consistent with a secondary analysis of the ASPRE trial, which demonstrated that aspirin reduced the rates of pregnancies with SGA and/or PE delivered < 32 weeks and < 37 weeks [20]. However, it had no significant effect on pregnancies with SGA and/or PE delivered ≥ 37 weeks [20]. We fitted a model including SGA to reflect the hypothesis of an aspirin‐related delaying effect, as PD has been commonly associated with abnormal fetal growth.
We propose a universal integrated risk assessment and targeted aspirin prophylaxis for women at high risk of preterm‐PE. This policy is safe and it can be implemented directly, provided that a first‐trimester assessment is already in place [24, 32, 43, 44]. Adding PlGF in the model comes with a marginal cost as it would be just an extension to the already existing biochemical testing infrastructure [24, 32, 43, 44]. Our analysis shows that women with PD could significantly benefit from pregnancy prolongation, especially those with more severe placental insufficiency. This effect could be clinically translated to improved perinatal outcomes and health economics, considering the exponential increase in the rate of adverse outcomes and associated costs with preterm birth [4, 6, 45, 46, 47]. Aspirin appears to delay the onset of a severe, unpredictable consequence of PD, namely placental abruption, which is challenging to diagnose clinically due to its nonspecific symptoms. This effect may positively impact neonatal outcomes by reducing the risk of adverse neonatal outcomes associated with hypoxia and prematurity‐related complications, such as asphyxia, respiratory distress syndrome and apnoea.
This aspirin shift model considers that the women at risk for PD‐related complications could benefit from a personalised pathway of care [21]. An important prerequisite for this notion is the integration of first‐trimester algorithms for placental disease into current clinical recommendations [3, 48, 49], using appropriate risk factors in alignment with the evidence [50]. This methodology will utilise the beneficial effect of aspirin that appears to be mediated through a birth delaying effect. We gain more time that will possibly improve the perinatal outcome, mainly by reducing the prematurity burden. However, a new clinical necessity arises as effective stratification of the obstetric population, increased surveillance, and maternal awareness are becoming important elements on the clinical front. In particular, timely extra ultrasound scans, blood pressure monitoring and control and maternal education to increase awareness for symptoms such as vaginal bleeding or reduced fetal movements, in the high‐risk group, will probably enhance aspirin's positive effect. There is an urgent need to formalise this new clinical mindset in the upcoming guidelines advocating universal models, which are the basis for applying preventive strategies and managing pregnancy‐specific needs [21].
5. Conclusion
In this secondary explanatory analysis of a cluster randomised clinical trial, we present evidence suggesting that women at higher risk of PE who are expected to develop a spectrum of PD‐related conditions may benefit from a longer pregnancy duration as a result of the aspirin administration starting as early as 11 weeks of gestation. This is achieved through a delivery delaying effect, which is stronger for pregnancies with a higher degree of PD that require delivery at an earlier gestational age.
Author Contributions
L.C.P. conceptualised the trial. L.C.P. and M.K.C.C. contributed to the trial design. L.C.P. supervised trial implementation. L.C.P., L.N.‐H., D.S.S. supervised data collection. M.K.C.C. was the lead statistician and led the development of the statistical analysis plan. L.C.P., I.P., Y.C., M.K.C.C., L.N.‐H. directly accessed and verified the underlying data reported in the manuscript and analysed the data. L.C.P., I.P. and Y.C. led the interpretation of the data. I.P. and Y.C. drafted the tables and figures. I.P., Y.C., L.C.P. wrote the first draft of the manuscript. I.P., Y.C., L.N.‐H., D.‐A.N., L.T.D., R.K.P., A.S., M.Z., Y.H., Y.W., A.K., P.Y., M.A.C., M.K., S.L., T.‐Y.C., N.C., T.N., Y.J., S.W.S., W.C.L., A.S.M., A.A., S.L.L., N.M.W.L., E.W.C.T., D.S.S., M.K.C.C., and L.C.P. reviewed the manuscript and approved the final draft. All authors had full access to all the data in the trial and had final responsibility for the decision to submit for publication.
Conflicts of Interest
L.C. Poon has received speaker fees and consultancy payments from Roche Diagnostics, Ferring Pharmaceuticals, Shenzhen Mindray Bio‐Medical Electronics Co. Ltd. and Samsung Healthcare. In addition, she serves as the CEO of PregnaSense Co. Limited and has received in‐kind contributions from Roche Diagnostics, Revvity Inc. (formerly PerkinElmer Life and Analytical Sciences), ThermoFisher Scientific, Ningbo Aucheer Biological Technology Co. Ltd., Samsung Healthcare and GE Healthcare. D.S. Sahota has received in‐kind contributions from Revvity Inc. (formerly PerkinElmer Life and Analytical Sciences), Thermo Fisher Scientific, Roche Diagnostics, Diabetomics and Ningbo Aucheer Biological Technology Co. Ltd. R.K. Pooh is the CEO of Ritz Medical Co. Ltd., a genetic testing company, holds shares in it, and receives executive compensation from it. Other authors declare no conflicts of interest.
Supporting information
Data S1.
Appendix S1.
Acknowledgements
We thank all the participants and their attending obstetricians, nurses, midwives, research assistants sonographers and the following medical professionals who helped in the recruitment and follow‐up of participants. We extend our sincere gratitude to Iok Seng Wong, Angela S.T. Tai, Xueqin Wang, Sakita Moungmaithong, Xiaohong Lu, Jing Lin, Ada W.T. Tse, Lo Wong, Fangzi Liu (Department of Obstetrics and Gynaecology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China); Bin Li (Kunming Angel Women and Children's Hospital, the Teaching Hospital of Kunming University of Science and Technology, Kunming, China); Huishu Liu (Department of Obstetrics, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China); Arundhati Gosavi (Yong Loo Lin School of Medicine, National University of Singapore, Singapore); Piengbulan Yapan (Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand); Akihide Ohkuchi (Jichi Medical University, Shimotsuke, Japan), Osamu Shimokawa (Clinical Laboratory, Ritz medical Co. Ltd., Osaka, Japan).
Funding: A start‐up grant from the Chinese University of Hong Kong.
Contributor Information
Ioannis Papastefanou, Email: jdpap2@yahoo.co.uk, Email: ioannis.papastefanou@kcl.ac.uk.
Liona C. Poon, Email: liona.poon@cuhk.edu.hk.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Brosens I., Pijnenborg R., Vercruysse L., and Romero R., “The ‘Great Obstetrical Syndromes’ Are Associated With Disorders of Deep Placentation,” American Journal of Obstetrics and Gynecology 204, no. 3 (2011): 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ananth C. V., “Ischemic Placental Disease: A Unifying Concept for Preeclampsia, Intrauterine Growth Restriction, and Placental Abruption,” Seminars in Perinatology 38, no. 3 (2014): 131–132. [DOI] [PubMed] [Google Scholar]
- 3. Morris R. K., Johnstone E., Lees C., Morton V., Smith G., and Royal College of Obstetricians and Gynaecologists , “Investigation and Care of a Small‐For‐Gestational‐Age Fetus and a Growth Restricted Fetus (Green‐Top Guideline No. 31),” BJOG 131, no. 9 (2024): e31–e80. [DOI] [PubMed] [Google Scholar]
- 4. von Dadelszen P., Syngelaki A., Akolekar R., Magee L. A., and Nicolaides K. H., “Preterm and Term Pre‐Eclampsia: Relative Burdens of Maternal and Perinatal Complications,” BJOG: An International Journal of Obstetrics and Gynaecology 130, no. 5 (2023): 524–530, 10.1111/1471-0528.17370. [DOI] [PubMed] [Google Scholar]
- 5. Papastefanou I., Ashoor G., Syngelaki A., Akolekar R., and Nicolaides K. H., “Relation of Antepartum Stillbirth to Birthweight and Gestational Age: Prospective Cohort Study,” BJOG: An International Journal of Obstetrics and Gynaecology 131, no. 2 (2024): 200–206. [DOI] [PubMed] [Google Scholar]
- 6. Papastefanou I., Gyokova E., Gungil B., Syngelaki A., and Nicolaides K. H., “Prediction of Adverse Perinatal Outcome at Midgestation,” Ultrasound in Obstetrics & Gynecology 62, no. 2 (2023): 195–201. [DOI] [PubMed] [Google Scholar]
- 7. Duley L., “The Global Impact of Pre‐Eclampsia and Eclampsia,” Seminars in Perinatology 33, no. 3 (2009): 130–137. [DOI] [PubMed] [Google Scholar]
- 8. Mol B. W. J., Roberts C. T., Thangaratinam S., Magee L. A., de Groot C. J. M., and Hofmeyr G. J., “Pre‐Eclampsia,” Lancet 387, no. 10022 (2016): 999–1011. [DOI] [PubMed] [Google Scholar]
- 9. Roberts J. M., Rich‐Edwards J. W., McElrath T. F., Garmire L., and Myatt L., “Subtypes of Preeclampsia: Recognition and Determining Clinical Usefulness,” Hypertension 77, no. 5 (2021): 1430–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Dadelszen P., Payne B., Li J., et al., “Prediction of Adverse Maternal Outcomes in Pre‐Eclampsia: Development and Validation of the fullPIERS Model,” Lancet 377, no. 9761 (2011): 219–227. [DOI] [PubMed] [Google Scholar]
- 11. Ogge G., Chaiworapongsa T., Romero R., et al., “Placental Lesions Associated With Maternal Underperfusion Are More Frequent in Early‐Onset Than in Late‐Onset Preeclampsia,” Journal of Perinatal Medicine 39, no. 6 (2011): 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee A. C., Katz J., Blencowe H., et al., “National and Regional Estimates of Term and Preterm Babies Born Small for Gestational Age in 138 Low‐Income and Middle‐Income Countries in 2010,” Lancet Global Health 1, no. 1 (2013): e26–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz J., Lee A. C., Kozuki N., et al., “Mortality Risk in Preterm and Small‐For‐Gestational‐Age Infants in Low‐Income and Middle‐Income Countries: A Pooled Country Analysis,” Lancet 382, no. 9890 (2013): 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papastefanou I., Menenez M., Szczepkowska A., Gungil B., Syngelaki A., and Nicolaides K. H., “Comparison of Competing‐Risks Model With Angiogenic Factors in Midgestation Screening for Preterm Growth‐Related Neonatal Morbidity,” Ultrasound in Obstetrics & Gynecology 63, no. 5 (2024): 613–618. [DOI] [PubMed] [Google Scholar]
- 15. Karagianni P., Kyriakidou M., Mitsiakos G., et al., “Neurological Outcome in Preterm Small for Gestational Age Infants Compared to Appropriate for Gestational Age Preterm at the Age of 18 Months: A Prospective Study,” Journal of Child Neurology 25, no. 2 (2010): 165–170. [DOI] [PubMed] [Google Scholar]
- 16. Hong Y. H. and Chung S., “Small for Gestational Age and Obesity Related Comorbidities,” Annals of Pediatric Endocrinology & Metabolism 23, no. 1 (2018): 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arcangeli T., Thilaganathan B., Hooper R., Khan K. S., and Bhide A., “Neurodevelopmental Delay in Small Babies at Term: A Systematic Review,” Ultrasound in Obstetrics & Gynecology 40, no. 3 (2012): 267–275. [DOI] [PubMed] [Google Scholar]
- 18. Ashoor G., Syngelaki A., Papastefanou I., Nicolaides K. H., and Akolekar R., “Development and Validation of Model for Prediction of Placental Dysfunction‐Related Stillbirth From Maternal Factors, Fetal Weight and Uterine Artery Doppler at Mid‐Gestation,” Ultrasound in Obstetrics & Gynecology 59, no. 1 (2022): 61–68. [DOI] [PubMed] [Google Scholar]
- 19. Nicolaides K. H., Papastefanou I., Syngelaki A., Ashoor G., and Akolekar R., “Predictive Performance for Placental Dysfunction Related Stillbirth of the Competing Risks Model for Small‐For‐Gestational‐Age Fetuses,” BJOG: An International Journal of Obstetrics and Gynaecology 129, no. 9 (2022): 1530–1537. [DOI] [PubMed] [Google Scholar]
- 20. Tan M. Y., Poon L. C., Rolnik D. L., et al., “Prediction and Prevention of Small‐For‐Gestational‐Age Neonates: Evidence From SPREE and ASPRE,” Ultrasound in Obstetrics & Gynecology 52, no. 1 (2018): 52–59. [DOI] [PubMed] [Google Scholar]
- 21. Papastefanou I., Wright D., Syngelaki A., Akolekar R., and Nicolaides K. H., “Personalized Stratification of Pregnancy Care for Small for Gestational Age Neonates From Biophysical Markers at Mid‐Gestation,” American Journal of Obstetrics and Gynecology 229 (2022): 57.e1. [DOI] [PubMed] [Google Scholar]
- 22. Brosens I. A., Robertson W. B., and Dixon H. G., “The Role of the Spiral Arteries in the Pathogenesis of Preeclampsia,” Obstetrics and Gynecology Annual 1 (1972): 177–191. [PubMed] [Google Scholar]
- 23. Maiti K., Sultana Z., Aitken R. J., et al., “Evidence That Fetal Death Is Associated With Placental Aging,” American Journal of Obstetrics and Gynecology 217, no. 4 (2017): 441.e1–441.e14. [DOI] [PubMed] [Google Scholar]
- 24. Rolnik D. L., Wright D., Poon L. C., et al., “Aspirin Versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia,” New England Journal of Medicine 377, no. 7 (2017): 613–622. [DOI] [PubMed] [Google Scholar]
- 25. Roberge S., Bujold E., and Nicolaides K. H., “Aspirin for the Prevention of Preterm and Term Preeclampsia: Systematic Review and Metaanalysis,” American Journal of Obstetrics and Gynecology 218, no. 3 (2018): 287–293.e1. [DOI] [PubMed] [Google Scholar]
- 26. Wright D. and Nicolaides K. H., “Aspirin Delays the Development of Preeclampsia,” American Journal of Obstetrics and Gynecology 220, no. 6 (2019): 580.e1–580.e6. [DOI] [PubMed] [Google Scholar]
- 27. Henderson J. T., Vesco K. K., Senger C. A., Thomas R. G., and Redmond N., “Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force,” Journal of the American Medical Association 326, no. 12 (2021): 1192–1206. [DOI] [PubMed] [Google Scholar]
- 28. Roberge S., Bujold E., and Nicolaides K. H., “Meta‐Analysis on the Effect of Aspirin Use for Prevention of Preeclampsia on Placental Abruption and Antepartum Hemorrhage,” American Journal of Obstetrics and Gynecology 218, no. 5 (2018): 483–489. [DOI] [PubMed] [Google Scholar]
- 29. Bujold E., Roberge S., and Nicolaides K. H., “Low‐Dose Aspirin for Prevention of Adverse Outcomes Related to Abnormal Placentation,” Prenatal Diagnosis 34, no. 7 (2014): 642–648. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen‐Hoang L., Dinh L. T., Tai A. S. T., et al., “Implementation of First‐Trimester Screening and Prevention of Preeclampsia: A Stepped Wedge Cluster‐Randomized Trial in Asia,” Circulation 150, no. 16 (2024): 1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campbell M. K., Elbourne D. R., and Altman D. G., “CONSORT Statement: Extension to Cluster Randomised Trials,” British Medical Journal 328, no. 7441 (2004): 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaemsaithong P., Pooh R. K., Zheng M., et al., “Prospective Evaluation of Screening Performance of First‐Trimester Prediction Models for Preterm Preeclampsia in an Asian Population,” American Journal of Obstetrics and Gynecology 221, no. 6 (2019): 650.e1–650.e16. [DOI] [PubMed] [Google Scholar]
- 33. Poon L. C., Shennan A., Hyett J. A., et al., “The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Pre‐Eclampsia: A Pragmatic Guide for First‐Trimester Screening and Prevention,” International Journal of Gynaecology and Obstetrics 145, no. Suppl 1 (2019): 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sahota D. S., Kagan K. O., Lau T. K., Leung T. Y., and Nicolaides K. H., “Customized Birth Weight: Coefficients and Validation of Models in a UK Population,” Ultrasound in Obstetrics & Gynecology 32, no. 7 (2008): 884–889. [DOI] [PubMed] [Google Scholar]
- 35. Magee L. A., Brown M. A., Hall D. R., et al., “The 2021 International Society for the Study of Hypertension in Pregnancy Classification, Diagnosis & Management Recommendations for International Practice,” Pregnancy Hypertension 27 (2022): 148–169. [DOI] [PubMed] [Google Scholar]
- 36. Downes K. L., Grantz K. L., and Shenassa E. D., “Maternal, Labor, Delivery, and Perinatal Outcomes Associated With Placental Abruption: A Systematic Review,” American Journal of Perinatology 34, no. 10 (2017): 935–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oyelese Y. and Ananth C. V., “Placental Abruption,” Obstetrics and Gynecology 108, no. 4 (2006): 1005–1016. [DOI] [PubMed] [Google Scholar]
- 38. Morris T. P., Walker A. S., Williamson E. J., and White I. R., “Planning a Method for Covariate Adjustment in Individually Randomised Trials: A Practical Guide,” Trials 23, no. 1 (2022): 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holmberg M. J. and Andersen L. W., “Adjustment for Baseline Characteristics in Randomized Clinical Trials,” JAMA 328, no. 21 (2022): 2155–2156. [DOI] [PubMed] [Google Scholar]
- 40. Altman D. G. and Bland J. M., “Interaction Revisited: The Difference Between Two Estimates,” BMJ 326, no. 7382 (2003): 219, 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. R Development Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013), https://www.r‐project.org. [Google Scholar]
- 42. Li X., Milosavljevic A., Elsea S. H., et al., “Effective Aspirin Treatment of Women at Risk for Preeclampsia Delays the Metabolic Clock of Gestation,” Hypertension 78, no. 5 (2021): 1398–1410. [DOI] [PubMed] [Google Scholar]
- 43. Nicolaides K. H., Syngelaki A., Poon L. C., et al., “First‐Trimester Prediction of Preterm Pre‐Eclampsia and Prophylaxis by Aspirin: Effect on Spontaneous and Iatrogenic Preterm Birth,” BJOG: An International Journal of Obstetrics and Gynaecology 131, no. 4 (2024): 483–492. [DOI] [PubMed] [Google Scholar]
- 44. Nzelu D., Palmer T., Stott D., et al., “First Trimester Screening for Pre‐Eclampsia and Targeted Aspirin Prophylaxis: A Cost‐Effectiveness Cohort Study,” BJOG: An International Journal of Obstetrics and Gynaecology 131, no. 2 (2024): 222–230. [DOI] [PubMed] [Google Scholar]
- 45. Larroque B., Ancel P. Y., Marret S., et al., “Neurodevelopmental Disabilities and Special Care of 5‐Year‐Old Children Born Before 33 Weeks of Gestation (The EPIPAGE Study): A Longitudinal Cohort Study,” Lancet 371, no. 9615 (2008): 813–820. [DOI] [PubMed] [Google Scholar]
- 46. Moster D., Lie R. T., and Markestad T., “Long‐Term Medical and Social Consequences of Preterm Birth,” New England Journal of Medicine 359, no. 3 (2008): 262–273. [DOI] [PubMed] [Google Scholar]
- 47. Pierrat V., Marchand‐Martin L., Arnaud C., et al., “Neurodevelopmental Outcome at 2 Years for Preterm Children Born at 22 to 34 Weeks' Gestation in France in 2011: EPIPAGE‐2 Cohort Study,” BMJ 358 (2017): j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Papastefanou I. and Nicolaides K. H., “A Comment on Green Top Guideline No. 31: Investigating and Care in the Small‐For‐Gestational‐Age and Growth Restricted Foetus,” BJOG: An International Journal of Obstetrics and Gynaecology (2024), 10.1111/1471-0528.17972 (Online Ahead of Print). [DOI] [PubMed] [Google Scholar]
- 49. Atkins B. and Siassakos D., “Optimising Aspirin Use for Pre‐Eclampsia Prevention: The Critical Role of Dose, Timing and Adherence,” BJOG 132, no. 5 (2025): 547–551. [DOI] [PubMed] [Google Scholar]
- 50. Elawad T., Scott G., Bone J. N., et al., “Risk Factors for Pre‐Eclampsia in Clinical Practice Guidelines: Comparison With the Evidence,” BJOG 131, no. 1 (2024): 46–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


