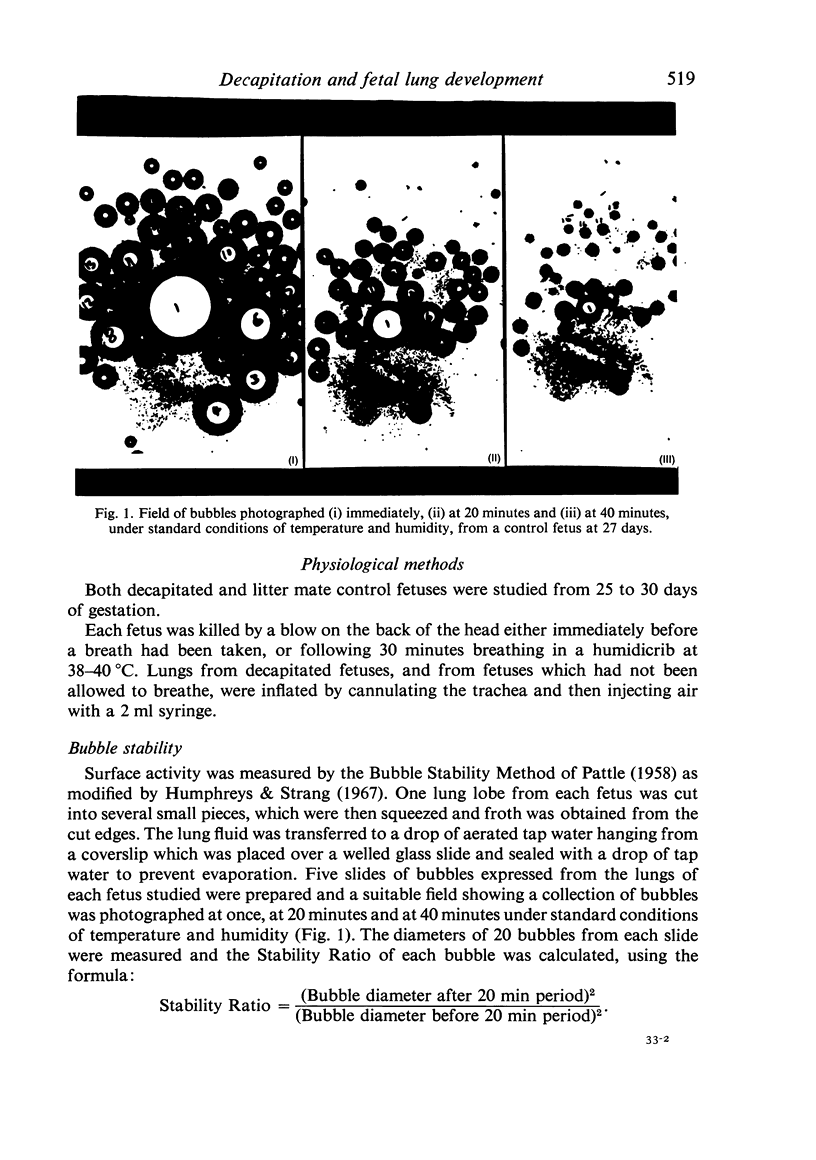
Abstract
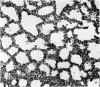
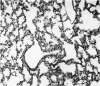
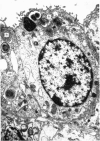
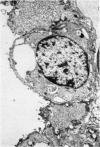
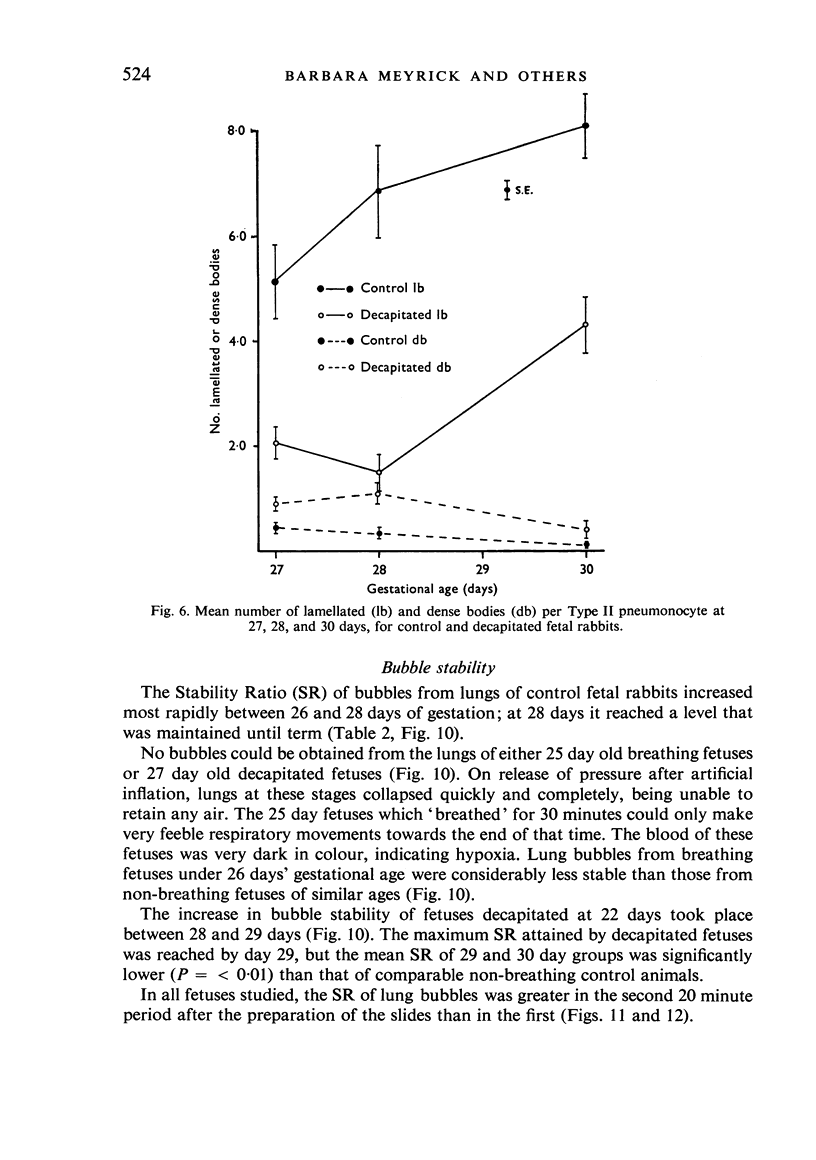
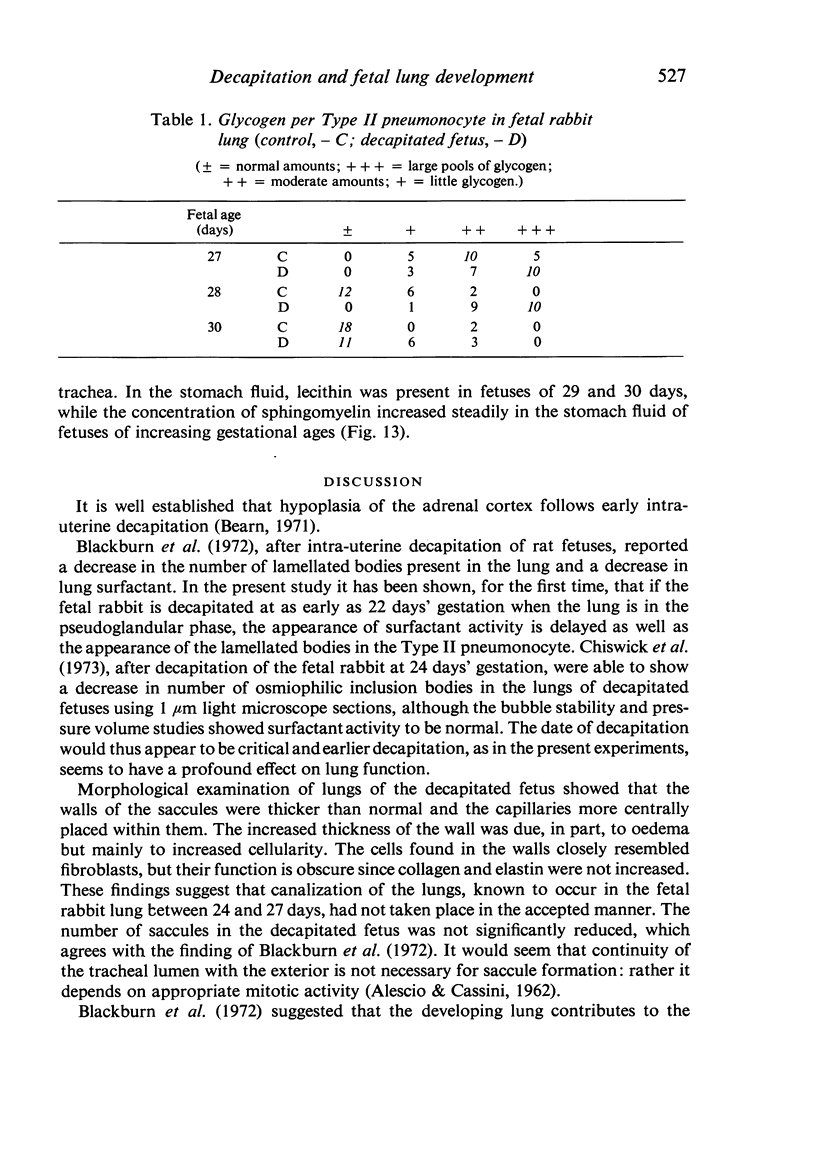
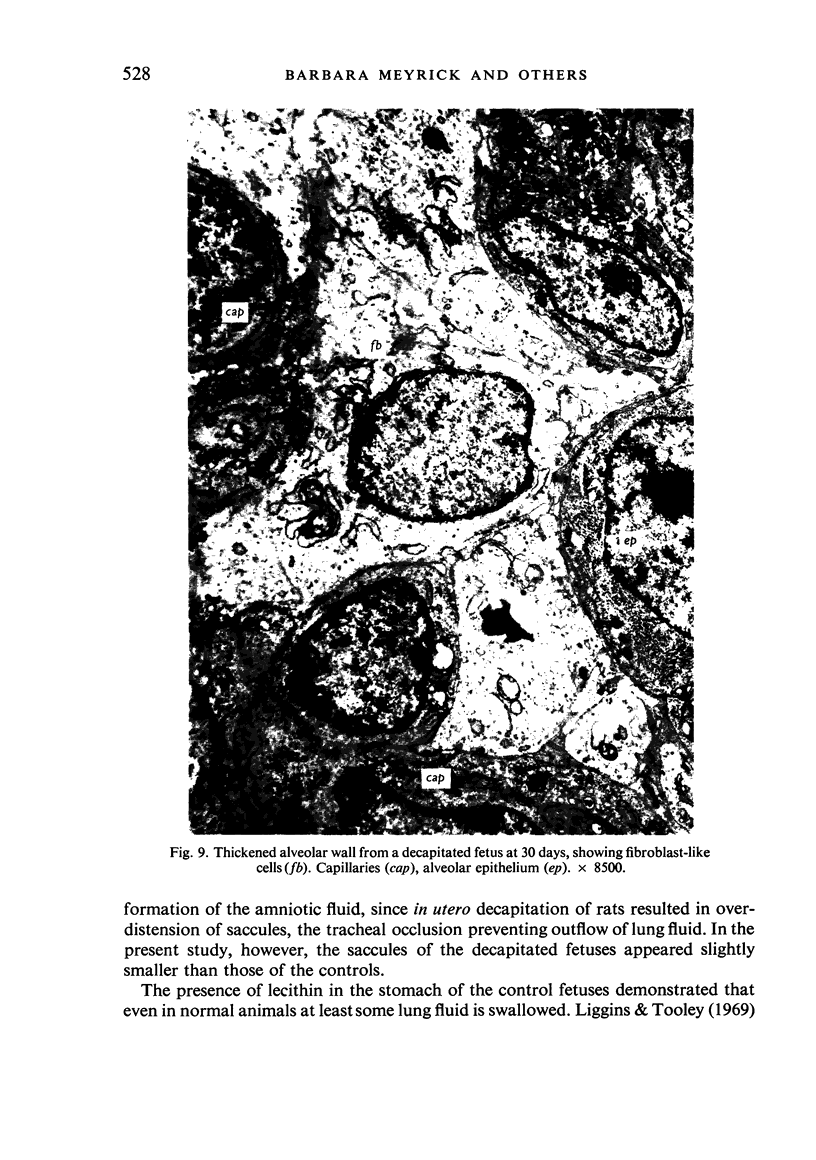
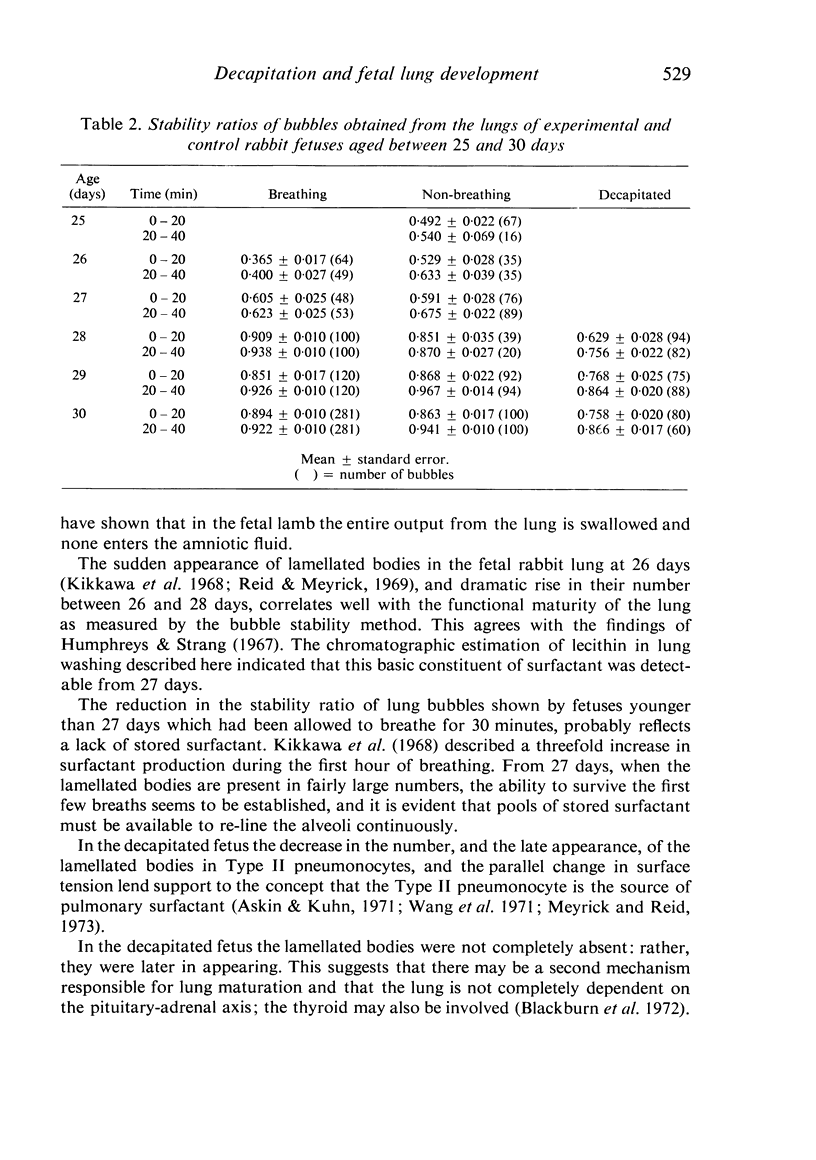
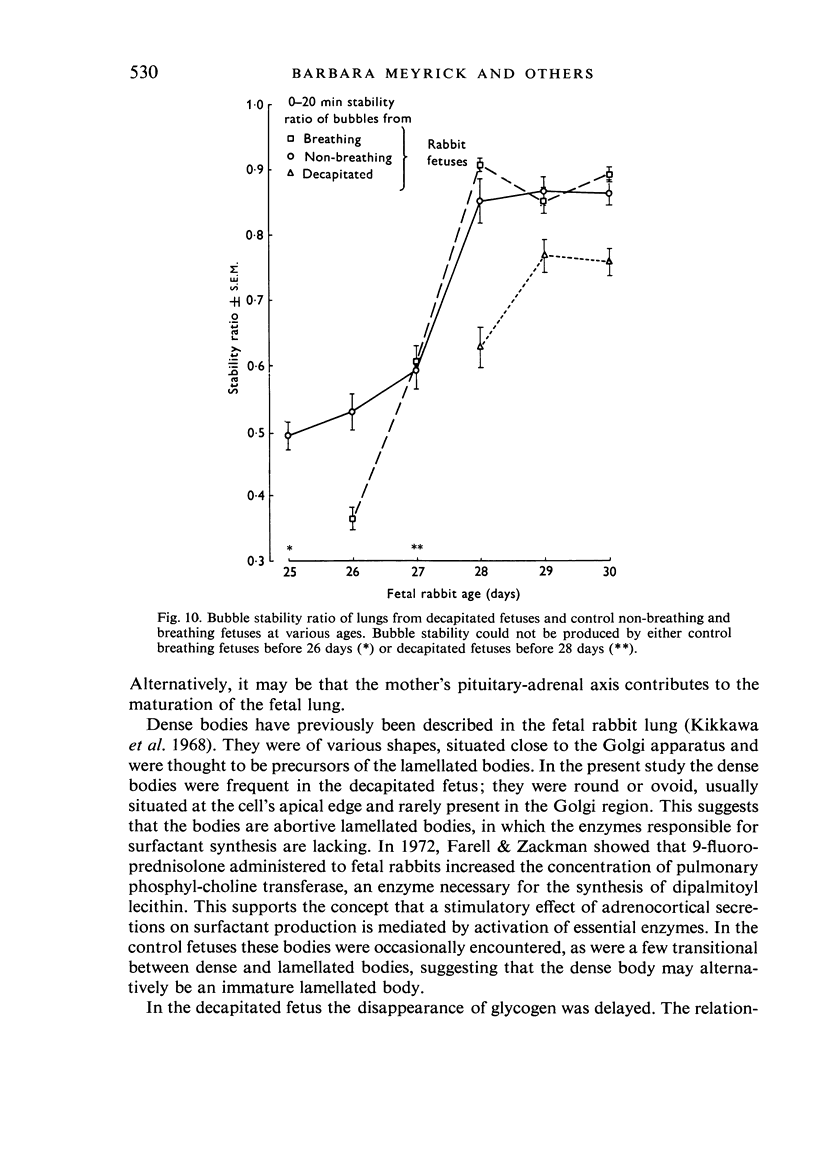
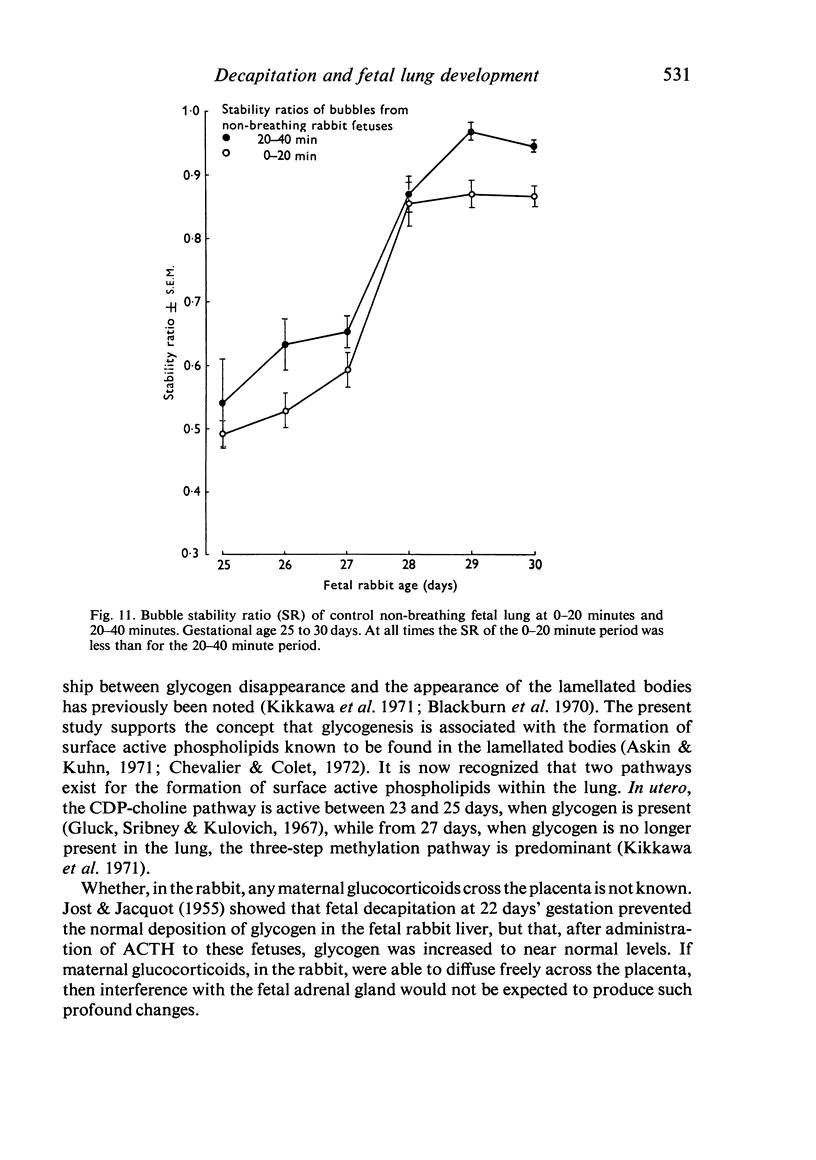
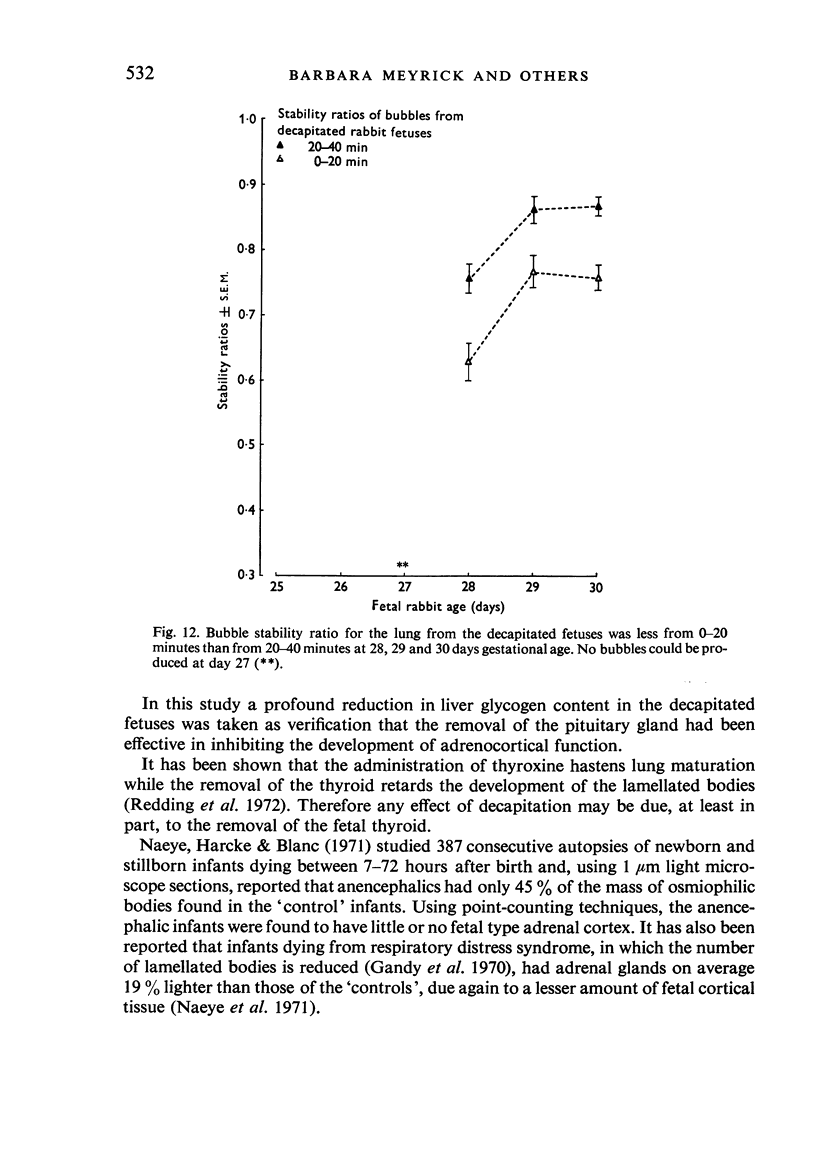
A study has been made of the consequences of in utero decapitation on the morphological and physiological development of the fetal lung. Fetal rabbits were decapitated in situ at 22 days, without losing any amniotic fluid, and allowed to continue their development with their undamaged littermates as controls. Such decapitation, of course, removes the pituitary and so interferes with adrenal cortical development. Morphological studies showed an interference with lung development in that, although the number of alveolar saccules increased normally, their walls failed to thin. In the decapitated fetuses, a reduction in the number of lamellated bodies per Type II pneumonocyte was found at each age studied; while dense, homogeneous bodies were more numerous. The normal disappearance of glycogen in the Type II pneumonocytes of the decapitated fetuses was retarded. Physiological studies supported these findings. In control fetuses allowed to breathe for a while the Bubble Stability Ratio increased rapidly from day 26 to reach a maximum at 28 days; whereas, in the decapitated ones, bubble stability was not apparent before day 28 and by the 29th day had reached a maximum which was lower than that of the controls. In the control fetuses, lecithin was detected in lung fluid from 26 days on, and in stomach fluid from 29 days. It is argued that lung development must be, at least in part, under the control of the fetus' own pituitary-adrenal axis.
Full text
PDF




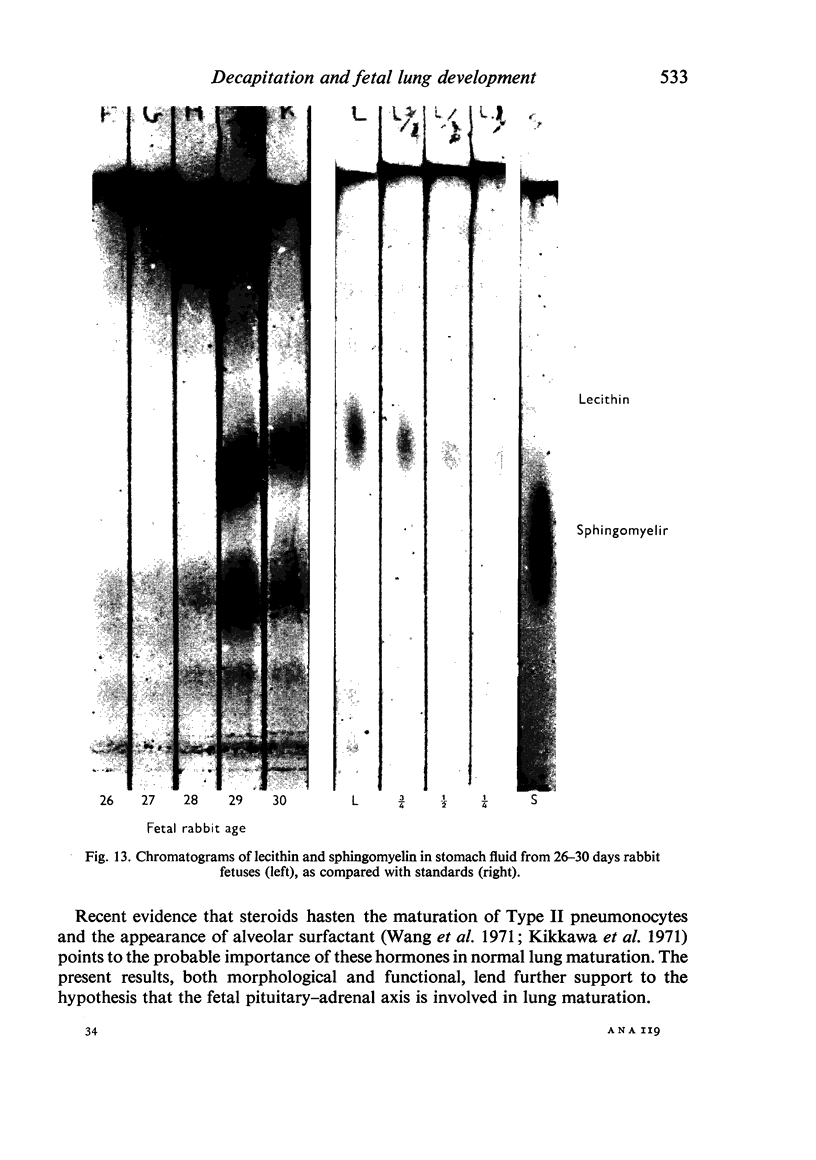


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALESCIO T., CASSINI A. Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J Exp Zool. 1962 Jul;150:83–94. doi: 10.1002/jez.1401500202. [DOI] [PubMed] [Google Scholar]
- Askin F. B., Kuhn C. The cellular origin of pulmonary surfactant. Lab Invest. 1971 Sep;25(3):260–268. [PubMed] [Google Scholar]
- BEARN J. G., PILKINGTON T. R. Hormonal control of the metabolism of cholesterol in the rabbit foetus. Nature. 1963 Jun 8;198:1005–1006. doi: 10.1038/1981005a0. [DOI] [PubMed] [Google Scholar]
- Blackburn W. R., Travers H., Potter D. M. The role of the pituitary-adrenal-thyroid axes in lung differentiation. I. Studies of the cytology and physical properties of anencephalic fetal rat lung. Lab Invest. 1972 Mar;26(3):306–318. [PubMed] [Google Scholar]
- Chevalier G., Collet A. J. In vivo incorporation of choline- 3 H, leucine- 3 H and galactose- 3 H in alveolar type II pneumocytes in relation to surfactant synthesis. A quantitative radoautographic study in mouse by electron microscopy. Anat Rec. 1972 Nov;174(3):289–310. doi: 10.1002/ar.1091740303. [DOI] [PubMed] [Google Scholar]
- Chiswick M. L., Ahmed A., Jack P. M., Milner R. D. Control of fetal lung development in the rabbit. Arch Dis Child. 1973 Sep;48(9):709–713. doi: 10.1136/adc.48.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy G., Jacobson W., Gairdner D. Hyaline membrane disease. I. Cellular changes. Arch Dis Child. 1970 Jun;45(241):289–310. doi: 10.1136/adc.45.241.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck L., Kulovich M. V., Borer R. C., Jr, Brenner P. H., Anderson G. G., Spellacy W. N. Diagnosis of the respiratory distress syndrome by amniocentesis. Am J Obstet Gynecol. 1971 Feb 1;109(3):440–445. doi: 10.1016/0002-9378(71)90342-5. [DOI] [PubMed] [Google Scholar]
- Gluck L., Sribney M., Kulovich M. V. The biochemical development of surface activity in mammalian lung. II. The biosynthesis of phospholipids in the lung of the developing rabbit fetus and newborn. Pediatr Res. 1967 Jul;1(4):247–265. doi: 10.1203/00006450-196707000-00002. [DOI] [PubMed] [Google Scholar]
- Humphreys P. W., Strang L. B. Effects of gestation and prenatal asphyxia on pulmonary surface properties of the foetal rabbit. J Physiol. 1967 Sep;192(1):53–62. doi: 10.1113/jphysiol.1967.sp008287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOST A., JACQUOT R. Recherches sur les facteurs endocriniens de la charge en glycotène du foie foetal chez le lapin (avec des indications sur le glycogène placentaire). Ann Endocrinol (Paris) 1955;16(6):849–872. [PubMed] [Google Scholar]
- Kikkawa Y., Kaibara M., Motoyama E. K., Orzalesi M. M., Cook C. D. Morphologic development of fetal rabbit lung and its acceleration with cortisol. Am J Pathol. 1971 Aug;64(2):423–442. [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Motoyama E. K., Gluck L. Study of the lungs of fetal and newborn rabbits. Morphologic, biochemical, and surface physical development. Am J Pathol. 1968 Jan;52(1):177–210. [PMC free article] [PubMed] [Google Scholar]
- MEAD J., WHITTENBERGER J. L., RADFORD E. P., Jr Surface tension as a factor in pulmonary volume-pressure hysteresis. J Appl Physiol. 1957 Mar;10(2):191–196. doi: 10.1152/jappl.1957.10.2.191. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Electron microscopic aspects of surfactant secretion. Proc R Soc Med. 1973 Apr;66(4):386–387. [PMC free article] [PubMed] [Google Scholar]
- Naeye R. L., Harcke H. T., Jr, Blanc W. A. Adrenal gland structure and the development of hyaline membrane disease. Pediatrics. 1971 Apr;47(4):650–657. [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function, and origin of the alveolar lining layer. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):217–240. doi: 10.1098/rspb.1958.0015. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding R. A., Douglas W. H., Stein M. Thyroid hormone influence upon lung surfactant metabolism. Science. 1972 Mar 3;175(4025):994–996. doi: 10.1126/science.175.4025.994. [DOI] [PubMed] [Google Scholar]
- Reid L., Meyrick B. Etude au microscope électronique du poumon foetal de lapin. Poumon Coeur. 1969;25(3):201–206. [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Kotas R. V., Avery M. E., Thurlbeck W. M. Accelerated appearance of osmiophilic bodies in fetal lungs following steroid injection. J Appl Physiol. 1971 Mar;30(3):362–365. doi: 10.1152/jappl.1971.30.3.362. [DOI] [PubMed] [Google Scholar]