Abstract
Corticobulbar projections have been studied in the American opossum by both degeneration and autoradiographic methods and, for the most part, the results confirm our earlier observations (Martin & West, 1967; Martin, 1968). However, we have obtained evidence for certain connexions not previously described and have delineated the origin(s) of several connexions more precisely by paying particular attention to the degeneration present at thalamic levels in all cases and by the use of autoradiography. When our results are collated and correlated with new somatosensory cortical maps arrived at by microelectrode techniques (Pubols et al. 1975), it is obvious that corticolbulbar connexions in the North American opossum are remarkably similar to those in the monkey and differ mainly in quantity, relative origins and distribution and in the fact that some of them arise from spatially co-extensive motor-sensory areas (Lende, 1963a, b). In the light of our findings on the American opossum we have examined a large collection of brush-tailed possum material (as well as some from the potoroo and Tasmanian native cat) and have been able to extend our previous findings (Martin et al. 1971; Martin & Megirian, 1972) to a more precise evaluation of the origin of projections from the limb, face motor-sensory cortex. Differences between these representatives of the marsupial radiation, as well as features which are common to all, are described.
Full text
PDF

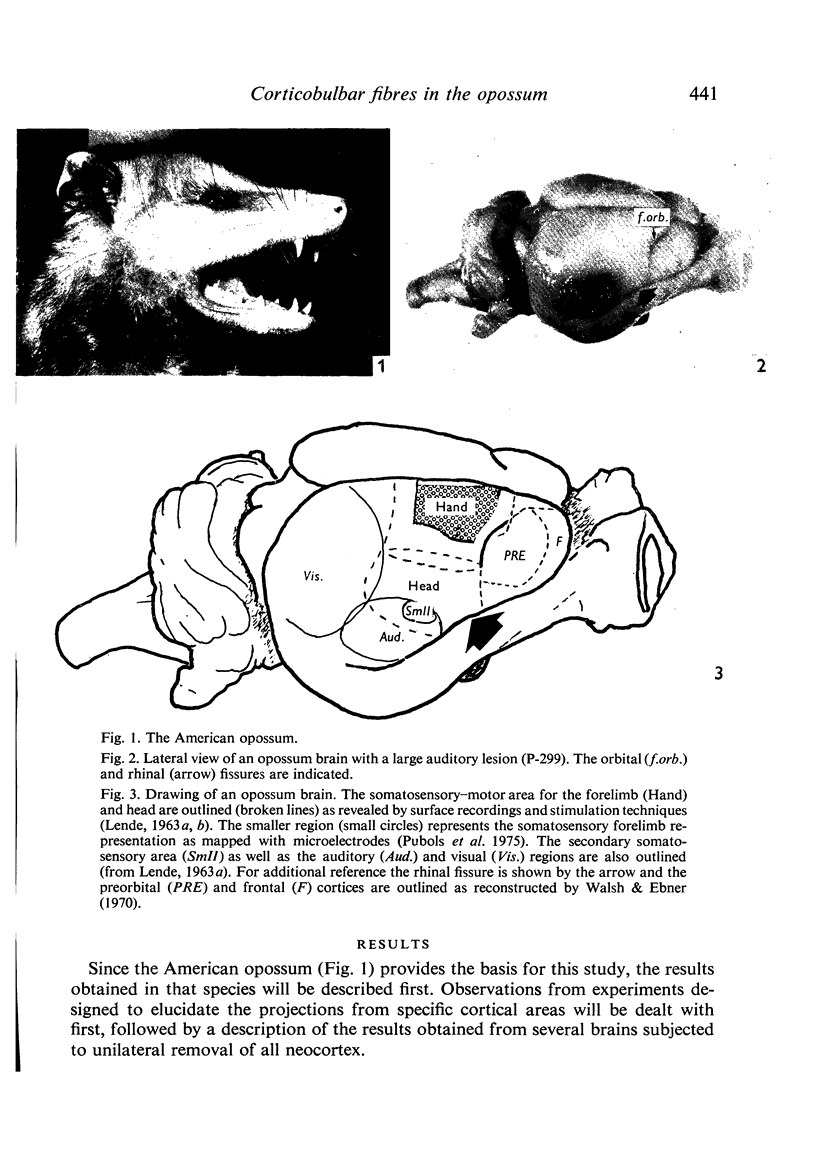
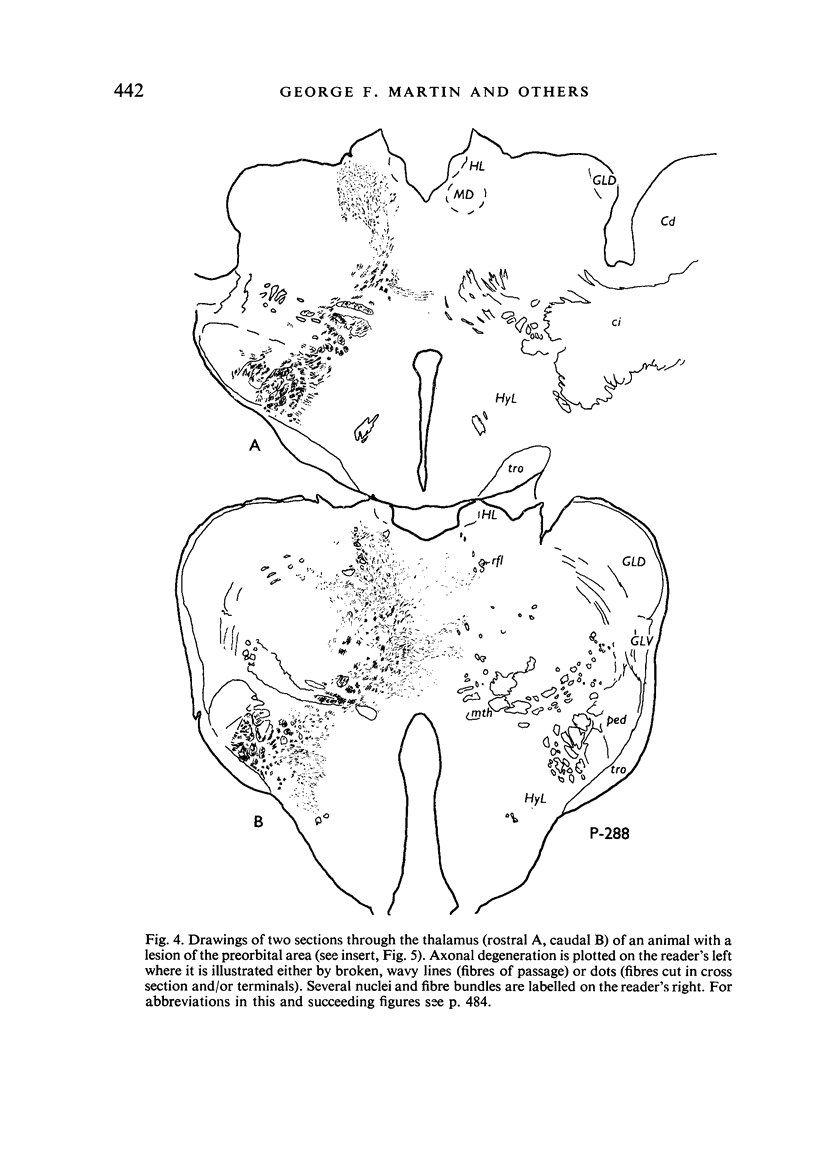
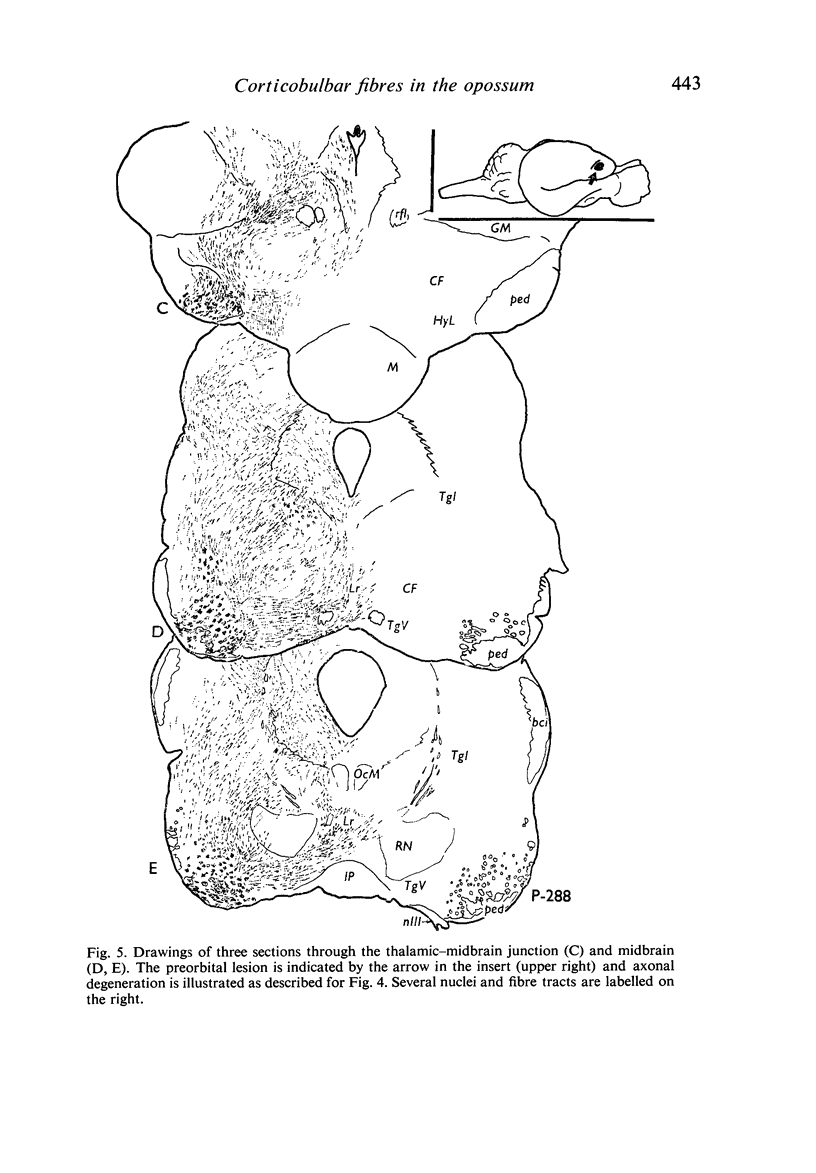



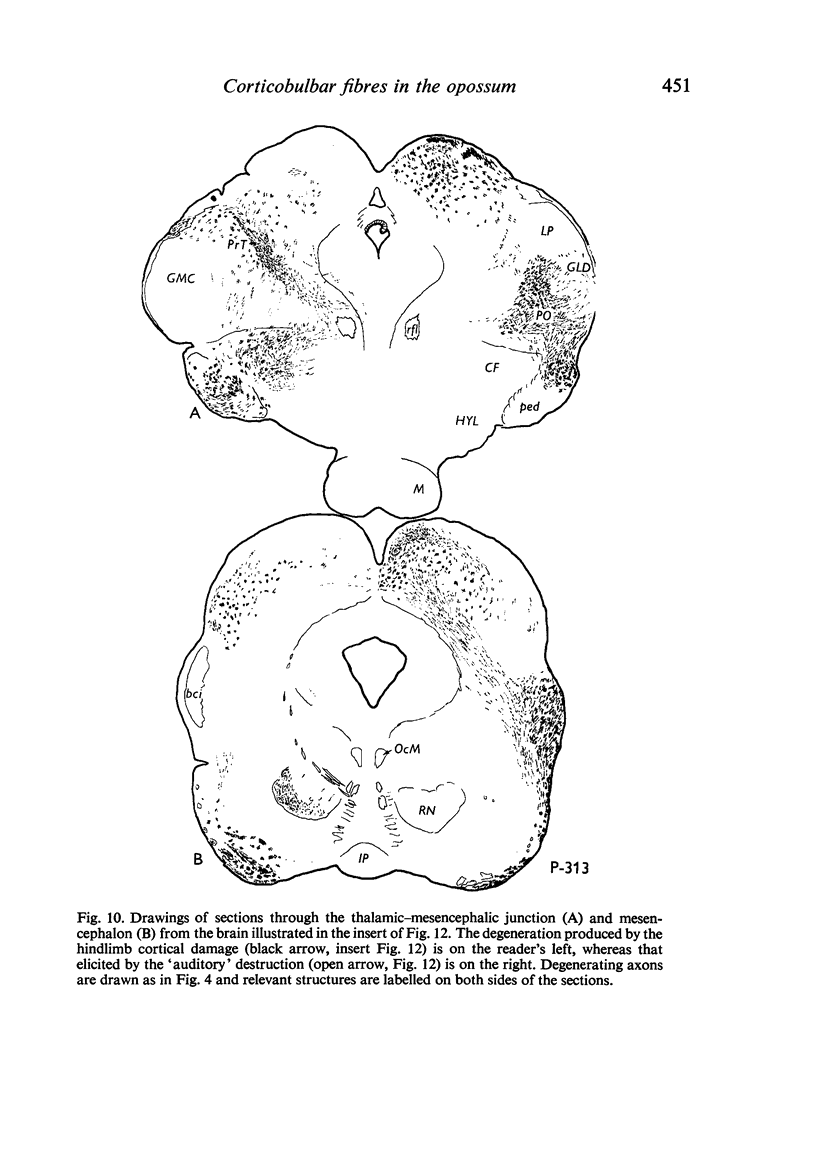
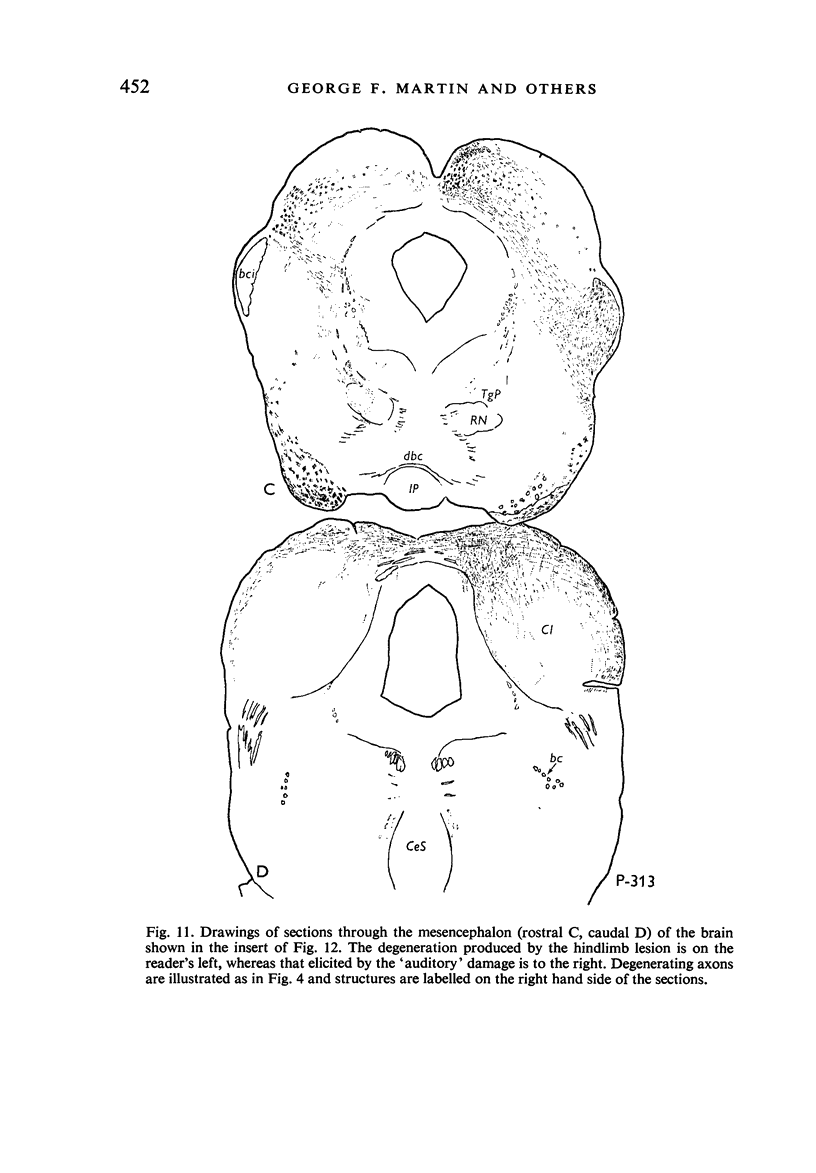
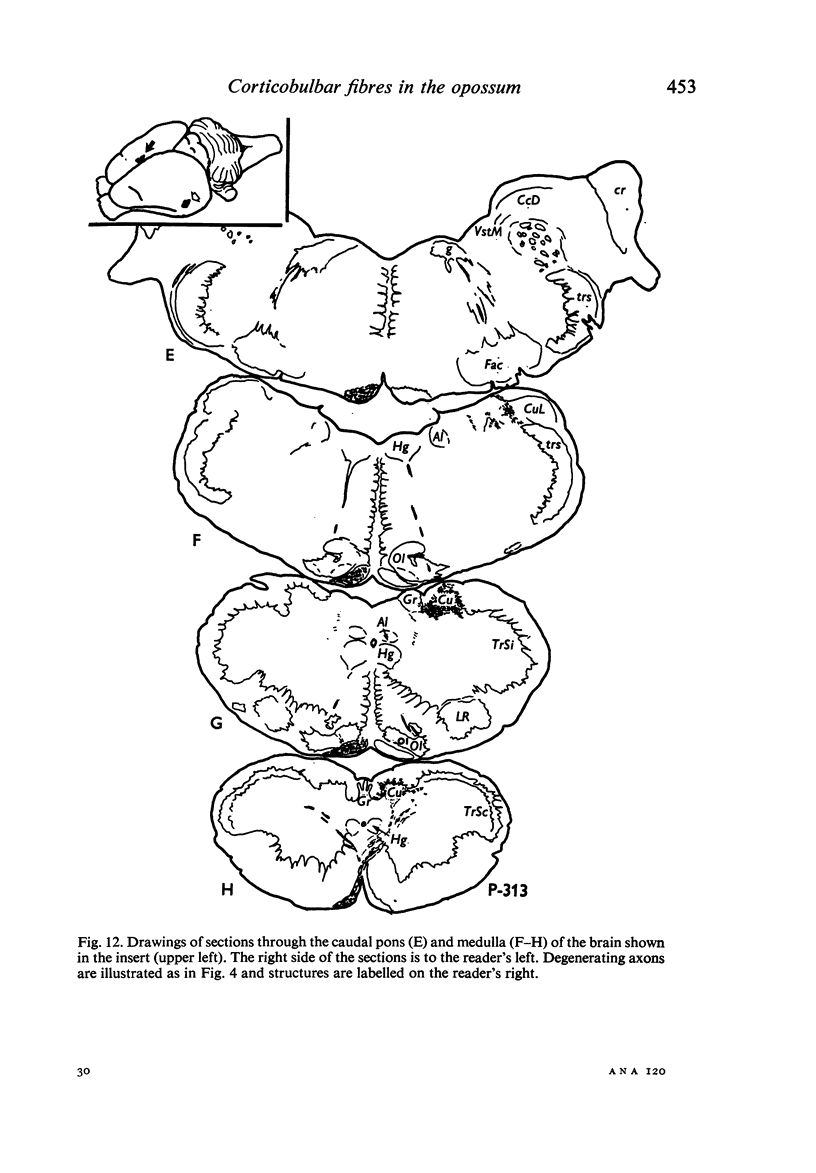

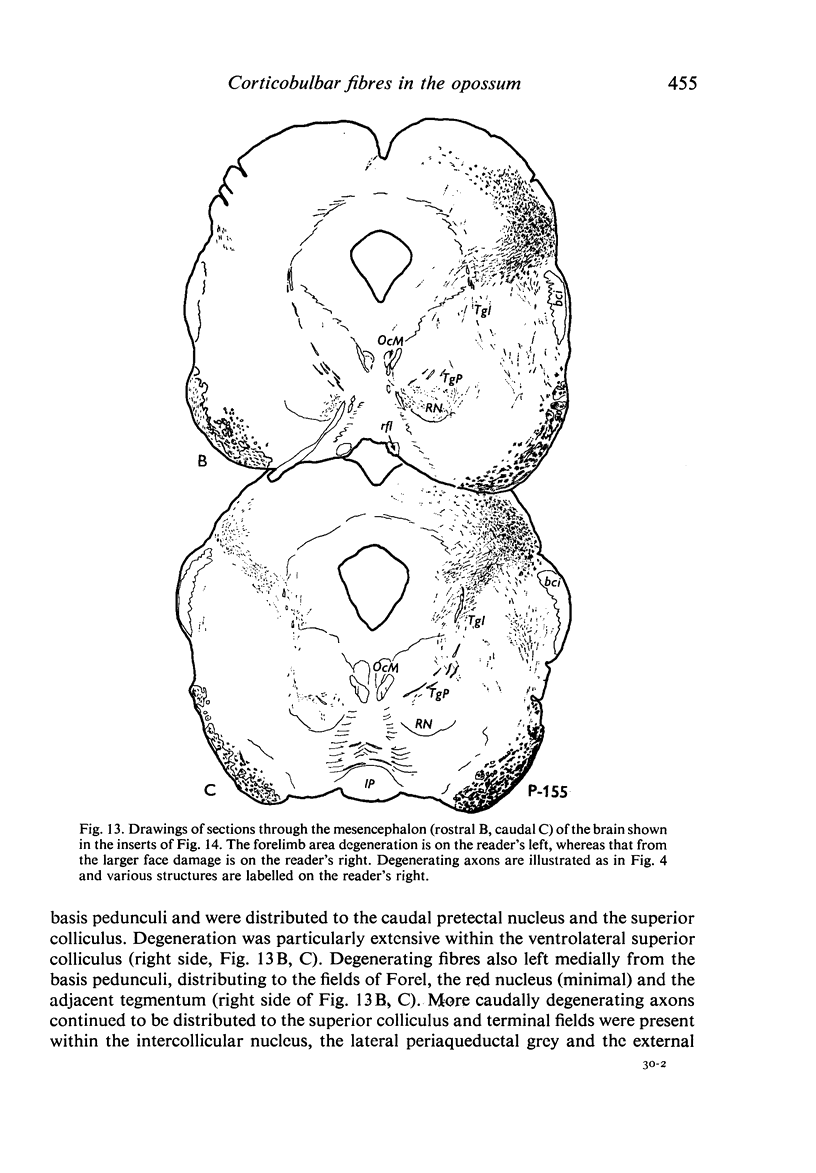
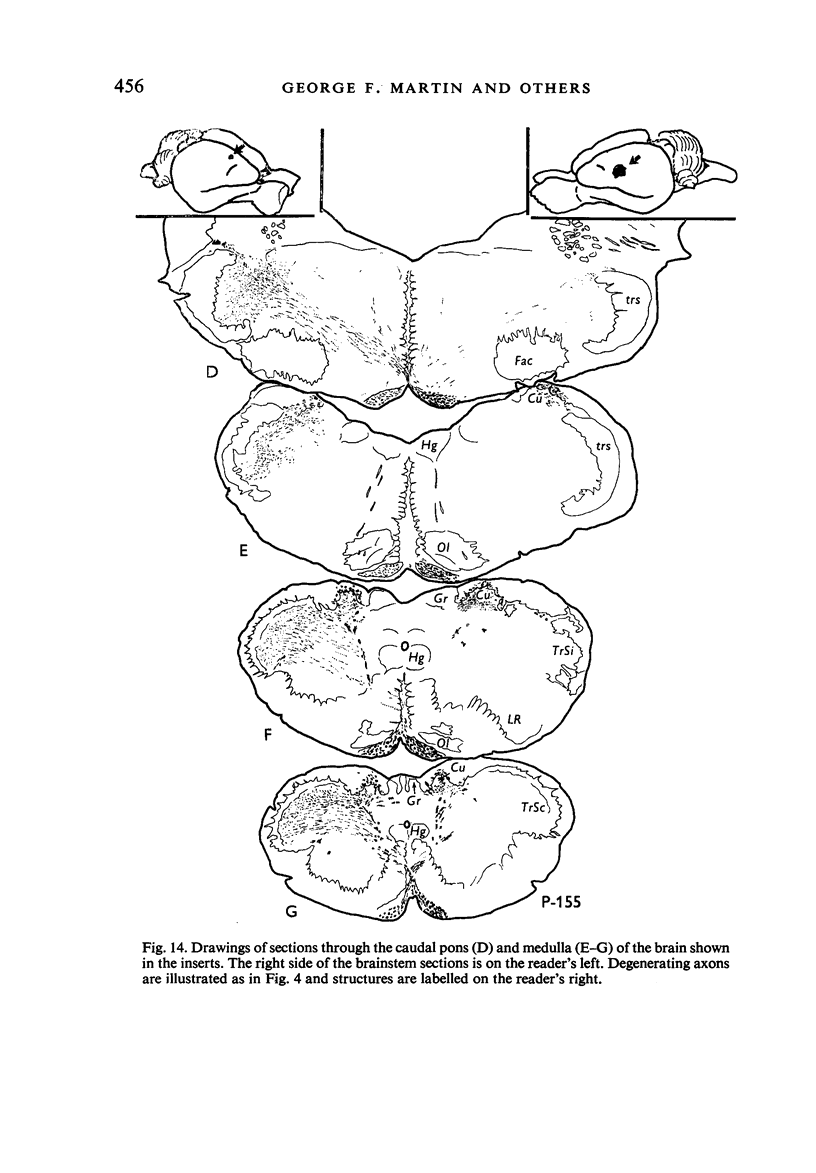

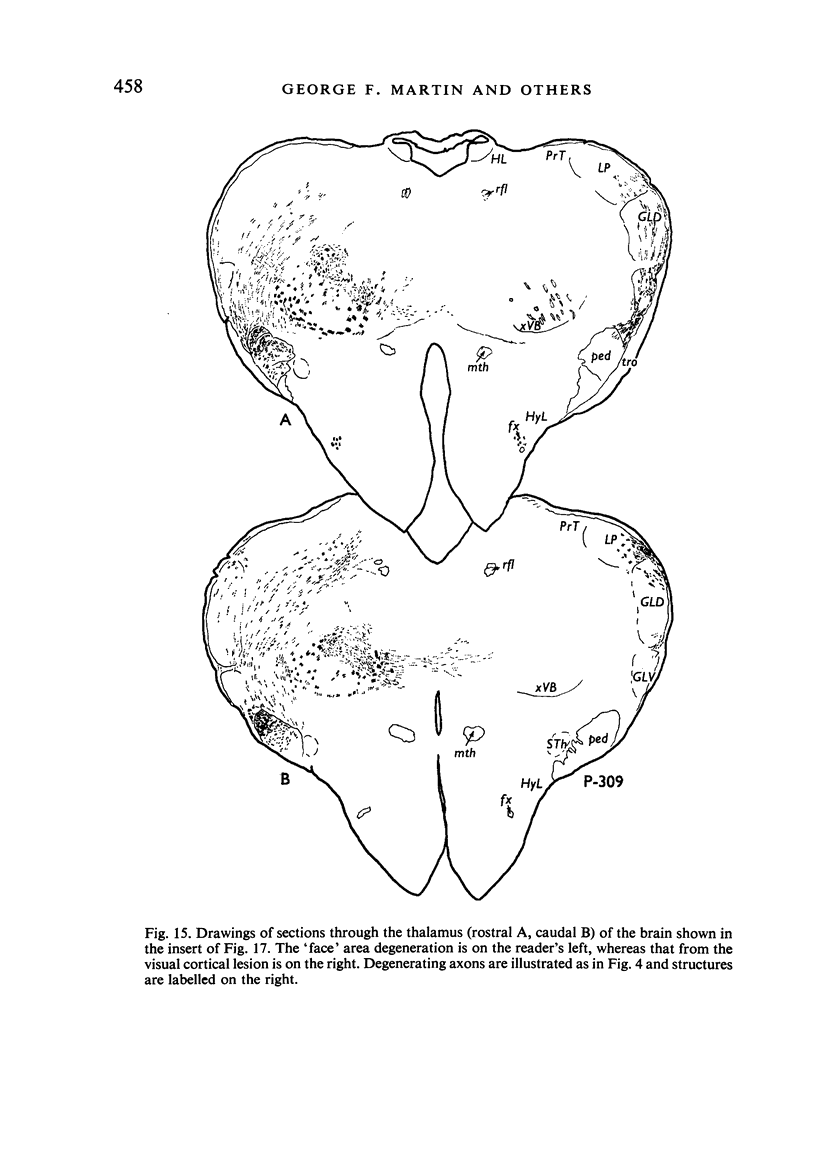
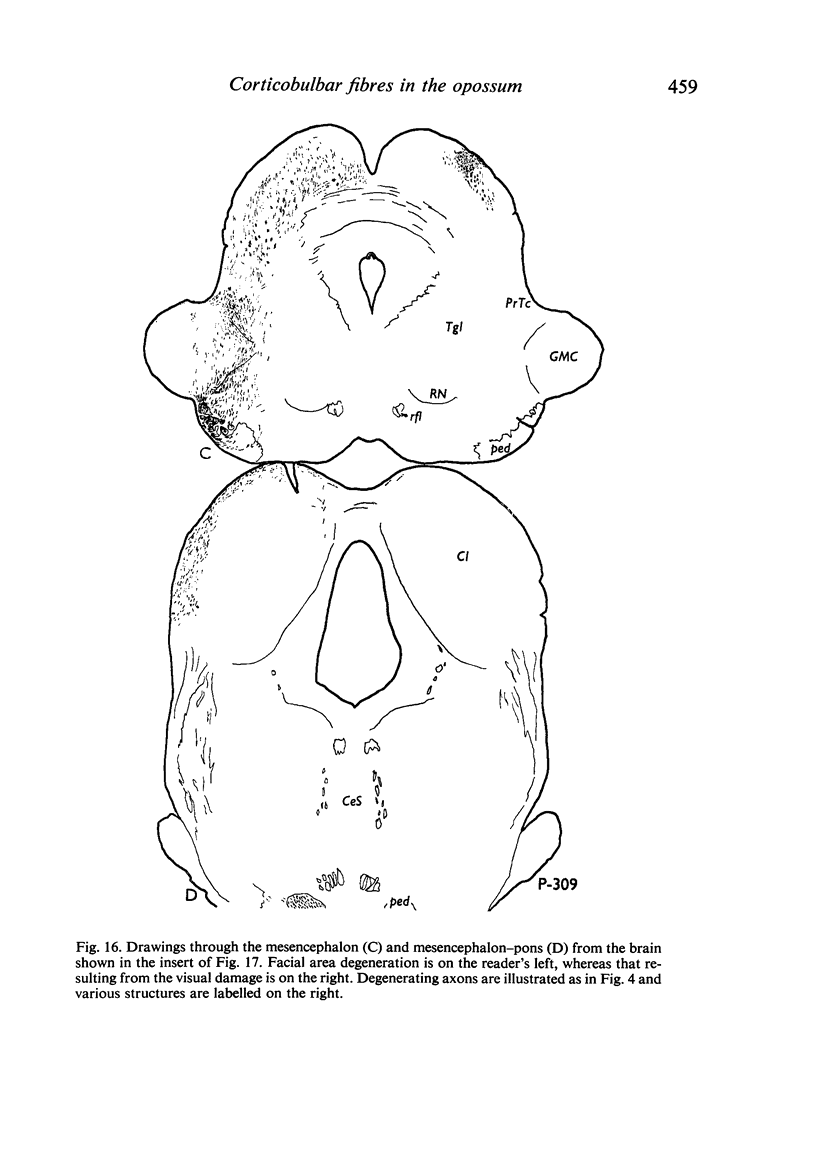
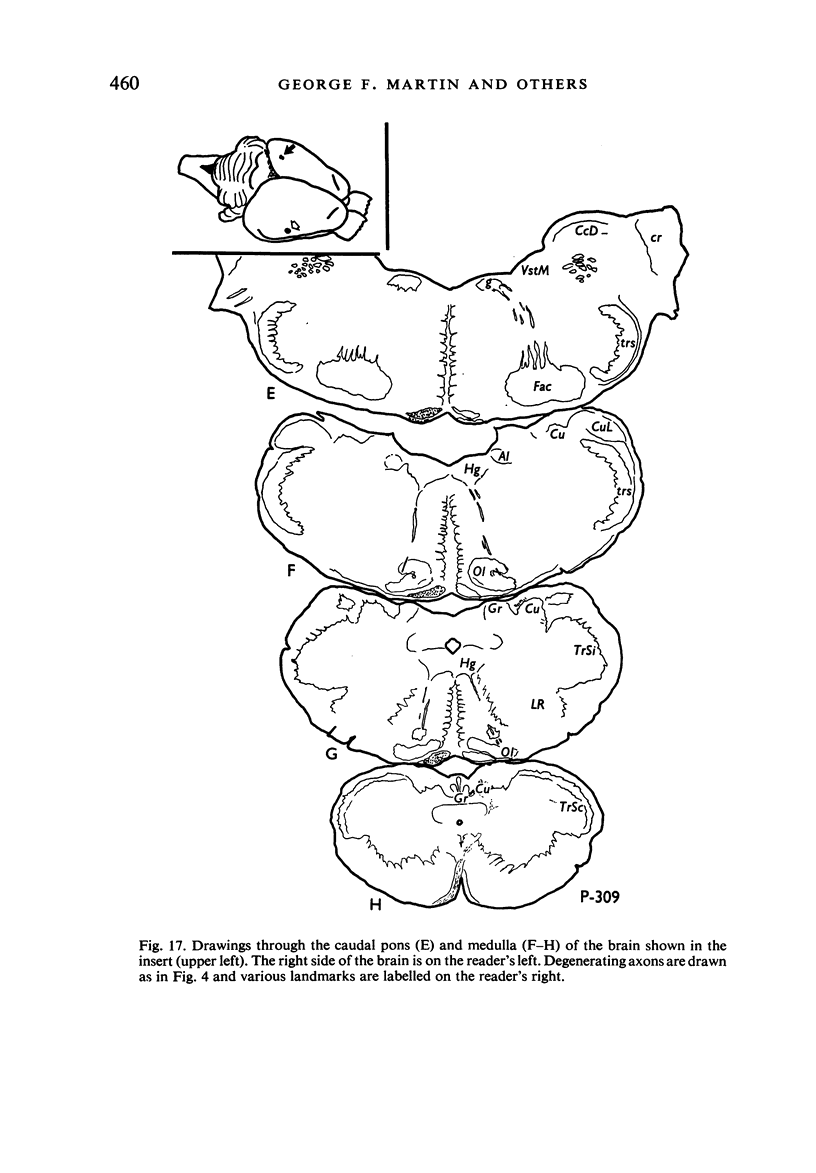




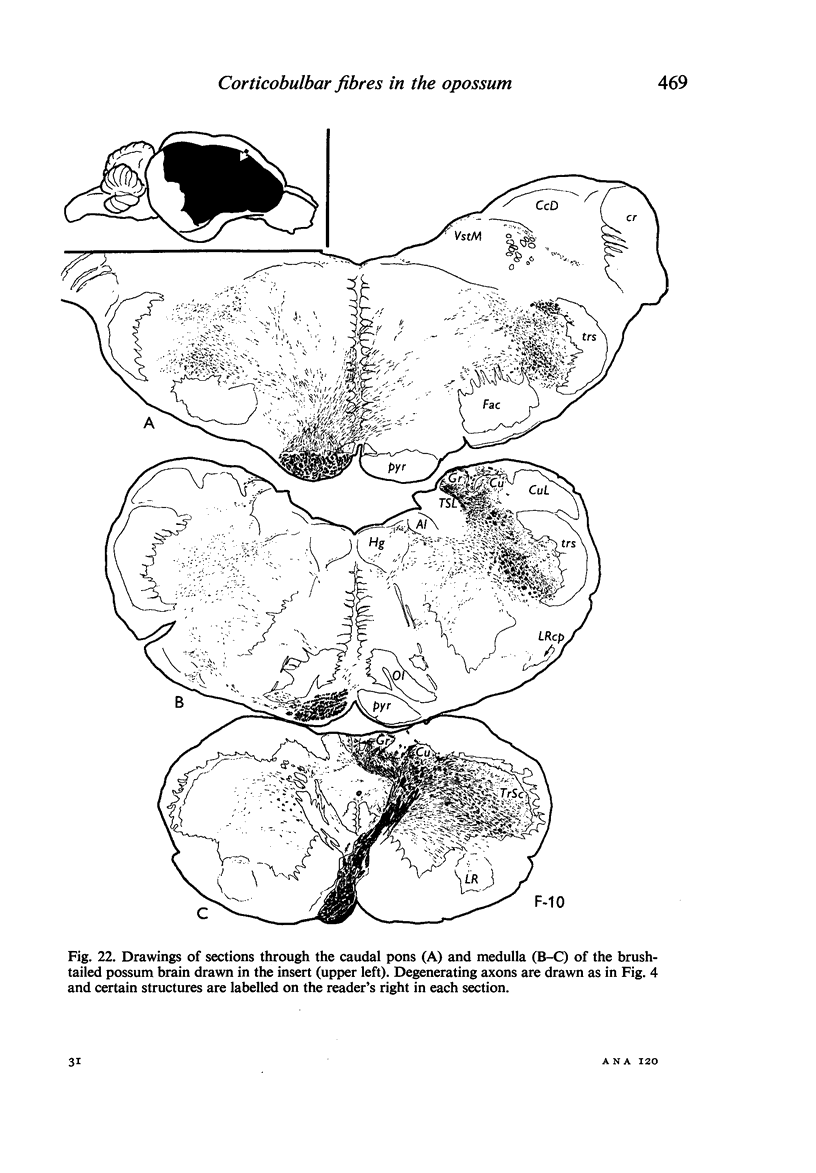
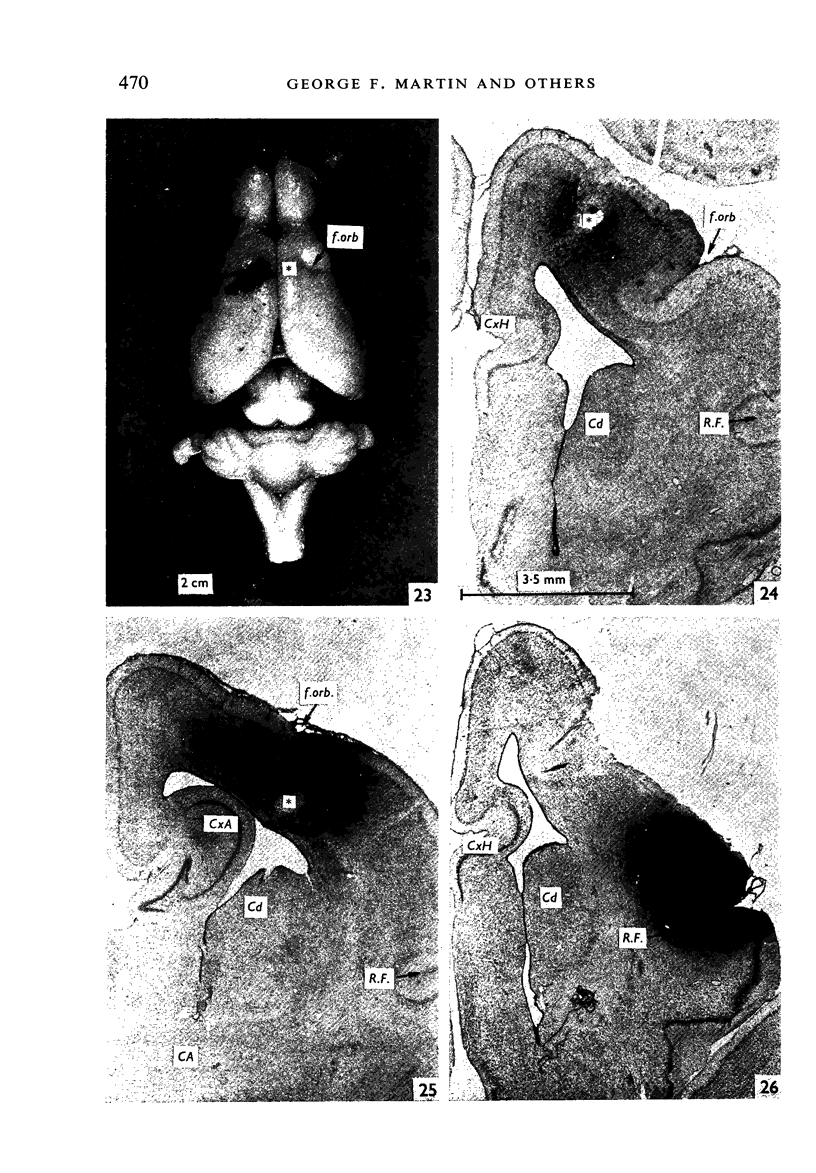

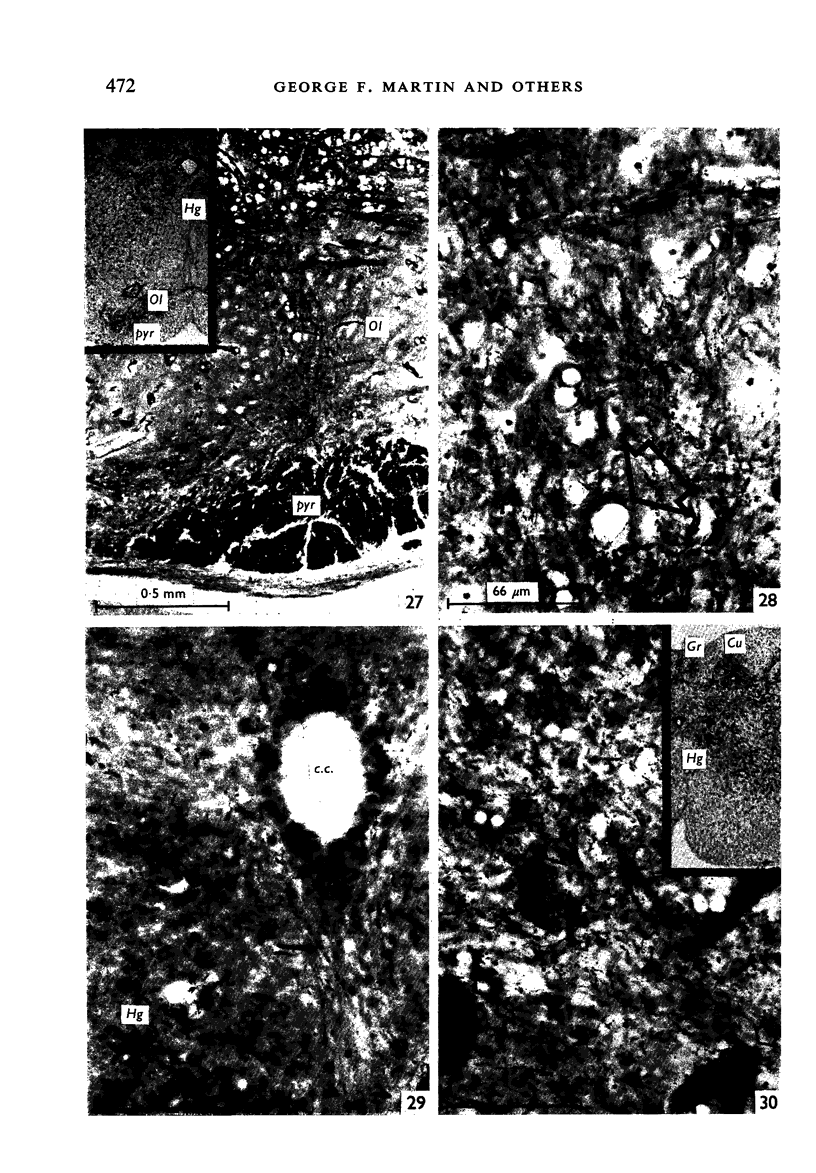


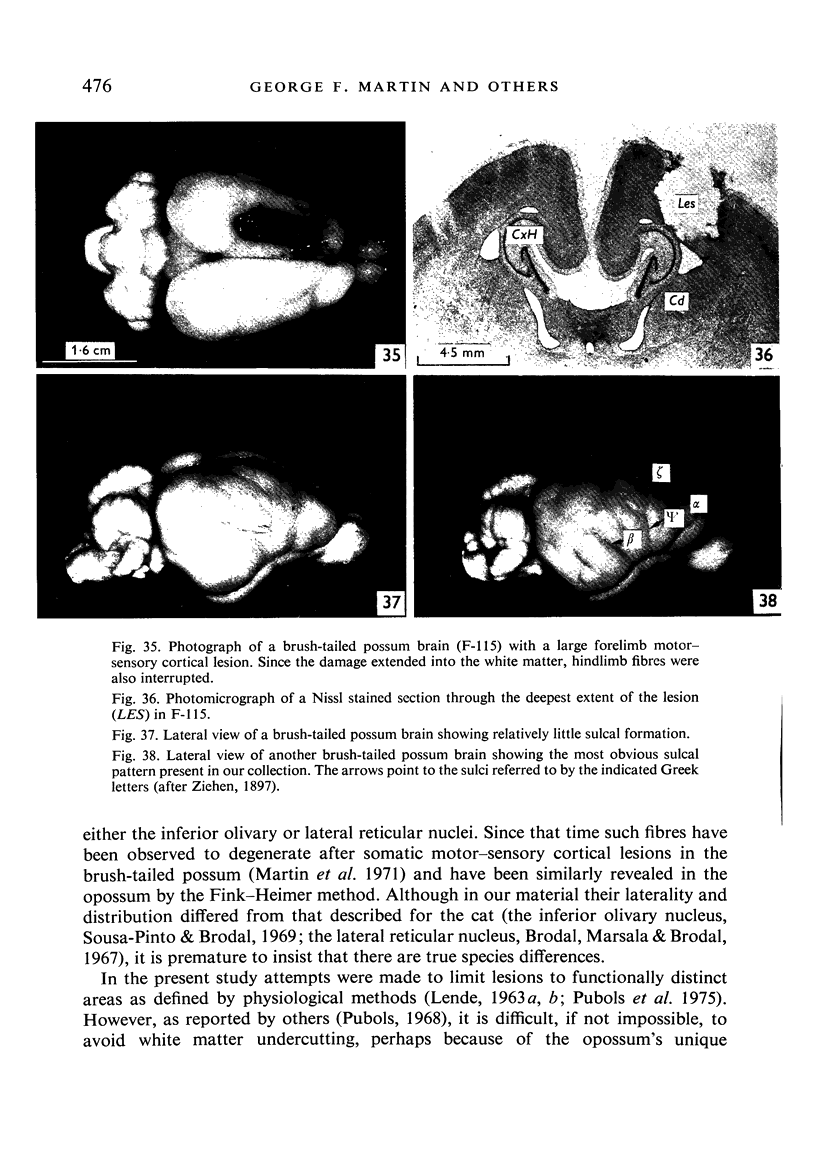








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADEY W. R., KERR D. I. The cerebral representation of deep somatic sensibility in the marsupial phalanger and the rabbit; an evoked potential and histological study. J Comp Neurol. 1954 Jun;100(3):597–624. doi: 10.1002/cne.901000307. [DOI] [PubMed] [Google Scholar]
- Bautista N. S., Matzke H. A. A degeneration study of the course and extent of the pyramidal tract of the opossum. J Comp Neurol. 1965 Jun;124(3):367–375. doi: 10.1002/cne.901240307. [DOI] [PubMed] [Google Scholar]
- Beran R. L., Martin G. F. Reticulospinal fibers of the opossum, Didelphis virginiana. I. Origin. J Comp Neurol. 1971 Apr;141(4):453–465. doi: 10.1002/cne.901410404. [DOI] [PubMed] [Google Scholar]
- Bowman J. P., Sladek J. R., Jr Morphology of the inferior olivary complex of the rhesus monkey (Macaca mulatta). J Comp Neurol. 1973 Dec 1;152(3):299–316. doi: 10.1002/cne.901520306. [DOI] [PubMed] [Google Scholar]
- Brodal A., Destombes J., Lacerda A. M., Angaut P. A cerebellar projection onto the pontine nuclei. An experimental antaomical study in the cat. Exp Brain Res. 1972;16(2):115–139. doi: 10.1007/BF00233993. [DOI] [PubMed] [Google Scholar]
- Brodal P., Marsala J., Brodal A. The cerebral cortical projection to the lateral reticular nucleus in the cat, with special reference to the sensorimotor cortical areas. Brain Res. 1967 Oct;6(2):252–274. doi: 10.1016/0006-8993(67)90195-3. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Gottlieb D. I., Hendrickson A. E., Price J. L., Woolsey T. A. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res. 1972 Feb 11;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Dom R., Falls W., Martin G. F. The motor nucleus of the facial nerve in the opossum (Didelphis marsupialis virginiana). Its organization and connections. J Comp Neurol. 1973 Dec 15;152(4):373–401. doi: 10.1002/cne.901520405. [DOI] [PubMed] [Google Scholar]
- Dom R., King S., Martin G. F. Evidence for two direct cerebello-olivary connections. Brain Res. 1973 Jul 27;57(2):498–501. doi: 10.1016/0006-8993(73)90156-x. [DOI] [PubMed] [Google Scholar]
- ERICKSON R. P., JANE J. A., WAITE R., DIAMOND I. T. SINGLE NEURON INVESTIGATION OF SENSORY THALAMUS OF THE OPOSSUM. J Neurophysiol. 1964 Nov;27:1026–1047. doi: 10.1152/jn.1964.27.6.1026. [DOI] [PubMed] [Google Scholar]
- Edwards S. B. The ascending and descending projections of the red nucleus in the cat: an experimental study using an autoradiographic tracing method. Brain Res. 1972 Dec 24;48:45–63. doi: 10.1016/0006-8993(72)90170-9. [DOI] [PubMed] [Google Scholar]
- Fink R. P., Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967 Apr;4(4):369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Nauta H. J., Lasek R. J., Nauta W. J. A cerebello-olivary pathway in the cat: an experimental study using autoradiographic tracing technics. Brain Res. 1973 Aug 17;58(1):205–211. doi: 10.1016/0006-8993(73)90833-0. [DOI] [PubMed] [Google Scholar]
- Haight J. R., Weller W. L. Proceedings: Neocortical topography in the brush-tailed possum: variability and functional significance of sulci. J Anat. 1973 Dec;116(Pt 3):473–474. [PubMed] [Google Scholar]
- Hamilton T. C., Johnson J. I. Somatotopic organization related to nuclear morphology in the cuneate-gracile complex of opossums Didelphis marsupialis virginiana. Brain Res. 1973 Mar 15;51:125–140. doi: 10.1016/0006-8993(73)90368-5. [DOI] [PubMed] [Google Scholar]
- Hazlett J. C., Dom R., Martin G. F. Spino-bulbar, spino-thalamic and medical lemniscal connections in the American opossum, Didelphis marsupialis virginiana. J Comp Neurol. 1972 Sep;146(1):95–118. doi: 10.1002/cne.901460107. [DOI] [PubMed] [Google Scholar]
- Jane J. A., Schroeder D. M. A comparison of dorsal column nuclei and spinal afferents in the European hedgehog (Erinaceus europeaus). Exp Neurol. 1971 Jan;30(1):1–17. doi: 10.1016/0014-4886(71)90216-0. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G. Central cortical projections to motor and somato-sensory cell groups. An experimental study in the rhesus monkey. Brain. 1960 Mar;83:161–184. doi: 10.1093/brain/83.1.161. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chimpanzee. J Comp Neurol. 1958 Oct;110(2):221–255. doi: 10.1002/cne.901100205. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., TUERK J. D. THE DISTRIBUTION OF THE CORTICAL FIBRES WITHIN THE NUCLEI CUNEATUS AND GRACILIS IN THE CAT. J Anat. 1964 Apr;98:143–162. [PMC free article] [PubMed] [Google Scholar]
- Killackey H., Ebner F. Convergent projection of three separate thalamic nuclei on to a single cortical area. Science. 1973 Jan 19;179(4070):283–285. doi: 10.1126/science.179.4070.283. [DOI] [PubMed] [Google Scholar]
- King J. S., Martin G. F., Biggert T. P. The basilar pontine gray of the opossum (Didelphis virginiana). I. Morphology. J Comp Neurol. 1968 Aug;133(4):439–445. doi: 10.1002/cne.901330404. [DOI] [PubMed] [Google Scholar]
- King J. S., Martin G. F., Conner J. B. A light and electron microscopic study of corticorubral projections in the opossum, Didelphis marsupialis virginiana. Brain Res. 1972 Mar 24;38(2):251–265. doi: 10.1016/0006-8993(72)90711-1. [DOI] [PubMed] [Google Scholar]
- LENDE R. A. MOTOR REPRESENTATION IN THE CEREBRAL CORTEX OF THE OPOSSUM (DIDELPHIS VIRGINIANA). J Comp Neurol. 1963 Dec;121:405–415. doi: 10.1002/cne.901210308. [DOI] [PubMed] [Google Scholar]
- LENDE R. A. SENSORY REPRESENTATION IN THE CEREBRAL CORTEX OF THE OPOSSUM (DIDELPHIS VIRGINIANA). J Comp Neurol. 1963 Dec;121:395–403. doi: 10.1002/cne.901210307. [DOI] [PubMed] [Google Scholar]
- Leonard C. M. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969 Feb;12(2):321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Dom R., Katz S., King J. S. The organization of projection neurons in the opossum red nucleus. Brain Res. 1974 Sep 20;78(1):17–34. doi: 10.1016/0006-8993(74)90350-3. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Dom R., King J. S., RoBards M., Watson C. R. The inferior olivary nucleus of the opossum (Didelphis marsupialis virginiana), its organization and connections. J Comp Neurol. 1975 Apr 15;160(4):507–533. doi: 10.1002/cne.901600407. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Dom R. Rubrobulbar projections of the opossum (Didelphis virginiana). J Comp Neurol. 1970 Jun;139(2):199–214. doi: 10.1002/cne.901390204. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Dom R. The rubro-spinal tract of the opposum (Didelphis virginiana). J Comp Neurol. 1970 Jan;138(1):19–30. doi: 10.1002/cne.901380103. [DOI] [PubMed] [Google Scholar]
- Martin G. F. Efferent tectal pathways of the opossum (Didelphis virginiana). J Comp Neurol. 1969 Feb;135(2):209–224. doi: 10.1002/cne.901350206. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Fisher A. M. A further evaluation of the origin, the course and the termination of the opossum corticospinal tract. J Neurol Sci. 1968 Jul-Aug;7(1):177–187. doi: 10.1016/0022-510x(68)90012-9. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Jr The pattern of neocortical projections to the mesencephalon of the opossum, Didelphis virginiana. Brain Res. 1968 Dec;11(3):593–610. doi: 10.1016/0006-8993(68)90148-0. [DOI] [PubMed] [Google Scholar]
- Martin G. F., King J. S. The basilar pontine gray of the opossum (Didelphis virginiana). II. Experimental determination of neocortical input. J Comp Neurol. 1968 Aug;133(4):447–461. doi: 10.1002/cne.901330405. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Megirian D., Conner J. B. The origin, course and termination of the corticospinal tracts of the Tasmanian potoroo (Potorous apicalis). J Anat. 1972 Feb;111(Pt 2):263–281. [PMC free article] [PubMed] [Google Scholar]
- Martin G. F., Megirian D. Corticobulbar projections of the marsupial phalanger (Trichosurus vulpecula). II. Projections to the mesencephalon. J Comp Neurol. 1972 Feb;144(2):165–192. doi: 10.1002/cne.901440203. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Megirian D., Roebuck A. Corticobulbar projections of the marsupial phalanger (Trichosurus vulpecula). I. Projections to the pons and medulla oblongata. J Comp Neurol. 1971 Jul;142(3):275–295. doi: 10.1002/cne.901420303. [DOI] [PubMed] [Google Scholar]
- Martin G. F., Megirian D., Roebuck A. The corticospinal tract of the marsupial phalanger (Trichosurus vulpecula). J Comp Neurol. 1970 Jun;139(2):245–258. doi: 10.1002/cne.901390207. [DOI] [PubMed] [Google Scholar]
- Martin G. F., West H. J. Efferent neocortical projections to sensory nuclei in the brain stem of the opossum (Didelphys virginiana). J Neurol Sci. 1967 Sep-Oct;5(2):287–302. doi: 10.1016/0022-510x(67)90137-2. [DOI] [PubMed] [Google Scholar]
- Matano S., Shigenaga Y., Hiura T., Sakai A. The cortico-thalamic and -bulbar projections from the somatic sensory face area in the rat. Okajimas Folia Anat Jpn. 1972 Nov;49(4):249–269. doi: 10.2535/ofaj1936.49.4_249. [DOI] [PubMed] [Google Scholar]
- NAUTA W. J., GYGAX P. A. Silver impregnation of degenerating axons in the central nervous system: a modified technic. Stain Technol. 1954 Mar;29(2):91–93. doi: 10.3109/10520295409115448. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr, Pubols L. M. Somatic sensory representation in the thalamic ventrobasal complex of the Virginia opossum. J Comp Neurol. 1966 May;127(1):19–34. doi: 10.1002/cne.901270103. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr Retrograde degeneration study of somatic sensory thalamocortical connections in brain of Virginia opossum. Brain Res. 1968 Feb;7(2):232–251. doi: 10.1016/0006-8993(68)90101-7. [DOI] [PubMed] [Google Scholar]
- Rees S., Hore J. The motor cortex of the brush-tailed possum (Trichosurus vulpecula): motor representation, motor function and the pyramidal tract. Brain Res. 1970 Jun 15;20(3):439–451. doi: 10.1016/0006-8993(70)90173-3. [DOI] [PubMed] [Google Scholar]
- Rockel A. J., Heath C. J., Jones E. G. Afferent connections to the diencephalon in the marsupial phalanger and question of sensory convergence in the "posterior group" of the thalamus. J Comp Neurol. 1972 May;145(1):105–129. doi: 10.1002/cne.901450107. [DOI] [PubMed] [Google Scholar]
- Rockel A. J., Jones E. G. The neuronal organization of the inferior colliculus of the adult cat. I. The central nucleus. J Comp Neurol. 1973 Jan 1;147(1):11–60. doi: 10.1002/cne.901470103. [DOI] [PubMed] [Google Scholar]
- Rockel A. J., Jones E. G. The neuronal organization of the inferior colliculus of the adult cat. II. The pericentral nucleus. J Comp Neurol. 1973 Jun 1;149(3):301–334. doi: 10.1002/cne.901490303. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the nucleus gracilis from the lumbar cord of the ct. Brain Res. 1973 Mar 15;51:81–95. doi: 10.1016/0006-8993(73)90366-1. [DOI] [PubMed] [Google Scholar]
- Schroeder D. M., Jane J. A. Projection of dorsal column nuclei and spinal cord to brainstem and thalamus in the tree shrew, Tupaia glis. J Comp Neurol. 1971 Jul;142(3):309–350. doi: 10.1002/cne.901420305. [DOI] [PubMed] [Google Scholar]
- Sousa-Pinto A., Brodal A. Demonstration of a somatotopical pattern in the cortico-olivary projection in the cat. An experimental-anatomical study. Exp Brain Res. 1969;8(4):364–386. doi: 10.1007/BF00234382. [DOI] [PubMed] [Google Scholar]
- Tobias T. J., Ebner F. F. Thalmocortical projections from the mediodorsal nucleus in the virginia opossum. Brain Res. 1973 Mar 30;52:79–96. doi: 10.1016/0006-8993(73)90651-3. [DOI] [PubMed] [Google Scholar]
- Walsh T. M., Ebner F. F. Distribution of cerebellar and somatic lemniscal projections in the ventral nuclear complex of the Virginia opossum. J Comp Neurol. 1973 Feb 15;147(4):427–446. doi: 10.1002/cne.901470402. [DOI] [PubMed] [Google Scholar]
- Walsh T. M., Ebner F. F. The cytoarchitecture of somatic sensory-motor cortex in the opossum (Didelphis marsupialis virginiana): a Golgi study. J Anat. 1970 Jul;107(Pt 1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Warner G., Watson C. R. The rubrospinal tract in a diprotodont marsupial (Trichosurus vulpecula). Brain Res. 1972 Jun 8;41(1):180–183. doi: 10.1016/0006-8993(72)90625-7. [DOI] [PubMed] [Google Scholar]
- Watson C. R. An experimental study of the corticospinal tract of the kangaroo. J Anat. 1971 Dec;110(Pt 3):501–501. [PubMed] [Google Scholar]
- Watson C. R., Symons M. C. Ascending pathways to the brain stem of the phalanger (Trichosurus vulpecula). J Anat. 1971 Dec;110(Pt 3):501–501. [PubMed] [Google Scholar]
- Watson C. R. The corticospinal tract of the quokka wallaby (Setonix brachyurus). J Anat. 1971 May;109(Pt 1):127–133. [PMC free article] [PubMed] [Google Scholar]
- Yuen H., Dom R. M., Martin G. F. Cerebellopontine projections in the American opossum. A study of their origin, distribution and overlap with fibers from the cerebral cortex. J Comp Neurol. 1974 Apr 1;154(3):257–285. doi: 10.1002/cne.901540304. [DOI] [PubMed] [Google Scholar]