Abstract
Francisella tularensis is the causative agent of the zoonotic disease tularemia. We investigated a pathogenic factor of F. tularensis subsp. novicida (F. novicida). Accordingly, we established a novel infection model using HeLa cells. F. novicida usually infects macrophage lineage cells and less frequently epithelial cells. We successfully infected HeLa cells expressing the Fc receptor (HeLa–FcγRII cells) using F. novicida supplemented with mouse serum containing F. novicida antibodies. A total of 2,232 transposon mutants of F. novicida were screened to determine the relatively fewer cytotoxic strains of the HeLa–FcγRII cells, and 13 strains were thus isolated. Sequencing analysis of transposon insertion sites identified 13 genes, including FTN_0096. We focused on FTN_0096. Although the F. novicida wild-type strain proliferated in HeLa–FcγRII and THP-1 cells, the number of intracellular FTN_0096 mutant decreased. FTN_0096 mutant cannot escape from phagolysosomes in the initial phases of infection. Moreover, FTN_0096 mutant was detected in the mitochondria and Golgi complex. These findings indicate the importance of FTN_0096 of F. novicida for intracellular replication in the cells.
Introduction
Francisella tularensis is a gram-negative coccobacillus that infects animals and humans through contact with infected arthropods or animals, consumption of contaminated food or water, or inhalation of bacterial aerosols [1,2]. Taxonomy of F. tularensis consists of some sub-species, including tularensis (type A), holarctica (type B), mediaasiatica, and novicida. Cottontail rabbits (Sylvilagus spp.) and ticks are the primary reservoirs of type A [3]. F. tularensis is a highly pathogenic facultative intracellular bacteria that can cause tularemia—a zoonosis disease [4]. F. tularensis is a dangerous pathogen that can incur a high risk of morbidity and mortality. F. tularensis causes typhoidal and pneumonic systemic tularemia, which has a fatality rate of up to 60% [5]. F. tularensis has been classified as a Tier 1 select agent by the Centers for Disease Control and Prevention (CDC) due to its extremely low infectious dose, potential for broad dissemination, and documented history of use as a biological weapon [6].
F. tularensis subsp. novicida is similar to F. tularensis but is a less virulent subspecies, as it can cause severe illness in mice but does not, usually, have much impact on the immunocompetence of humans. F. novicida is characterized as an uncommon opportunistic human infecting agent, capable of inducing morbidity and fatality in case of severe pathologies in either immunocompromised or medically weakened patients. F. novicida, which is regarded as a more environmentally adapted species, also shares several similarities to more virulent subspecies, including the LVS and Schu S4, a human virulent type A strain. F. novicida has a similar intracellular replication cycle within macrophages, characterized by swift phagosome escape and vigorous cytosolic replication, making it a commonly employed mouse model for studying the pathogenic mechanisms of tularemia [7,8].
For F. tularensis to effectively develop an infection and cause disease, it needs to replicate within the host cells. The intracellular lifecycle of F. tularensis has been observed in vitro with fixed and live-cell microscopy. Phagosome escape is necessary for intracellular replication, after which the organism must adapt to and replicate inside the host cytoplasm. When F. tularensis is internalized by a host cell, it enters macrophages via the phagocytosis process and evades lysosomal fusion, then the bacterium degrades the phagosome within 30 min and proliferates in the cytosol [9–11]. Phagosome escape is a crucial phase in F. tularensis lifecycle, and mutations in the Francisella pathogenicity island (FPI), which is responsible for encoding an alternate secretion pathway, lead to failed phagosome escape [12]. A characteristic of F. tularensis pathogenicity is its ability to remain immunologically silent during infection in both types of hosts (i.e., mice and people) and at the cellular level [13]. F. tularensis virulence factors, intracellular lifestyle, and interaction with different cell organelles are poorly known [14].
F. novicida infections rarely involve them due to their significantly lower infection efficiency in epithelial cell compared to macrophage-like cells. Research involving epithelial cell lines is markedly limited when compared to that of macrophage cell lines [15]. The efficiency of F. novicida uptake by macrophages is strongly influenced by opsonization. Opsonized bacteria, whether by serum or antibodies, are internalized by macrophages at levels that are 10-fold greater than their unopsonized counterparts [16]. Macrophages derived from the bone marrow of mice lacking Fc-gamma receptors (FcγR) show a 90% decrease in their ability to take up immunoglobulin G (IgG)-opsonized bacteria. Consequently, FcγR-mediated uptake could be a promising strategy for facilitating F. novicida infection in epithelial cells. Based on a previous research, HeLa–FcγRII cells were used as a new model to study how F. novicida infects epithelial cells [15].
The FTN_0096 protein product is a conserved hypothetical membrane protein. FTN_0096 is a member of the DUF1275 superfamily of proteins [17]. Members of this family are found across all three domains of life, making them highly widespread. DUF1275 belongs to a large family characterized by a consistent structure of six—and occasionally seven—transmembrane segments (TMSs), which aligns with what’s typically seen in half-sized proteins of the Major Facilitator Superfamily (MFS). Some of these proteins are encoded by genes located near what appears to be a YtcJ-like metallo–amido–hydrolase, suggesting a possible role in transporting peptides or other amido compounds, or exporting their breakdown products. However, none of these proteins have been functionally studied in detail, and their transport mechanisms remain unclassified [18].
In this study, we generated a transposon mutant library of F. novicida and screened it by infecting the mutant to the epithelial cells, HeLa cells expressing the Fc-gamma receptor (HeLa–FcγRII). This cell has the potential to enhance the efficiency of cell-based infection assays, such as large-scale genetic screening, and to offer novel insights into Francisella infection in epithelial cells, which has been difficult to analyze in phagocytic cells [15]. We focused on FTN_0096 and discovered that the FTN_0096 gene is a pathogenic factor in F. novicida.
Materials and methods
Bacterial strains and culture conditions
F. novicida U112 was sourced from the Pathogenic Microorganism Genetic Resource Stock Center (Gifu University). The bacterium was cultured aerobically at 37°C using either a chemically defined medium (CDM) [19] or a brain–heart infusion broth (Becton, Dickinson and Company, Franklin Lakes, NJ) enriched with cysteine (BHIc) [20] which included 1.5% agar (Wako Laboratory Chemicals, Osaka, Japan).
Cell culture
HeLa–FcγRII cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) enriched with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, MA, USA) and incubated at 37°C in a humidified atmosphere with 5% CO₂. THP-1 cells, a human monocytic cell line, were cultured in RPMI 1640 medium (Sigma-Aldrich) enriched 10% heat-inactivated FBS under the same temperature and CO₂ conditions.
Construction of a transposon mutant library
The transposon mutant library was developed using the Ez-Tn5 transposon system (Epicentre, Madison, WI). To construct pMOD3-FtKm, the multiple cloning sites of pMOD3 were digested with HindIII and EcoRI, and the kanamycin-resistance cassette of pKEK1140 [21] was inserted into these sites to generate pMOD3-FtKm. The transposon region of pMOD3-FtKm was then amplified by PCR, purified, and mixed with transposase following the protocol. This mixture was used to transform F. novicida via cryotransformation. The resulting transformants were plated on BHIc agar supplemented with 50 μg/mL of kanamycin.
Screening of transposon mutant library
Microscopic observations were used to screen a mutant library of Francisella novicida to identify mutants with less cytotoxicity toward HeLa-FcγRII cells. To validate the cytotoxicity, lactate dehydrogenase (LDH) release assays were performed. HeLa–FcγRII cells (5 × 104 cells/well) were seeded into 48-well tissue culture plate for 24 h. F. novicida strains preincubated with mouse serum containing specific antibodies were used at a multiplicity of infection (MOI) of 1.0. The cultured plates were centrifuged at 300 × g for 10 minutes at room temperature, followed by incubation at 37°C for a designated period. After incubation, the cells were washed thrice with DMEM medium, and extracellular bacteria were eliminated by treating the cultures with gentamicin (50 μg/mL) for 1 hour. To measure LDH release as a marker of cytotoxicity, cells were incubated in the DMEM medium at 37°C for the specified time. The release of LDH into the culture supernatant was quantified using the LDH Cytotoxicity Detection Kit (Takara Bio, Shiga, Japan).
Sequence analysis of transposon mutants
The plasmid pMOD3 contains the R6Kγ origin of replication from Escherichia coli. Genomic DNA from F. novicida transposon mutants was extracted using the PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific) and digested with a combination of restriction enzymes XhoI, BglII, EcoRI, SalI, NotI, BamHI, PstI, and SphI. The resulting DNA fragments were blunt-ended using the DNA-Blunting Kit (Takara Bio) and subsequently ligated using Ligation High Ver. 2 (Toyobo). The ligation products were used to transform One-Shot PIR1 Chemically Competent E. coli (Thermo Fisher Scientific). Transformants were selected on kanamycin-containing plates, and plasmid DNA was purified from the resistant colonies. Sequence analysis was performed using a primer described in the Ez-Tn5 transposon system manual.
GFP-expression of F. novicida and complimentary strains
The GFP-expressing plasmid pOM5-GFP was constructed in accordance with the procedures published elsewhere [22]. This plasmid was introduced into the wild-type and FTN_0096 strains of F. novicida via electroporation. To create pOM5–0096 or its complementary, FTN_0096 gene along with its native promoter region (300-bp upstream) was cloned into the pOM5 vector. pOM5–0096 was then used to transform into the FTN_0096 mutant of F. novicida via electroporation.
Intracellular growth assay
THP-1 cells (4 × 105 cells/well) were seeded into 24-well tissue culture plate and treated with 200 nM phorbol 12-myristate 13-acetate (PMA) for 48 h to induce differentiation. F. novicida strains were added at an MOI of 1. HeLa–FcγRII cells (1 × 105 cells/well) were incubated overnight in 24-well tissue culture plates. F. novicida strains were incubated with mouse serum containing F. novicida antibodies and infected at an MOI of 1 to the HeLa–FcγRII cells. The THP-1 cells were washed thrice with RPMI1640 medium, HeLa–FcγRII cells were washed thrice with DMEM medium, and extracellular bacteria were eliminated by treating the cultures with gentamicin at the concentration of 50 μg/mL for 1 h. The cells were incubated in a fresh medium at 37˚C for the desired time points. The cells were washed with phosphate-buffered saline (PBS) and then lysed with 0.1% Triton X-100 in CDM to determine the intracellular growth. Colony-forming units (CFUs) were determined by plating serial dilutions of the lysates on BHIc agar plates.
Fluorescence microscopy
THP-1 cells (4 × 10⁵ cells per well) were seeded onto 12-mm glass coverslips placed in 48-well tissue culture plates and treated with 100 nM of PMA for 48 h. HeLa–FcγRII cells (1 × 105 cells/well) were plated onto 12-mm glass coverslips in 24-well tissue culture plates and incubated overnight. THP-1 cells were infected with GFP-expressing F. novicida strains and incubated for the specified times. Separately, GFP-expressing F. novicida strains were opsonized with mouse serum and used to infect HeLa–FcγRII cells at a multiplicity of infection (MOI) of 1.0, followed by incubation at the indicated time points. The cells were stained with LysoTracker Red DND-99, MitoTracker® Deep Red FM, and BODIPY TR (Thermo Fisher Scientific) to visualize lysosomes, mitochondria, and Golgi complex as per the source’s instruction manual. To detect lysosomal-associated membrane protein 1 (LAMP-1), the cells were fixed using the PLP Solution Set (Wako Laboratory Chemicals) containing 5% sucrose at 37°C for 1 h, followed by permeabilized with cold methanol for 10 s. The cells were treated with an anti-LAMP-1 antibody (ab25245, 1:100, Abcam) and subsequently stained with FITC-conjugated anti-rat IgG (1:1000, Abcam). The percentage of colocalizing bacteria was calculated manually by counting the total colocalized and non-colocalized bacteria in at least 100 infected cells. Confocal images were acquired using a FluoView FV100 confocal laser scanning microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical significance between groups was analyzed with the Bonferroni/Dunnett or the Student’s t-test, P < 0.01 was considered statistically significant.
Results
Identification of F. novicida genes with reduced cytotoxicity to HeLa–FcγRII cells
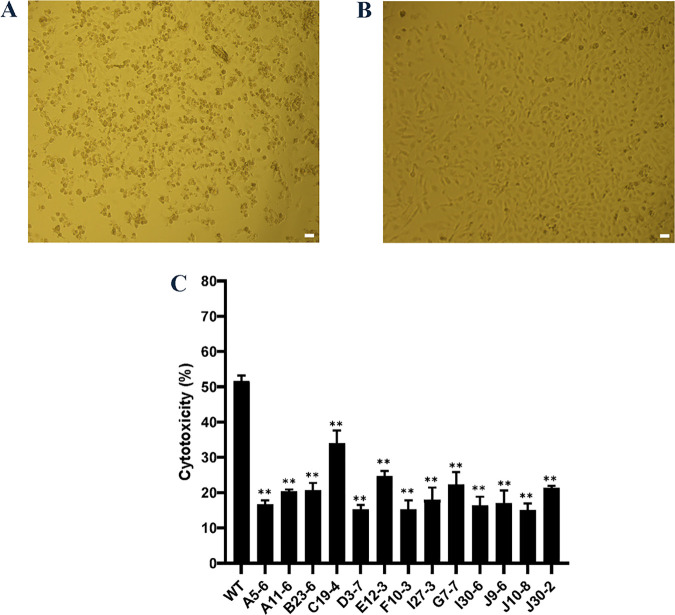
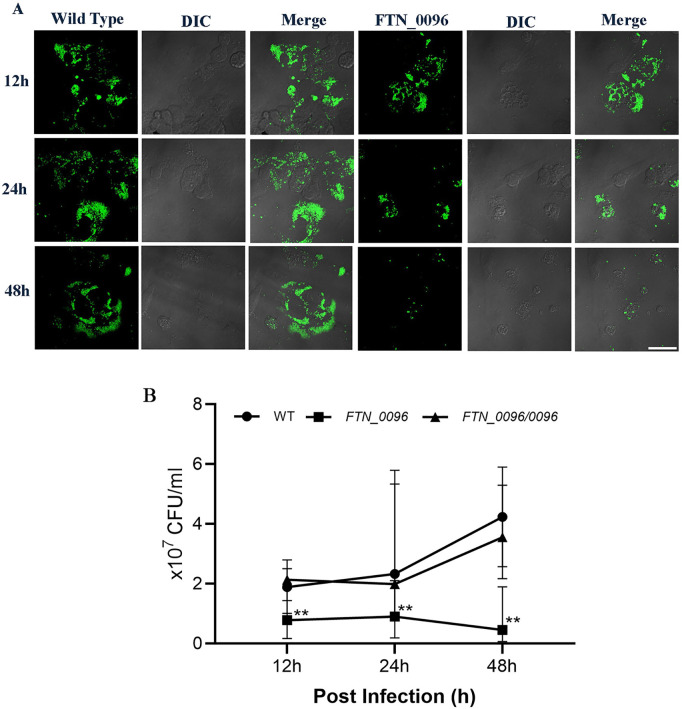
We previously developed new infection model of F. novicida using HeLa cells expressing mouse FcγRII (HeLa-FcγRII). HeLa-FcγRII cells enhanced F. novicida infection efficiency in epithelial cells and enabled more detailed study of Francisella’s intracellular behavior [15]. Using this new infection model, we screened the F. novicida transposon mutant library to identify the new virulence factor of F. novicida. Considering that intracellular invasion of bacteria was mediated by antibodies and FcγRII in this model, we expected to elucidate new intracellular growth factors that could not be detected via conventional analysis using macrophages. We demonstrated in a previous study that F. novicida infection was cytotoxic to HeLa–FcγRII and induced cell death. Therefore, we made microscopic observations to screen the mutant library of F. novicida that lacked cytotoxicity (Fig 1A and 1B). Among 2,232 transposon mutants examined, 13 were identified as being less cytotoxic. LDH assays were conducted to validate these findings. When compared to the wild-type strain, these mutants reduced the extent of LDH release from HeLa–FcγRII cells (Fig 1C). The transposon-insertion sites in the mutant strains were identified through sequencing to identify the genes. We focused on mutants with the code number E12-3, where the sequencing results encoded FTN_0096 and the function of FTN_0096 were analyzed.
Fig 1. Screening of the Transposon Mutant Library.
(A) HeLa–FcγRII cells were infected with F. novicida wild-type strain, preincubated with mouse serum containing F. novicida antibodies and incubated at 37°C for 24 h. Scale bar = 20 μm. (B) HeLa–FcγRII cells were infected with E12-3 transposon mutant of F. novicida, preincubated with mouse serum containing F. novicida antibodies, and incubated at 37°C for 24 h. Scale bar = 20 μm. (C) The transposon mutant library was screened using LDH cytotoxicity assay. HeLa–FcγRII cells were infected with the wild-type strain of F. novicida and transposon mutants of F. novicida preincubated with mouse serum containing F. novicida antibodies and incubated at 37°C for 24 h. LDH release was measured as an indicator of cytotoxicity. Data represent the mean and standard deviations of three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01.
Effect of FTN_0096 mutation on intracellular growth and cytotoxicity in HeLa–FcγRII cells
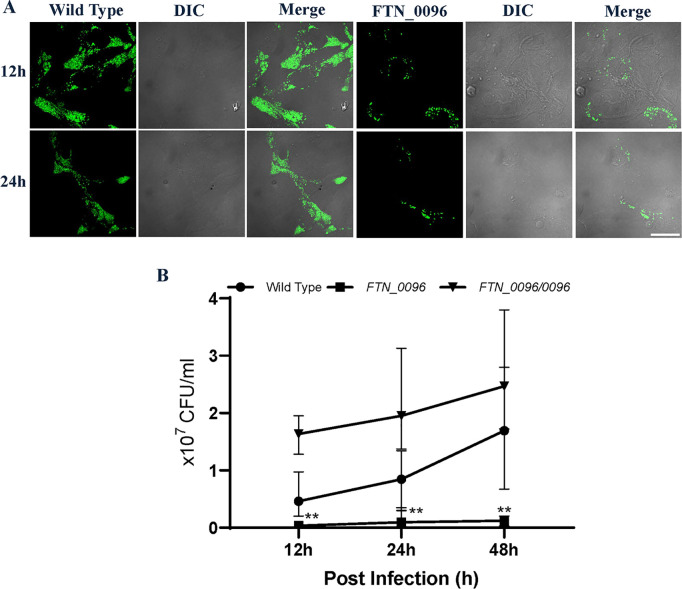
To analyze the function of FTN_0096 on intracellular growth and cytotoxicity, we infected the FTN_0096 transposon mutant of F. novicida to HeLa–FcγRII cells. The FTN_0096 mutant (E12-3) induced a decreased LDH-release in comparison with the wild-type strain in HeLa–FcγRII cells (Fig 1C). To investigate the mechanism underlying cytotoxicity, we assessed the intracellular growth of the FTN_0096 mutant in HeLa–FcγRII cells. While the wild-type F. novicida strain exhibited robust intracellular proliferation, the FTN_0096 mutant showed significantly reduced intracellular growth in HeLa–FcγRII cells (Fig 2B). Replication was reinstated to the wild-type level in the complemented strains (Fig 2B). We also detected intracellular bacteria using GFP-expressing F. novicida strains. We examined the intracellular growth of the wild type and FTN_0096 mutant at 12 and 24 h after infection in HeLa–FcγRII cells (Fig 2A). These results indicated that FTN_0096 was essential for the cytotoxicity and intracellular growth of F. novicida using HeLa–FcγRII cells.
Fig 2. Intracellular growth and cytotoxicity of the wild-type strain and FTN_0096 mutant in HeLa–FcγRII cells.
(A) HeLa–FcγRII cells were infected with F. novicida wild-type strain and FTN_0096 mutant, preincubated with mouse serum containing F. novicida antibodies at MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. The cells were fixed and examined at 12 and 24 h post-infection. Scale bar = 20 μm. (B) HeLa–FcγRII cells were infected with F. novicida wild-type, FTN_0096 mutant, and complementary strain (FTN_0096/0096), preincubated with mouse serum containing F. novicida antibodies at MOI = 1, and following treatment with 50 μg/mL of gentamicin for 1 h. At 12, 24, and 48 h post-infection, cells were lysed with 0.1% Triton X-100, and plating serial dilutions on BHIc agar. Data represent the mean and standard deviations of three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01.
Escape of the bacterial cells from phagolysosomes in HeLa–FcγRII cells
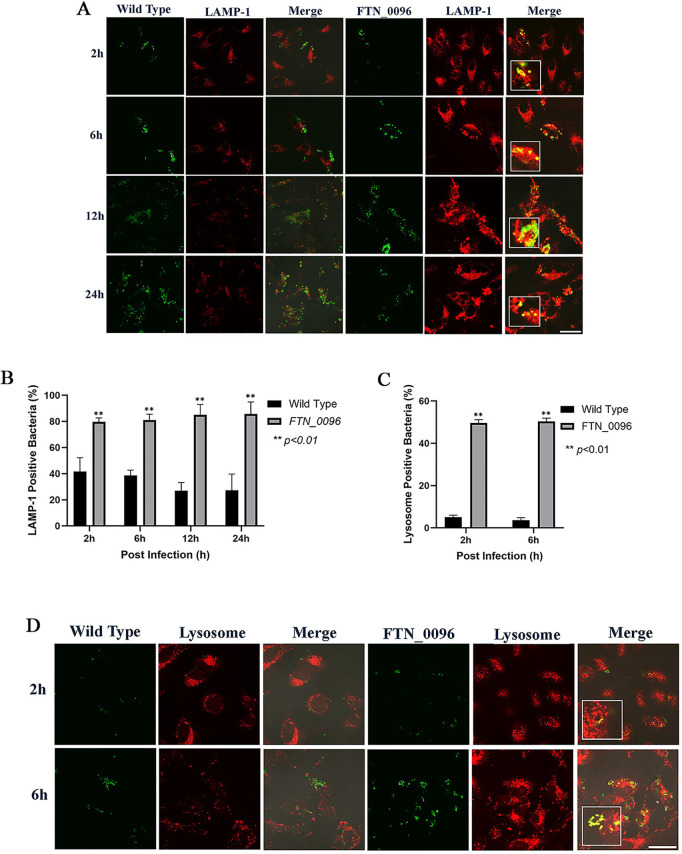
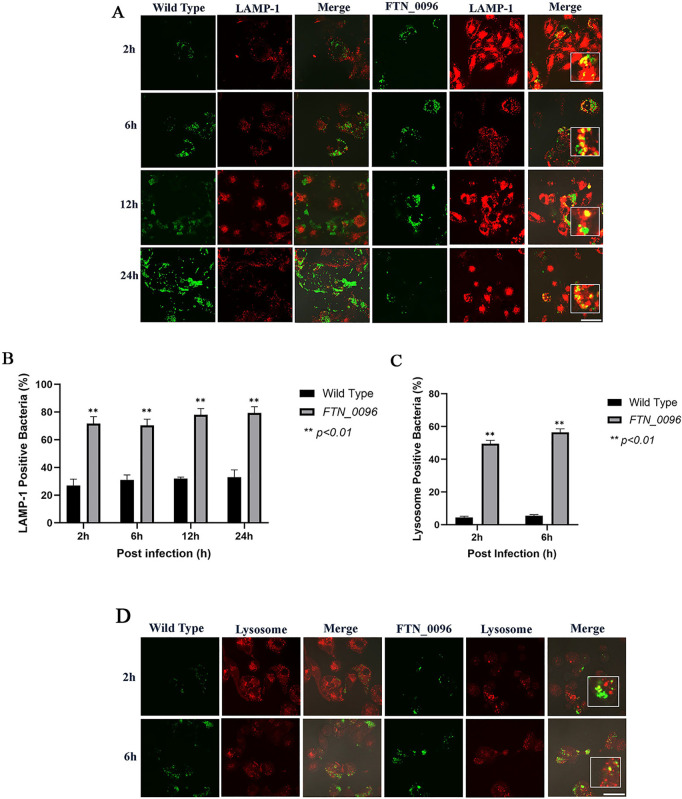
To investigate the function of FTN_0096 in intracellular growth, the ability of F. novicida strains to escape from phagolysosomes was measured. HeLa–FcγRII cells were infected with both the wild-type and FTN_0096 mutant, and lysosomes were identified using an antibody against the lysosome marker LAMP-1 (Fig 3A). The wild-type strain exhibited continuous intracellular replication from 2 to 24 h post-infection, in line with the number of intracellular bacteria. In contrast, the number of FTN_0096 mutant escalated until 12 h after the infection. However, the bacterial count diminished 24 h after the infection. In the case of infection with the wild-type strain, only a few numbers of bacteria showed colocalization with LAMP-1 in 27–42% of the cells (Fig 3B). On the other hand, the large numbers of FTN_0096 mutant were observed with LAMP-1 in 70–79% cells at 2, 6, 12, and 24-h post-infection.
Fig 3. Recognition of F. novicida strains by lysophagosomes and lysosomes in HeLa–FcγRII cells.
(A) HeLa–FcγRII cells were infected with F. novicida wild-type strain and FTN_0096 mutant preincubated with mouse serum containing F. novicida antibodies at MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. At 2 to 24 h post-infection, cells were treated with an anti-LAMP-1 antibody and visualized using a TRITC-conjugated anti-rat IgG. Scale bar = 20 μm. (B) The percentage of F. novicida wild-type strain and FTN_0096 mutant colocalized with LAMP-1. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (C) The percentage of F. novicida wild-type strain and FTN_0096 mutant colocalized with lysosomes. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (D) HeLa–FcγRII cells were infected with F. novicida wild-type strain and FTN_0096 mutant preincubated with mouse serum containing F. novicida antibodies at MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. At 2 to 6 h post-infection, cells were stained with LysoTracker™ Red DND-99 to visualize lysosomes. Scale bar = 20 μm.
In order to further investigate the role of FTN_0096 in the escape from phagolysosomes in HeLa–FcγRII cells, we observed the acidic organelles using Lysotracker. HeLa–FcγRII cells were infected with GFP-expressing F. novicida strains to examine their ability to escape from the phagolysosome during the early stages of infection (2 and 6 h post-infection). The cells were stained with LysoTracker and analyzed by confocal microscopy to visualize lysosomal localization. The co-localization of the Lysotracker of the wild type was observed in only 4–5% of infected cells, but the colocalization of FTN_0096 mutant and Lysotracker was observed in 49–50% of the cells (Fig 3C). Few wild-type bacteria colocalized with lysosome, and several from the FTN_0096 mutant colocalized with lysosome in HeLa–FcγRII cells (Fig 3D). These observations indicated that the wild-type bacteria evaded the phagolysosomes, whereas FTN_0096 mutant failed to escape from them in HeLa–FcγRII cells.
Effect of FTN_0096 mutant on the mitochondria of HeLa–FcγRII cells
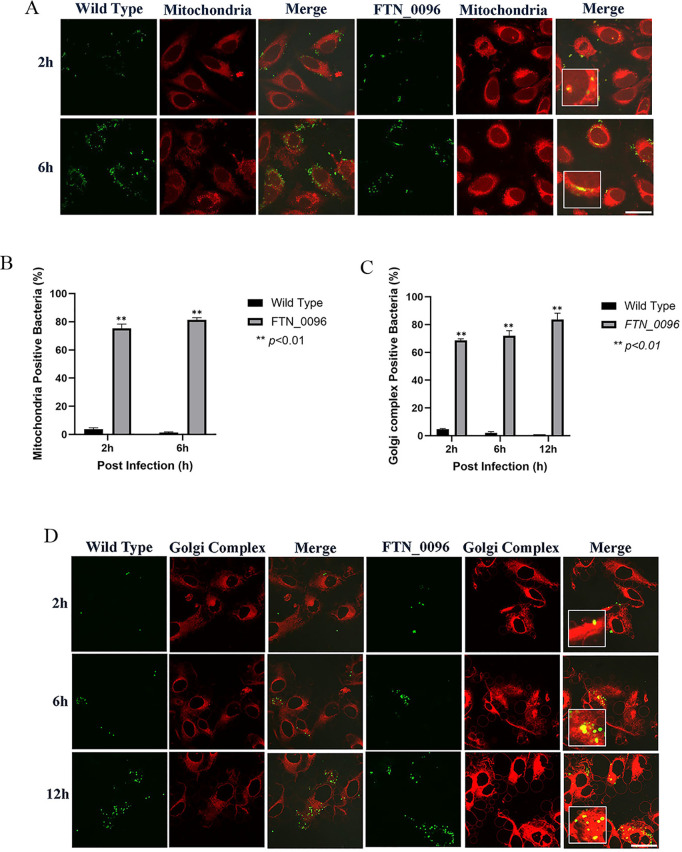
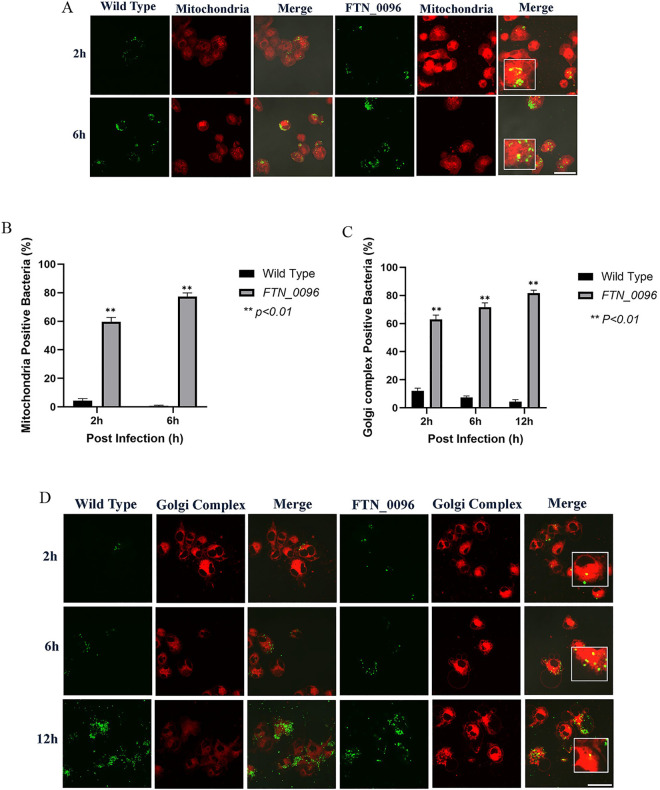
To further investigate the functions of FTN_0096 in the mitochondria, HeLa–FcγRII were infected with the wild-type strains and the FTN_0096 mutant. The mitochondria were visualized by using MitoTracker® Deep Red FM (Fig 4A) to observe whether the mitochondria can colocalize wild-type and FTN_0096 mutant. The wild-type strain infected the HeLa–FcγRII cells, after which the intracellular bacterial cells were examined at 2–6 h post-infection. Nonetheless, a few cells colocalized with the mitochondria (Fig 4B). However, FTN_0096 mutant was observed from 2 and 6 h after the infection, and several of the bacteria colocalized with the mitochondria, at approximately 75%–81% in HeLa–FcγRII cells (Fig 4B). These results suggest that FTN_0096 mutant cannot modulate the mitochondria, rather F. novicida wild-type can modulate the mitochondria function and maintain their replication.
Fig 4. Recognition of F. novicida strains by the mitochondria and Golgi complex in HeLa–FcγRII cells.
(A) HeLa–FcγRII cells were infected with F. novicida wild-type strain and FTN_0096 mutant preincubated with mouse serum containing F. novicida antibodies at MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. Cells were stained with Mitotracker® Deep Red FM at 2 and 6 h post-infection. Scale bar = 20 μm. (B) Percentage of F. novicida wild-type strain and FTN_0096 mutant that colocalized with the mitochondria. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks,**P < 0.01. (C) The percentage of F. novicida wild-type strain and FTN_0096 mutant that colocalized with the Golgi complex. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (D) HeLa–FcγRII cells were infected with F. novicida wild-type strain and FTN_0096 mutant preincubated with mouse serum containing F. novicida antibodies at MOI = 1, and treated with 50 μg/mL gentamicin. At 2, 6, and 12 h post-infection, cells were stained with BODIPY TR. Scale bar = 20 μm.
Effect of FTN_0096 mutant infection on the Golgi complex of HeLa–FcγRII cells
To analyze the function of FTN_0096 on the Golgi complex, we infected HeLa–FcγRII cells with the wild-type strains and FTN_0096 mutant. The Golgi complex was stained using BODIPY TR (Fig 4D) to observe whether the Golgi complex can colocalize with the wild-type strain and FTN_0096 mutant. The wild-type strain infected the HeLa–FcγRII cells, and the intracellular bacterial cells were then examined at 2, 6, and 12 h after infection. Few bacteria colocalized with the Golgi complex (Fig 4C). Nevertheless, the FTN_0096 mutant colocalized with the Golgi complex at all time points after the infection. Approximately 69%–84% of the bacteria from FTN_0096 mutant colocalized with the Golgi complex in HeLa–FcγRII cells (Fig 4C).
Intracellular growth of FTN_0096 mutant in THP-1 cells
To explore the function of FTN_0096 in THP-1 cells, we measured the intracellular growth of the FTN_0096 mutant in THP-1 cells. While the wild-type strain exhibited robust intracellular proliferation, in contrast to the FTN_0096 mutant, for which the numbers of intracellular growths reduced in THP-1 cells (Fig 5B). Replication was reinstated up to the wild-type level in the complemented strains (Fig 5B). We also detected intracellular bacteria using GFP-expressing F. novicida strains. Next, we observed the intracellular growth of the wild-type strain and FTN_0096 mutant at 12, 24, and 48 h post-infection in THP-1 cells, respectively (Fig 5A). These results indicate the importance of FTN_0096 for the intracellular growth of F. novicida in THP-1 cells.
Fig 5. Intracellular growth of the wild-type strain and FTN_0096 mutant in THP-1 cells.
(A) THP-1 cells were infected with F. novicida wild-type strain and FTN_0096 mutant at MOI = 1, following treatment with gentamicin (50 μg/mL) for 1 h. Cells were fixed and examined at 12, 24, and 48 h post-infection. Scale bar = 20 μm. (B) THP-1 cells were infected with F. novicida wild-type, FTN_0096 mutant, and FTN_0096/0096 at MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. At 12, 24, and 48 h post-infection, cells were lysed with 0.1% Triton X-100 and plating serial dilutions on BHIc agar. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01.
Escape of the bacteria from phagolysosomes in THP-1 cells
To assess ability of F. novicida to escape from the phagolysosome in THP-1 cells, we infected the wild-type and FTN_0096 mutant into THP-1 cells and detection of lysosomes was performed using a LAMP-1–specific antibody (Fig 6A). The wild-type strain showed robust intracellular proliferation from 2 to 24 h post-infection, in line with the number of intracellular bacteria. FTN_0096 mutant proliferated until 12 h after infection; however, the bacterial count diminished 24 h post-infection. In the case of wild-type infection, only a few numbers of bacteria showed colocalization with LAMP-1 in 27–33% of infected cells (Fig 6B). However, a large number of colocalization of FTN_0096 mutant with LAMP-1was observed in 70−79% of the cells at 2, 6, 12, and 24 h post-infection, indicating significant colocalization of the mutant with LAMP-1 (Fig 6B).
Fig 6. Recognition of F. novicida strains by lysophagosomes and lysosomes in THP-1 cells.
(A) THP-1 cells were infected with F. novicida wild-type strain and FTN_0096 mutant at MOI = 1, following treatment with gentamicin (50 μg/mL) for 1 h. Cells were treated with an anti-LAMP-1 antibody and stained with a TRITC-conjugated anti-rat IgG at 2–24 h after the infection. Scale bar = 20 μm. (B) The percentage of F. novicida wild-type strain and FTN_0096 mutant colocalized with LAMP-1. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (C) The percentage of F. novicida wild-type strain and FTN_0096 mutant colocalized with lysosomes. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (D) THP-1 cells were infected with F. novicida wild-type strain and FTN_0096 mutant MOI = 1, following treatment with gentamicin (50 μg/mL) for 1 h. Cells were treated with LysoTracker™ Red DND-99 from 2 to 6 h after the infection. Scale bar = 20 μm.
To further investigate the role of FTN_0096 in the escape from phagolysosomes in THP-1 cells, the cells were infected with GFP-expressing wild-type strain and FTN_0096 mutant and acidic organelles were stained using Lysotracker. The wild-type strain showed colocalization with Lysotracker only in 5−6% of the cells, whereas the FTN_0096 mutant was colocalized with Lysotracker in 50−57% of the cells (Fig 6C). A few wild-type bacteria colocalized with lysotracker and several the FTN_0096 mutant had colocalized with lysotracker in THP-1 cells (Fig 6D). These observations indicated that the wild-type bacteria evaded the phagolysosomes, whereas FTN_0096 mutant failed to escape from them in THP-1 cells.
Effect of FTN_0096 mutant on the mitochondria of THP-1 cells
To further investigate the functions of FTN_0096 in the mitochondria of THP-1 cells, THP-1 cells were infected with the wild-type strains and FTN_0096 mutant. The mitochondria were visualized using MitoTracker® Deep Red FM (Fig 7A) to observe whether the mitochondria colocalized the wild-type strain and FTN_0096 mutant. The wild-type strain infected the THP-1 cells, and intracellular bacterial cells were examined 2–6 h post-infection. Nonetheless, few cells colocalized with the mitochondria (Fig 7B). However, the FTN_0096 mutant was observed from 2 to 6 h post-infection, and several cells (57%–77%) had colocalized with the mitochondria in THP-1 cells (Fig 7B).
Fig 7. Recognition of F. novicida strains by the mitochondria and Golgi complex in THP-1 cells.
(A) THP-1 cells were infected with F. novicida wild-type strain and FTN_0096 mutant, MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. Cells were stained with the Mitotracker® Deep Red FM at 2 and 6 h post-infection. Scale bar = 20 μm. (B) The percentage of F. novicida wild-type strain and FTN_0096 mutant that colocalized with mitochondria. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (C) The percentage of F. novicida wild-type strain and FTN_0096 mutant that colocalized with the Golgi complex. The data represent the mean and standard deviation from three identical experiments. Significant differences were evaluated in comparison to the wild-type strain using multiple comparison analyses as indicated by asterisks, **P < 0.01. (D) THP-1 cells were infected with F. novicida wild-type strain and FTN_0096, MOI = 1, and following treatment with gentamicin (50 μg/mL) for 1 h. Cells were stained with BODIPY TR at 2, 6, and 12 h post-infection. Scale bar = 20 μm.
Effect of FTN_0096 mutant infection on the Golgi complex of THP-1 cells
To analyze the functions of FTN_0096 in the Golgi complex, we infected THP-1 cells with wild-type strains and FTN_0096 mutant. The Golgi complex was stained with the BODIPY TR (Fig 7D) to observe whether the Golgi complex can colocalize with the wild-type strain and FTN_0096 mutant. The wild-type strain infected the THP-1 cells, and intracellular bacterial cells were examined at 2, 6, and 12 h post-infection. Few bacterial cells colocalized with the Golgi complex (Fig 7C). Nevertheless, the FTN_0096 mutant colocalized with the Golgi complex from 2, 6, and 12 h post-infection. Around 63%–82% of the bacterial cells from FTN_0096 mutant colocalized with the Golgi complex in THP-1 cells (Fig 7C).
Discussion
Intracellular bacteria, such as Francisella, possess mechanisms that help evade the host immune system and aid survival in the host cells. Macrophage-based cell lines, such as bone marrow-derived macrophage cells, phorbol myristate acetate (PMA)-differentiated U937, THP-1, and J774 cells have been widely used to study Francisella infection [7,23–25]. We previously developed an infection model of F. novicida using HeLa cells expressing mouse FcγRII (HeLa–FcγRII). When using the HeLa–FcγRII cells with antibody-treated bacteria, the efficiency of infection will be 100-fold greater compared to the normal HeLa cells [15]. This model promoted the discovery of novel factors vital for intracellular proliferation that could not be found through conventional analysis using macrophages. Accordingly, we constructed a transposon mutant library of F. novicida and screened it to identify mutants that were relatively less cytotoxic to the HeLa–FcγRII. As such, 13 mutants that showed decreased LDH release in HeLa–FcγRII cells were compared to the wild-type strain, and transposon-inserted genes were determined. Contrary to our initial expectations of finding novel virulence factors, the identified gene set largely consisted of previously characterized virulence genes such as Slt, IglC, pdpA, and pdpB. Although previous comprehensive analyses had implicated FTN_0096 as a potentially important factor for intracellular proliferation within macrophages [17], its detailed function remains to be elucidated. Consequently, we selected FTN_0096 for further investigation. In this research, we had difficulty making homologous recombination, which is one of the steps to make deletion mutants for this gene. It was suggested that complete gene deletion of FTN_0096 might result in lethal. Therefore, we attempted to directly analyze FTN_0096 from the transposon mutant library of F. novicida. A detailed examination of the dynamics of the FTN_0096 transposon mutant was accordingly performed. The observed dynamics of the mutant were consistent in both HeLa–FcγRII cells and the macrophage-derived THP-1 cell line. These findings implied that the infection model using HeLa–FcγRII accurately reflected the behavior of macrophage-derived cells, making it a valuable platform for the study of virulence factors of F. novicida.
FTN_0096 encodes a conserved hypothetical membrane protein and is a member of the DUF1275 superfamily, which includes proteins found in a wide range of bacterial species. While the function of DUF1275 remains unidentified, some of its members are encoded near genes for a predicted metallo–amido–hydrolase, suggesting a role related to the transport of peptides or other amido compounds, or in exporting of their hydrolysis products [18]. Nonetheless, the pathogenicity mechanism of FTN_0096 of F. novicida remains unidentified.
Francisella enters the macrophages through phagocytosis and avoids lysosomal fusion by escaping from the resultant phagosome in 1–4 h [10]. Upon phagosomal escape, Francisella localizes to the host cytosol, an environment rich in nutrients and innate immune signaling components. Francisella manipulates the host cell metabolism to promote massive replication within the host cytosol [26]. The bacteria then re-enter the autophagosomes in the latter phase of infection [27].
F. novicida wild-type strain proliferated intracellularly well 12–48 h post-infection in HeLa–FcγRII and THP-1 cells. Nakamura et al. [28] demonstrated that the F. novicida wild-type strain proliferated intracellularly within THP-1 cells. In contrast, the FTN_0096 mutant proliferated intracellularly until 12 h post-infection in HeLa–FcγRII and THP-1 cells but decreased 24 and 48 h post-infection. The rate of intracellular growth of the wild-type strain differed significantly from that in the FTN_0096 mutant (P < 0.01).
The F. novicida wild-type strain colocalized with acidic organelles (LysoTracker) 4%–5% and LAMP-1 at 27%–42% in HeLa–FcγRII. Similarly, in THP-1 cells, LysoTracker colocalization ranged from 5%–6%, while LAMP-1 levels were between 27%–33%. F. novicida wild-type strain can escape from phagosomes and replicate within the cytosol of HeLa–FcγRII and THP-1 cells. FTN_0096 mutant was found within acidic compartments marked by LysoTracker during the initial phases of infection (2–6 h), and the mutant also localized with the lysosomal marker LAMP-1 during the later stages of the infection (12–48 h post-infection). The FTN_0096 mutant colocalized with LysoTracker at 49%–50% and LAMP-1 at 80%–86% in HeLa–FcγRII cells, while in THP-1 cells colocalized by LysoTracker 50%–57% and LAMP-1 70%–79%. These results demonstrate that FTN_0096 plays a critical role in the intracellular lifecycle of F. novicida, particularly in the ability to escape from the phagosome into the host cytosol. As successful escape into the host cytosol is essential for intracellular replication and the induction of cytotoxicity, the impaired ability of the FTN_0096 mutant to escape from the phagosome likely accounts for its reduced intracellular proliferation and attenuated cytotoxic effects.
Lysosomes are membrane-bound organelles containing hydrolytic enzymes that facilitate the breakdown of diverse biological macromolecules. They exhibit considerable dynamic behavior and are capable of fusing with vesicles formed through endocytosis, auto-phagocytosis, and phagocytosis [29,30]. Lysosomes are essential for cellular waste disposal, digestion of foreign substances, recycling of cellular components, and the regulation of various signaling pathways. Dysfunction in lysosomal activity is associated with lysosomal storage disorders and can significantly impair cellular homeostasis [31].
Previous data have revealed that F. novicida can evade the phagosome and proliferate within the cytosol after the infection of mammalian cells. Intracellular pathogens have developed several methods to survive and evade the phagosome–lysosome process [32,33]. The role of host intracellular acidity in facilitating F. novicida reproduction remains unclear. However, past studies have demonstrated that some bacteria become sequestered within lysosomes where the alkalized pH of the intracellular environment can inhibit their replication [34–36]. Perhaps, FTN_0096 may related to the mechanisms to survive in various pH of intracellular environment.
The FTN_0096 mutant colocalized with mitochondria stained with MitoTracker® during the initial stages of infection (2–6 h), whereas the wild-type strains were not colocalized with the mitochondria. The FTN_0096 mutant colocalized with the mitochondria (MitoTracker®) in HeLa–FcγRII cells at 75%–81% proportion and in THP-1 cells at 60%–77% proportion. These results indicate that the FTN_0096 of F. novicida plays an important role in escape from the mitochondria and survive intracellularly. The mitochondrion is a vital organelle that facilitates several functions of the immune system, particularly via metabolic regulation, calcium homeostasis, and mitochondrial ROS (mtROS) production and degradation [37]. A distinct metabolic signature is mitochondrial clearance via mitophagy, which is important for activating the macrophage. Mitophagy, a form of autophagy specific to mitochondria, is designed to remove damaged mitochondria, either in response to stress or as a part of targeted mitochondrial clearing [38–41]. Previous results have shown the capability of F. tularensis SchuS4 to modulate mitochondrial processes early in infection by increasing mitochondrial activity and disrupting the metabolic transition from oxidative phosphorylation to glycolysis. Virulent F. tularensis manipulates host mitochondrial metabolism as a central strategy to restrict inflammation and regulate cell death for optimal bacterial replication [42]. F. tularensis also acts at different levels to maintain mitochondrial integrity and modulate cytosolic regulatory factors that alter the mitochondria to suppress caspase activity [43]. Caspases are essential components in apoptosis and are responsible for activating dismantling of the cell. Apoptosis is one of the host defense mechanisms that restricts the spread of pathogens. Apoptotic cells maintain their contents inside and are cleared by phagocytes. Some intracellular pathogens, such as Rickettsia rickettsii, Legionella pneumophila, Salmonella enterica, and Chlamydia sp., prevent host cell apoptosis by acting on the mitochondria. Thus, the mitochondria are important targets for the regulation of apoptosis by intracellular pathogens [44,45]. Since FTN_0096 mutant captured in mitochondria in this study, these findings suggested that FTN_0096 may play an important role in intracellular growth by affecting the function of mitochondria.
To observe the activity of the F. novicida wild-type strain and FTN_0096 mutant in the Golgi complex, the infected HeLa–FcγRII and THP-1 cells were stained using Bodipy TR. The result revealed that the colocalization of the Golgi complex to F. novicida wild-type strain was not observed, and FTN_0096 mutant was observed. The percentage of wild-type strains that colocalized with the Golgi complex was 1%–5% in HeLa–FcγRII cells and 4%–12% in THP-1 cells. The FTN_0096 mutant colocalized with the Golgi complex (Bodipy TR) in HeLa–FcγRII cells at 69%–84% and in THP-1 cells at 63%–82%. These results suggest that the F. novicida wild-type strain can escape from the intracellular events related to Golgi complex, but the FTN_0096 mutant was detected within the Golgi complex, indicating that this mutant cannot escape from the events related to Golgi complex.
The Golgi complex, a vital cytoplasmic organelle, is the primary site for the post-translational modification, transport, and sorting of lipids and proteins synthesized by the endoplasmic reticulum. This complex ensures their transport to designated cellular locations via cytophagy and cytotoxicity. The typically compact structure of the Golgi complex can be disrupted, leading to various levels of disorganization and unstacking because of the cytotoxic agents. These changes in Golgi structure are often linked to problems with the transport and secretion of several essential hepatic glycoproteins. Furthermore, it engages in other biological processes, such as cell signaling, division, and apoptosis. Because of this primary function of the Golgi complex, pathogens try to develop strategies to disrupt and utilize these processes to facilitate the proliferation of pathogens [46–48]. The Golgi complex is essential for the induction of type I interferon (IFN-I) in response to several stimuli, such as Francisella infection, Toll-like receptors activation, and viral infection [49]. IFN-I is a class of cytokines that are deemed essential for viral and bacterial infections in regulating innate and adaptive immunity [50]. Previous research has suggested that IFN-I can impair the host’s ability to clear intracellular bacteria, such as Listeria monocytogenes [51], Mycobacterium tuberculosis [52], and F. tularensis [53]. Intracellular bacteria, which are similar to viruses, proliferate within the cytoplasm and can trigger IFN-I products through intracellular pathways. Intracellular bacterial infections can create a cellular environment that mimics viral infection, potentially helping pathogens evade immune surveillance. IFN-I helps limit neutrophil swarming as a mechanism to mitigate excessive tissue injury, whereas intracellular bacteria may utilize this process to elude immunological detection by neutrophils. Disruption of neutrophil swarming, caused by IFN-α, has been recognized as a significant contributor to increased susceptibility to bacterial infections [50]. These findings may suggest that FTN_0096 may affect the function of Golgi complex to survive intracellularly.
Although the detailed function of FTN_0096 in the mitochondria and the Golgi complex is not yet fully understood. The FTN_0096 mutant showed increased colocalization with these organelles, indicating a failure to escape host cell defenses. FTN_0096 may modulate host intracellular trafficking or stress response pathways that are regulated by these organelles, thereby promoting bacterial survival by interfering with host defense mechanisms. Comprehensive research is needed to determine whether FTN_0096 directly modulates mitochondrial dynamics or Golgi-associated pathways during infection.
In conclusion, we screened a transposon mutant library using HeLa–FcγRII cells and identified FTN_0096 as a pathogenic factor of F. novicida. FTN_0096 is required for intracellular replication in the cells. FTN_0096 contributes to bacterial survival by promoting escape from the phagosome and avoiding host organelle-mediated defenses, including those involving the mitochondria and Golgi complex. Further studies are however warranted to establish the detailed pathogenicity mechanisms of F. novicida, which may provide a basis for comprehending how Francisella expresses its pathogenicity. Moreover, the detailed mechanisms of FTN_0096 can contribute to developing vaccines that can prevent diseases caused by Francisella.
Supporting information
This file contains the raw measurements for the experiments described in the manuscript, with separate tabs corresponding to each figure.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
TS: JSPS KAKENHI Grant Number 22K07054 MW: JSPS KAKENHI Grant Number 21H02360 The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Petersen JM, Mead PS, Jameson JL, Fauci AS, Kasper DL, Hauser SL. Tularemia. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s principles of internal medicine. 20e ed. New York, NY: McGraw-Hill Education; 2018. p. 1196–9. [Google Scholar]
- 2.Nelson C, Kugeler K, Petersen J, Mead P. Tularemia-United States, 2001—2010. MMWR Morb Mortal Wkly Rep. 2013;62:963–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Telford SR 3rd, Goethert HK. Ecology of Francisella tularensis. Annu Rev Entomol. 2020;65:351–72. doi: 10.1146/annurev-ento-011019-025134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freudenberger Catanzaro KC, Inzana TJ. The Francisella tularensis Polysaccharides: What Is the Real Capsule? Microbiol Mol Biol Rev. 2020;84(1):e00065-19. doi: 10.1128/MMBR.00065-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeni DK, Büyük F, Ashraf A, Shah MSUD. Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol (Praha). 2021;66(1):1–14. doi: 10.1007/s12223-020-00827-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurin M. Tularemia. In: Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP, editors. Hunter’s tropical medicine and emerging infectious diseases. London: Elsevier; 2020. p. 630–5. [Google Scholar]
- 7.Guo Y, Mao R, Xie Q, Cheng X, Xu T, Wang X, et al. Francisella novicida Mutant XWK4 Triggers Robust Inflammasome Activation Favoring Infection. Front Cell Dev Biol. 2021;9:743335. doi: 10.3389/fcell.2021.743335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingry LC, Petersen JM. Comparative review of Francisella tularensis and Francisella novicida. Front Cell Infect Microbiol. 2014;4:35. doi: 10.3389/fcimb.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong A, Wehrly TD, Nair V, Fischer ER, Barker JR, Klose KE, et al. The early phagosomal stage of Francisella tularensis determines optimal phagosomal escape and Francisella pathogenicity island protein expression. Infect Immun. 2008;76(12):5488–99. doi: 10.1128/IAI.00682-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford MK, Elkins KL. Immune lymphocytes halt replication of Francisella tularensis LVS within the cytoplasm of infected macrophages. Sci Rep. 2020;10(1):12023. doi: 10.1038/s41598-020-68798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjöstedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71(10):5940–50. doi: 10.1128/IAI.71.10.5940-5950.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bröms JE, Sjöstedt A, Lavander M. The Role of the Francisella Tularensis Pathogenicity Island in Type VI Secretion, Intracellular Survival, and Modulation of Host Cell Signaling. Front Microbiol. 2010;1:136. doi: 10.3389/fmicb.2010.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopping EJ, Benziger PT, Thanassi DG. TolC and EmrA1 contribute to Francisella novicida multidrug resistance and modulation of host cell death. J Bacteriol. 2024;206(9):e0024624. doi: 10.1128/jb.00246-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubelkova K, Macela A. Francisella and Antibodies. Microorganisms. 2021;9(10):2136. doi: 10.3390/microorganisms9102136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Shimizu T, Nishinakama N, Takahashi R, Arasaki K, Uda A, et al. A novel method of Francisella infection of epithelial cells using HeLa cells expressing fc gamma receptor. BMC Infect Dis. 2024;24(1):1171. doi: 10.1186/s12879-024-10083-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geier H, Celli J. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect Immun. 2011;79(6):2204–14. doi: 10.1128/IAI.01382-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llewellyn AC, Jones CL, Napier BA, Bina JE, Weiss DS. Macrophage replication screen identifies a novel Francisella hydroperoxide resistance protein involved in virulence. PLoS One. 2011;6(9):e24201. doi: 10.1371/journal.pone.0024201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SC, Davejan P, Hendargo KJ, Javadi-Razaz I, Chou A, Yee DC, et al. Expansion of the Major Facilitator Superfamily (MFS) to include novel transporters as well as transmembrane-acting enzymes. Biochim Biophys Acta Biomembr. 2020;1862(9):183277. doi: 10.1016/j.bbamem.2020.183277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagle SC Jr, Anderson RE, Gary ND. Chemically defined medium for the growth of Pasteurella tularensis. J Bacteriol. 1960;79(4):566–71. doi: 10.1128/jb.79.4.566-571.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mc Gann P, Rozak DA, Nikolich MP, Bowden RA, Lindler LE, Wolcott MJ, et al. A novel brain heart infusion broth supports the study of common Francisella tularensis serotypes. J Microbiol Methods. 2010;80(2):164–71. doi: 10.1016/j.mimet.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez SA, Yu J-J, Davis G, Arulanandam BP, Klose KE. Targeted inactivation of Francisella tularensis genes by group II introns. Appl Environ Microbiol. 2008;74(9):2619–26. doi: 10.1128/AEM.02905-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu T, Otonari S, Suzuki J, Uda A, Watanabe K, Watarai M. Expression of Francisella pathogenicity island protein intracellular growth locus E (IglE) in mammalian cells is involved in intracellular trafficking, possibly through microtubule organizing center. Microbiologyopen. 2019;8(4):e00684. doi: 10.1002/mbo3.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura T, Shimizu T, Ikegaya R, Uda A, Watanabe K, Watarai M. Identification of pyrC gene as an immunosuppressive factor in Francisella novicida infection. Front Cell Infect Microbiol. 2022;12:1027424. doi: 10.3389/fcimb.2022.1027424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha SS, Uda A, Watanabe K, Shimizu T, Watarai M. RtxA like protein contributes to infection of Francisella novicida in silkworm and human macrophage THP-1. Microb Pathog. 2018;123:74–81. doi: 10.1016/j.micpath.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 25.Hare RF, Hueffer K. Francisella novicida pathogenicity island encoded proteins were secreted during infection of macrophage-like cells. PLoS One. 2014;9(8):e105773. doi: 10.1371/journal.pone.0105773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degabriel M, Valeva S, Boisset S, Henry T. Pathogenicity and virulence of Francisella tularensis. Virulence. 2023;14(1):2274638. doi: 10.1080/21505594.2023.2274638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong A, Wehrly TD, Child R, Hansen B, Hwang S, Virgin HW, et al. Cytosolic clearance of replication-deficient mutants reveals Francisella tularensis interactions with the autophagic pathway. Autophagy. 2012;8(9):1342–56. doi: 10.4161/auto.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Shimizu T, Uda A, Watanabe K, Watarai M. Soluble lytic transglycosylase SLT of Francisella novicida is involved in intracellular growth and immune suppression. PLoS One. 2019;14(12):e0226778. doi: 10.1371/journal.pone.0226778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajaj L, Lotfi P, Pal R, Ronza A di, Sharma J, Sardiello M. Lysosome biogenesis in health and disease. J Neurochem. 2019;148(5):573–89. doi: 10.1111/jnc.14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral O, Martins M, Oliveira AR, Duarte AJ, Mondragão-Rodrigues I, Macedo MF. The Biology of Lysosomes: From Order to Disorder. Biomedicines. 2023;11(1):213. doi: 10.3390/biomedicines11010213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akaaboune SR, Wang Y. Golgi defect as a major contributor to lysosomal dysfunction. Front Cell Dev Biol. 2024;12:1386149. doi: 10.3389/fcell.2024.1386149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marecic V, Shevchuk O, Link M, Viduka I, Ozanic M, Kostanjsek R, et al. Francisella novicida-Containing Vacuole within Dictyostelium discoideum: Isolation and Proteomic Characterization. Microorganisms. 2024;12(10):1949. doi: 10.3390/microorganisms12101949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7(5):333–40. doi: 10.1038/nrmicro2112 [DOI] [PubMed] [Google Scholar]
- 34.Qi X, Man SM, Malireddi RKS, Karki R, Lupfer C, Gurung P, et al. Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J Exp Med. 2016;213(10):2081–97. doi: 10.1084/jem.20151938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long ME, Lindemann SR, Rasmussen JA, Jones BD, Allen L-AH. Disruption of Francisella tularensis Schu S4 iglI, iglJ, and pdpC genes results in attenuation for growth in human macrophages and in vivo virulence in mice and reveals a unique phenotype for pdpC. Infect Immun. 2013;81(3):850–61. doi: 10.1128/IAI.00822-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, et al. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103(1):141–6. doi: 10.1073/pnas.0504271103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobet E, Letesson J-J, Arnould T. Mitochondria: a target for bacteria. Biochem Pharmacol. 2015;94(3):173–85. doi: 10.1016/j.bcp.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 38.Ramond E, Jamet A, Coureuil M, Charbit A. Pivotal Role of Mitochondria in Macrophage Response to Bacterial Pathogens. Front Immunol. 2019;10:2461. doi: 10.3389/fimmu.2019.02461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ney PA. Mitochondrial autophagy: Origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta. 2015;1853(10 Pt B):2775–83. doi: 10.1016/j.bbamcr.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 40.Gkikas I, Palikaras K, Tavernarakis N. The Role of Mitophagy in Innate Immunity. Front Immunol. 2018;9:1283. doi: 10.3389/fimmu.2018.01283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16(1):3–17. doi: 10.1080/15548627.2019.1603547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jessop F, Schwarz B, Heitmann E, Buntyn R, Wehrly T, Bosio CM. Temporal Manipulation of Mitochondrial Function by Virulent Francisella tularensis To Limit Inflammation and Control Cell Death. Infect Immun. 2018;86(8):e00044-18. doi: 10.1128/IAI.00044-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCracken JM, Kinkead LC, McCaffrey RL, Allen L-AH. Francisella tularensis Modulates a Distinct Subset of Regulatory Factors and Sustains Mitochondrial Integrity to Impair Human Neutrophil Apoptosis. J Innate Immun. 2016;8(3):299–313. doi: 10.1159/000443882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spier A, Stavru F, Cossart P. Interaction between Intracellular Bacterial Pathogens and Host Cell Mitochondria. Microbiol Spectr. 2019;7(2):10.1128/microbiolspec.bai-0016–2019. doi: 10.1128/microbiolspec.BAI-0016-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-González J, Gutiérrez-Kobeh L. Apoptosis and its pathways as targets for intracellular pathogens to persist in cells. Parasitol Res. 2023;123(1):60. doi: 10.1007/s00436-023-08031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casey CA, Thomes P, Manca S, Petrosyan A. Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells. Biomolecules. 2018;8(4):150. doi: 10.3390/biom8040150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi W, Kang S, Kim J. New insights into the role of the Golgi apparatus in the pathogenesis and therapeutics of human diseases. Arch Pharm Res. 2022;45(10):671–92. doi: 10.1007/s12272-022-01408-z [DOI] [PubMed] [Google Scholar]
- 48.Aistleitner K, Clark T, Dooley C, Hackstadt T. Selective fragmentation of the trans-Golgi apparatus by Rickettsia rickettsii. PLoS Pathog. 2020;16(5):e1008582. doi: 10.1371/journal.ppat.1008582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregory DJ, DeLoid GM, Salmon SL, Metzger DW, Kramnik I, Kobzik L. SON DNA-binding protein mediates macrophage autophagy and responses to intracellular infection. FEBS Lett. 2020;594(17):2782–99. doi: 10.1002/1873-3468.13851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Yao Q, Li J, Yang H, Qian R, Zheng M, et al. Inhibition of neutrophil swarming by type I interferon promotes intracellular bacterial evasion. Nat Commun. 2024;15(1):8663. doi: 10.1038/s41467-024-53060-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan JMJ, Garner ME, Regeimbal JM, Greene CJ, Márquez JDR, Ammendolia DA, et al. Listeria exploits IFITM3 to suppress antibacterial activity in phagocytes. Nat Commun. 2021;12(1):4999. doi: 10.1038/s41467-021-24982-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anes E, Azevedo-Pereira JM, Pires D. Role of Type I Interferons during Mycobacterium tuberculosis and HIV Infections. Biomolecules. 2024;14(7):848. doi: 10.3390/biom14070848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia A, Li X, Zhao C, Meng X, Kari G, Wang Y. For Better or Worse: Type I Interferon Responses in Bacterial Infection. Pathogens. 2025;14(3):229. doi: 10.3390/pathogens14030229 [DOI] [PMC free article] [PubMed] [Google Scholar]