Abstract
Hyperpolarization-activated nonselective cation channels (Ih channels) play an important role in the control of membrane excitability and rhythmic neuronal activity. The functional relevance of presynaptic Ih channels in regulating synaptic function, however, is not well established. Recently, it has been proposed [Mellor, J., Nicoll, R. A. & Schmitz, D. (2002) Science 295, 143–147] that presynaptic Ih channels are necessary for hippocampal mossy fiber long-term potentiation (LTP). This observation challenges an alternative model that suggests presynaptic forms of LTP are caused by a direct modification of the transmitter release machinery. Here, we assess the role of Ih in hippocampal mossy fiber LTP as well as cerebellar parallel fiber LTP, forms of potentiation that share common mechanisms. Our results show that after Ih blockade neither mossy fiber LTP nor parallel fiber LTP are affected. Furthermore, Ih does not significantly modify basal excitatory synaptic transmission in the hippocampus, whereas the organic Ih blockers ZD7288 and DK-AH 269 induce a large Ih-independent depression of synaptic transmission. In summary, our results indicate that Ih-mediated persistent changes in presynaptic excitability do not underlie presynaptic forms of LTP.
Voltage-dependent ion channels localized in presynaptic terminals are ideally suited for modulating transmitter release. Hyperpolarization-activated nonselective cationic channels, also known as If, Iq, or Ih channels, are widely distributed in the nervous system (1), and have been recently identified at different presynaptic terminals such as the crustacean neuromuscular junction (2), avian ciliary ganglion (3), the basket cell synapses on Purkinje cells in the cerebellum (4), and the calyx of Held in the brainstem (5). Although Ih channels are thought to have diverse functions in neuronal regulation (1), their contribution to neurotransmitter release is not fully understood.
Ih, first identified and characterized in the heart (6, 7), is a noninactivating inward cation current carried by Na+/K+ (−30 to −50 mV reversal potential) that slowly activates during hyperpolarization. Because of these functional properties, Ih channels have been postulated to contribute to the resting membrane potential (as a fraction of these channels is open at this potential), and control rhythmic activity in spontaneously active cells (8). In some neurons, like hippocampal CA1 pyramidal cells, Ih channels are highly expressed in the dendrites and participate in regulating cable properties and temporal summation of excitatory postsynaptic potential (EPSPs, refs. 5 and 6). Four genes differentially expressed throughout the brain (termed HCN1–4 for hyperpolarization-activated cyclic nucleotide-gated channels) encode for products that form the Ih channels that (11–13) display different activation kinetics (14). One interesting property of these channels is their strong regulation by cyclic nucleotides (7); i.e., cAMP positively modulates Ih by a change in the voltage-dependence of channel activation. Targeting Ih channels, cyclic nucleotides have been shown to play a key role in regulating neuronal excitability and rhythmic activity in the central nervous system (CNS) (15).
Ih channels have received much attention lately because of the idea that they may also modulate synaptic transmission and presynaptic forms of plasticity. In the crayfish neuromuscular junction for example, it has recently been postulated that presynaptic Ih, by means of cAMP modulation, enhances transmitter release by increasing the readily releasable vesicle pool (2). However, in two different mammalian synapses in the brain where Ih is present at the presynaptic terminal, no role of Ih channels in basal synaptic transmission has been identified (4, 5), casting doubt on the relevance of Ih in transmitter release. More recently, Mellor et al. (16) put forward the provocative idea that presynaptic Ih channels are necessary for hippocampal mossy fiber long-term potentiation (LTP). This form of plasticity is expressed presynaptically and requires cAMP/protein kinase A activation (17–19) and therefore, Ih channels are excellent candidates underlying mossy fiber LTP because of their means of modulation and possible presynaptic localization. Thus, LTP expression could be caused by an Ih-mediated persistent presynaptic depolarization resulting in a global change in excitability (16). In contrast to this model, previous observations that two presynaptic proteins, Rab3A and RIM1α, are necessary for mossy fiber LTP, strongly suggest that LTP results from a direct modification of the release machinery (20, 21). Therefore, we decided to reassess the role of Ih in mossy fiber LTP. We have further extended this study to cerebellar parallel fiber LTP, a form of potentiation that is also expressed presynapticaly, and depends on cAMP and requires RIM1α (21, 22). Because of the potential relevance of Ih channels in modulating transmitter release, we also explored the role of these channels in basal excitatory synaptic transmission in the hippocampus.
Methods
Tranverse hippocampal slices (400 μM thickness) and sagittal cerebellar slices (250–300 μm) were prepared from 3- to 4-week-old Long–Evans rats. In some experiments, to match the experimental conditions used by others (16), we also used 3- to 4-week-old Sprague–Dawley rats. Animals were killed by decapitation in accordance with local regulations. Slices were cut on a microslicer (Dosaka, Kyoto) in ice-cold extracellular solution in which sodium was virtually replaced by sucrose. This cutting solution contained 238 mM sucrose, 2.5 mM KCl, 10 mM glucose, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM CaCl2, and 1.3 mM MgCl2. For preparing cerebellar slices, the solution included 119 mM NaCl instead of sucrose and 1 mM Kynurenic acid to block excitatory glutamatergic transmission. The cutting medium was gradually switched to the recording solution that contained 119 mM NaCl, 2.5 mM KCl, 10 mM glucose, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM CaCl2, and 1.3 mM MgCl2. Cutting and recording solutions were both saturated with 95% O2/5% CO2, pH 7.4. The slices were kept at room temperature for at least 1.5 h before transfer to the recording chamber. In all experiments, two independent sets of presynaptic fibers were stimulated alternatively at 0.05 Hz with patch-pipettes (monopolar stimulation) filled with external solution. To induce excitatory synaptic responses in CA1, a stimulating-pipette was positioned in stratum radiatum. To activate two independent sets of mossy fiber synapses, two stimulating pipettes were positioned in the dentate gyrus cell body layer. Parallel fibers in the cerebellum were activated by stimulating pipettes that were placed 200–400 μm apart in the middle third of the cerebellar cortex molecular layer. For extracellular field potential recordings, a patch-pipette filled with 1 M NaCl was used to record field EPSPs (fEPSP) from stratum radiatum of CA1 and stratum lucidum of CA3. Mossy fiber synaptic responses were identified as described (21). For whole-cell voltage-clamp recording in the hippocampus, the internal solution contained 135 mM KMeS03, 5 mM KCl, 1 mM CaCl2, 5 mM EGTA-Na, 10 mM Hepes, and 10 mM glucose (pH 7.2; 280–290 mOsm). For Purkinje cell, the internal solution contained 123 mM cesium gluconate, 10 mM CsCl, 8 mM NaCl, 1 mM CaCl2, 10 mM EGTA, 10 mM Hepes, 10 mM glucose, 2 mM ATP, and 0.3 mM GTP (ph7.2; 280–290 mOsm). Series resistance (typically 10–20 MΩ) was monitored throughout each experiment with a −5 mV, 80-ms pulse before every afferent stimulus, and cells with more than 15% change in series resistance were excluded from analysis. Purkinje cells were viewed by using an upright microscope (Nikon E600) and infrared differential interference contrast videomicroscopy. All recordings were performed at 25.0 ± 0.1°C.
Mossy fiber LTP was induced after at least 20 min of stable baseline by tetanic stimulation including 2 trains (20-s interval) each containing 125 pulses at 25 Hz in presence of 50 μM d-APV (d-(−)-2-amino-5-phosphonovaleric acid). Parallel fiber LTP in the cerebellum was induced with a single train of 100 stimuli at 10 Hz. The magnitude of LTP was estimated 50–60 min and 20–30 min after tetanus for mossy fibers and parallel fibers, respectively. Whole-cell voltage-clamp recordings (holding potential −70 mV) were made from the soma of visually identified Purkinje cells in cerebellar slices as described (21). To block inhibitory inputs onto Purkinje cells, 100 μM picrotoxin was added to the external solution. Data were digitized (5 kHz) and analyzed online by using macros written in igorpro (Wavemetrics, Lake Oswego, OR). Results are reported as mean ± SEM. Statistical comparisons were performed by using Student's t test. All drugs were bath-applied after dilution into the external solution from concentrated stock solutions. ZD7288 and d-APV were obtained from Tocris-Cookson (St. Louis). DK-AH 269 was a gift from Boehringer-Ingelheim (Germany). All other chemicals and drugs were from Sigma.
Results
Role of Ih Channels in Basal Synaptic Transmission.
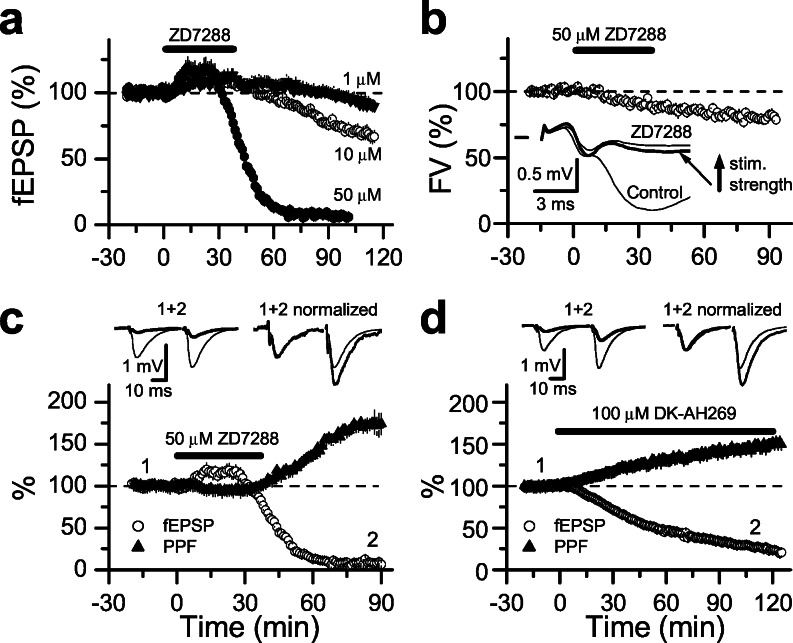
We first examined whether Ih channels modulate basal synaptic transmission in the well characterized synapse established between the Schaffer collateral and the CA1 pyramidal cell. We specifically studied the effect of two selective Ih channels blockers, ZD7288 (23) and DK-AH 269 (24), on synaptic transmission. As shown in Fig. 1a, 40-min application of 50 μM ZD7288 induced a transient and small increase in fEPSPs amplitude followed by a significant depression (91% ± 1% depression measured at 80–90 min after application, n = 4 slices) of synaptic transmission. This depression induced by ZD7288 was irreversible and dose-dependent (Fig. 1a). Given the magnitude of the synaptic depression induced by these Ih blockers, we decided to investigate its mechanism. Ih channels may control presynaptic excitability, and therefore it is conceivable that fewer fibers would be activated after blocking these channels. To directly explore this possibility, we estimated the number of activated fibers by measuring the amplitude of the fiber volley (FV) before and after ZD7288 bath application. To better distinguish the FV from the fEPSP, these experiments were performed after blocking synaptic transmission in the continuous presence of 10 μM NBQX [2,3-dioxo- 6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulfonamide]. In these conditions, the FV amplitude was reduced by 20% ± 1% (n = 5) after application of the blocker (Fig. 1b), but it is unlikely that this small reduction could account for the synaptic blockade induced by ZD7288. Furthermore, in those experiments in which NBQX was not added to the bath, an increase of the stimulus strength to match the initial fiber volley amplitude was clearly insufficient to restore synaptic transmission to control level in all cases (n = 4, see Fig. 1b Inset). Consequently, another mechanism unrelated to changes in excitability must underlie the ZD7288 dependent synaptic depression. It is likely that the mechanism resides on the presynaptic side because the Ih blocker-induced synaptic depression was associated with an increase in paired-pulse facilitation (PPF, Fig. 1c), a form of short-term presynaptic plasticity that inversely correlates with changes in transmitter release probability. Similar synaptic depression was also observed with DK-AH 269 (Fig. 1d), another organic Ih blocker with a different mechanism of action than ZD7288 (24). The synaptic depression induced by DK-AH 269 (100 μM) was also associated with a PPF enhancement but, in contrast to ZD7288, the effect of DK-AH 269 was fully reversible on washout (data not shown).
Figure 1.
Depression of basal synaptic transmission by organic Ih channel blockers. (a) Summary graph showing the effect of 1, 10, and 50 μM ZD7288 (n = 3, 3, and 4 slices, respectively) on field excitatory postsynaptic potentials (fEPSP) recorded from the CA1 area of the hippocampus. (b) Effects of 50 μM ZD7288 on the fiber volley (FV) amplitude measured in the continuous presence of 10 μM NBQX. Representative FV traces (averages of 10 individual responses) in the absence of NBQX (Inset) are superimposed before (Control), 90 min after ZD7288 bath application and after increasing stimulus strength to match the initial FV amplitude. (c and d) Summary graphs demonstrating that the depression of synaptic transmission (fEPSPs, open circles) induced by both 50 μM ZD7288 (c, n = 4) and 100 μM DK-AH 269 (d, n = 3) was associated with an increase in the PPF magnitude (filled triangles). Superimposed averaged traces before and after application of the organic Ih blockers are shown above. These synaptic responses are also shown normalized (right traces above on each c and d).
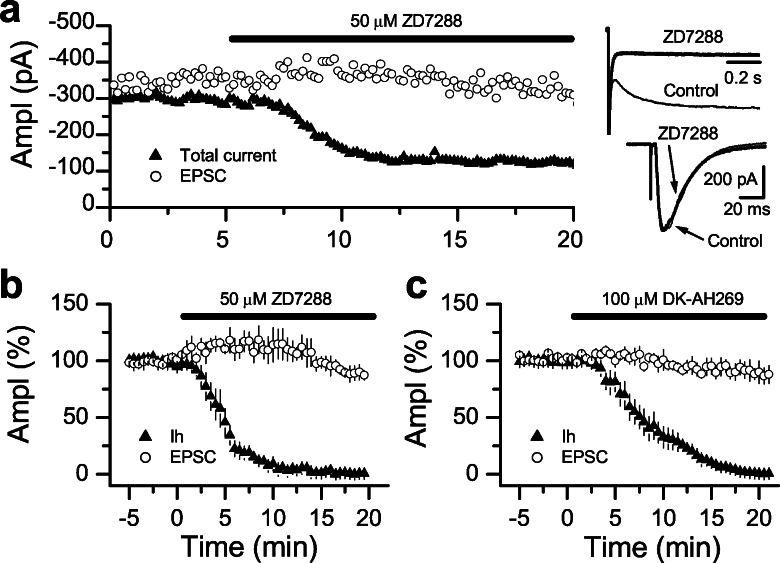
The fact that two different Ih blockers induced similar synaptic depression with a parallel PPF enhancement, suggests that Ih channels control transmitter release at these synapses. Ideally, this possibility would be tested by directly monitoring presynaptic Ih and correlating the blockade of this current with changes in synaptic transmission. Because Schaffer collateral presynaptic terminals are technically inaccessible for voltage-clamp recordings, one plausible alternative is to estimate the blockade of Ih at the postsynaptic cell instead. Thus, we monitored in the same CA1 pyramidal cell, the current induced by a hyperpolarizing step and the excitatory postsynaptic current (EPSC) induced by Schaffer collateral stimulation before and after ZD7288 or DK-AH 269 bath application. As shown in Fig. 2 a and b, 50 μM ZD7288 entirely blocked Ih in 20 min, whereas the EPSC amplitude was virtually unaffected. Identical results were also observed with 100 μM DK-AH 269 (Fig. 2c). This finding strongly suggests that Ih channels have no direct effect in basal synaptic transmission. Nevertheless, it is conceivable that Ih channel blockade may induce a delayed, indirect effect on synaptic transmitter release. Alternatively, both compounds could have a common side effect on synaptic transmission independent of Ih channels.
Figure 2.
Basal excitatory synaptic transmission is not modulated by Ih. (a Left) Single experiment from a CA1 pyramidal cell in which the total current (I leak + Ih) induced by a voltage step, from −50 mV to −90 mV, and the amplitude of EPSCs were simultaneously recorded and plotted over time. Bath application of 50 μM ZD7288 completely blocked Ih within 15–20 min, whereas the EPSC amplitude remained virtually unchanged. (Right) Sample traces of the total current recorded during the hyperpolarization step (Upper) and the EPSC (Lower) are shown before and after ZD7288 application. (b and c) Summary graphs of experiments performed as in a in 50 μM ZD7288 (b, n = 4) and 100 μM DK-AH 269 (c, n = 4).
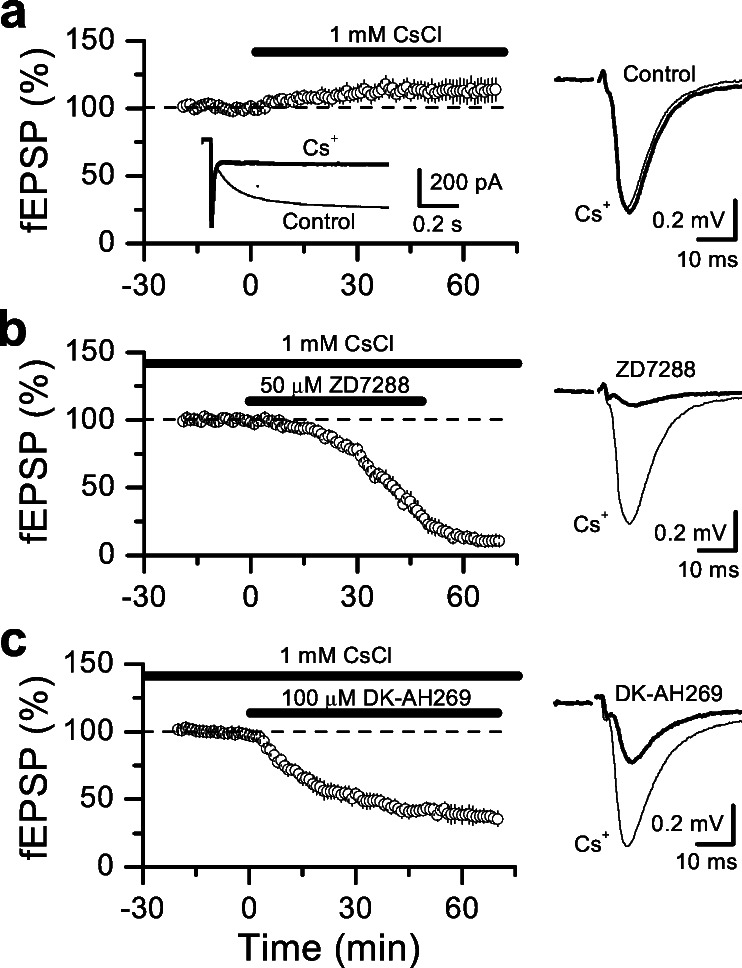
If synaptic depression induced by ZD7288 and DK-AH 269 is a consequence of blocking Ih channels, this depression should be mimicked by low concentrations of cesium (Cs+), a well known blocker of Ih (1, 25), and it should be also occluded by previous blockade of these channels with Cs+. Continuous application of 1 mM Cs+, which efficiently blocks Ih, induced no synaptic depression but rather a slight enhancement that stabilized in approximately 30 min (Fig. 3a, 13% ± 1%, n = 6 slices). Because Cs+ is a nonselective Ih channel blocker that also blocks some potassium channels, one may argue that the lack of synaptic depression was caused by a counteracting excitability enhancement induced by Cs+. This is unlikely to be the case because the fiber volley remained unchanged during Cs+ application (105% ± 2%, P > 0.05). More importantly, once synaptic responses stabilized and Ih was fully blocked in 1 mM Cs+, subsequent application of ZD7288 or DK-AH 269 in the same slice induced identical synaptic depression to that in control slices (Fig. 3 b and c). Interestingly, the transient increase in synaptic transmission induced by ZD7288 application was occluded by Cs+, suggesting that both blockers may share a common target, possibly involving Ih channels. Taken together, our results indicate that in contrast to extracellular Cs+, the organic Ih channel blockers, ZD7288 and DK-AH 269, induced a large Ih-independent reduction of synaptic transmission. Because of this effect, we took special care when using these blockers to study the role of Ih channels in long-term plasticity (see below).
Figure 3.
Synaptic depression induced by ZD7288 and DK-AH 269 is not mimicked by Cs+. (a) Bath-application of 1 mM Cs+, which effectively blocked Ih, did not depress synaptic transmission but rather induced a slight increase of synaptic responses. Superimposed sample traces before and after Cs+ application are shown on the right side of the panel. (b and c) In the same slices, after synaptic responses were stabilized in presence of cesium, subsequent application of 50 μM ZD7288 (b) or 100 μM DK-AH 269 (c) markedly depressed synaptic transmission. Sample traces before and after Ih blockers application are shown on the right.
Role of Ih in Mossy Fiber LTP.
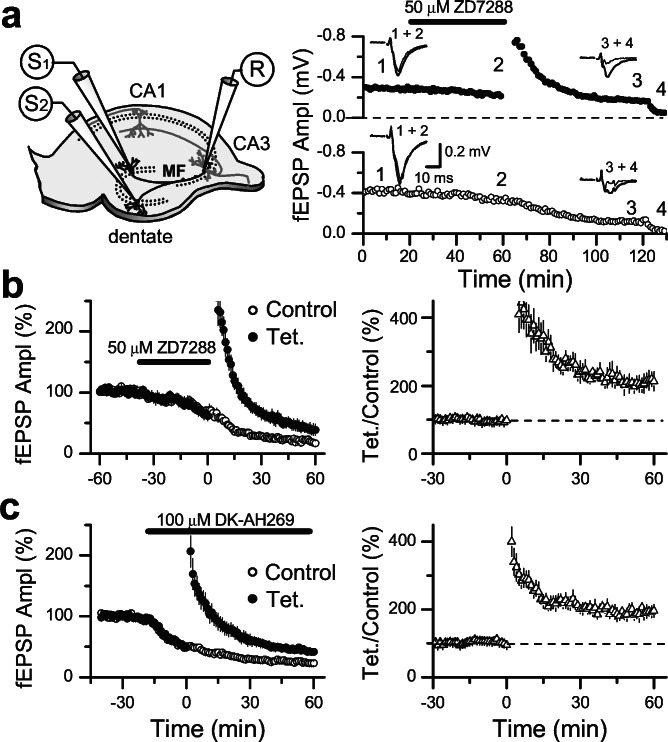
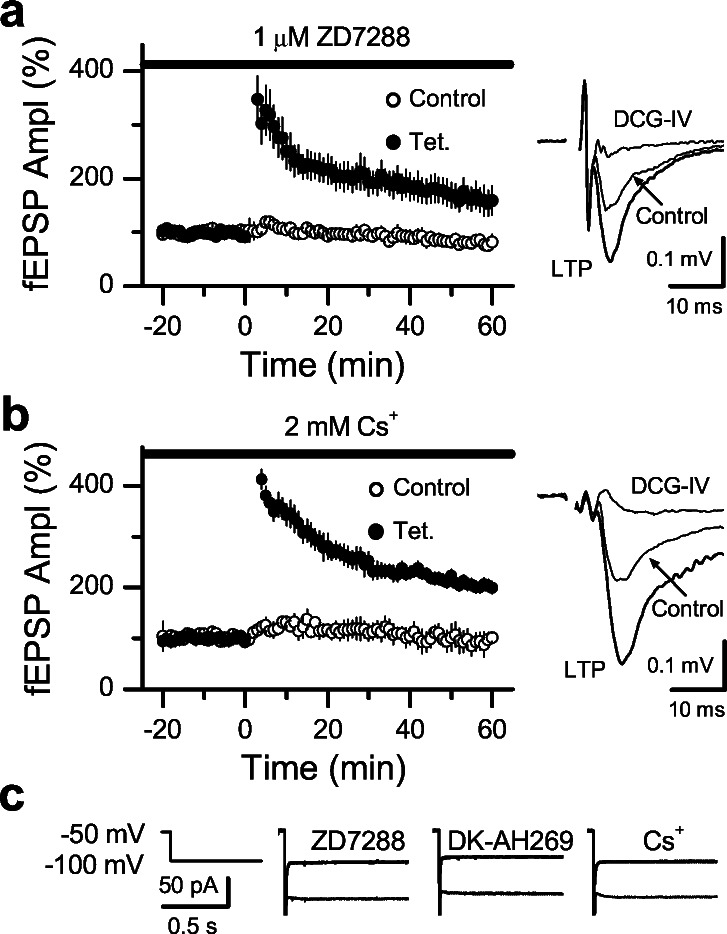
To explore whether Ih channels play any role in mossy fiber LTP, we tetanized these fibers after pharmacological blockade of Ih channels with three different blockers: ZD7288, DK-AH 269, and Cs+. We first verified, at the mossy fiber to CA3 pyramidal cell synapse, whether ZD7288 and DK-AH 269 induced similar synaptic depression to that observed in the CA1 area (Fig. 1). We found that after 1 h bath application of 50 μM ZD7288 or 100 μM DK-AH 269, mossy fiber fEPSPs were depressed to 20% ± 2% (n = 7 slices) and 24% ± 3% (n = 7 slices) of baseline, respectively. Thus, the depression induced by the Ih blockers is not synapse-specific because its magnitude at the mossy fiber to CA3 pyramidal cell synapse is similar to that observed at excitatory synapses in the CA1 area. This depression makes it difficult to analyze increases of synaptic transmission after induction of LTP. Therefore, in all our experiments we monitored two independent sets of mossy fibers; one was tetanized, whereas the naïve pathway served as control of the synaptic depression induced by the Ih blockers. The stimulus strength was set to induce similar synaptic responses in both pathways before drug application. An example of this experimental paradigm in which we applied ZD7288 is shown in Fig. 4a. Note in this example (see Upper Right) that if only the tetanized pathway was monitored without taking into account the effect of the blocker on the naïve pathway, we would have concluded that mossy fiber LTP was blocked in the presence of ZD7288. A summary graph of the experiments using ZD7288 is shown in Fig. 4b. In all cases (n = 7 slices), the normalized fEPSP amplitude was larger in the tetanized pathway vs. the naïve pathway at least 1 h after tetanus. Therefore, to control for the synaptic depression induced by ZD7288, the magnitude of mossy fiber LTP was estimated as the ratio of synaptic responses amplitudes between the tetanized and the naïve pathway for each individual experiment (Fig. 4b Right). Under these conditions, the magnitude of mossy fiber LTP (203% ± 3%, n = 7 slices) was entirely normal and identical to the LTP observed in a set of interleaved control slices (204% ± 2%, n = 4 slices). We used this same experimental paradigm and analysis, and also observed normal mossy fiber LTP in presence of 100 μM DK-AH 269 (Fig. 4c, 204% ± 3%, n = 7 slices). Our approach assumes that the effect of ZD7288 and DK-AH 269 is identical in the tetanized vs. naïve pathway. In fact, this assumption is hard to test and not necessarily correct. Thus, to minimize the depression induced by these blockers, we also performed experiments after 3–6 h of incubation and in the continuous presence of a low dose of ZD7288 (1 μM), which, in our hands, induces a slow run-down of synaptic transmission. To discard the rat strain as a source of potential discrepancies with previous work (16), these experiments were exclusively performed in Sprague–Dawley rats. As shown in Fig. 5a mossy fiber LTP was totally normal in this condition (167% ± 2%, n = 7 slices). The magnitude of LTP when measured as the ratio of fEPSP amplitudes between tetanized and nontetanized pathways was 203% ± 2%. Finally, we blocked Ih channels with extracellular application of 2 mM Cs+. At this dose, Cs+ also blocks potassium conductances, which enhances transmitter release and the global excitability of the CA3 network. To circumvent this problem, slice excitability was reduced by including 0.3 μM NBQX and changing the ratio of divalent ions in the perfusate to 4 mM Mg2+/4 mM Ca2+ instead of 1.3 mM Mg2+/2.5 mM Ca2+ as in the rest of this study. Under these conditions, mossy fiber LTP was also normal (Fig. 5b, 207% ± 2%, n = 4 slices).
Figure 4.
Organic Ih channel blockers do not block mossy fiber LTP, despite significant depression of basal synaptic transmission. (a Left) Scheme of the arrangement of electrodes for stimulating and recording. (Right) Representative experiment in which synaptic responses were induced by alternatively stimulating one of these pathways at 0.05 Hz (values are the mean of three successive points). ZD7288 (50 μM) was bath applied during 40 min before tetanizing one pathway. At the end of each experiment, 1 μM DCG-IV blocked transmission confirming that these were mossy fiber synaptic responses. Sample traces taken during baseline, in presence of ZD7288, 1hr after tetanus and in presence of DCG-IV are shown above. (b Left) Summary graph of 7 experiments performed as in a. (Right) Summary graph showing the magnitude of the LTP for each individual experiment that was estimated as the ratio of synaptic responses between the tetanized (Tet.) and nontetanized (Control) synapses. (c) DK-AH 269 (100 μM) also depressed synaptic transmission in 7 experiments but did not affect mossy fiber LTP.
Figure 5.
Mossy fiber LTP is independent of Ih channels. (a) Mossy fiber LTP was also normal in 7 slices that were incubated in 1 μM ZD7288 for 3–6 h and also applied during the experiment at the same dose. Sample traces from a single experiment during baseline, 50–60 min after tetanus and in 1 μM DCG-IV are shown on the right. (b) Summary graph of 4 experiments performed in presence of 2 mM Cs+. The LTP-inducing tetanus was applied at least 1 h after Cs+ bath application and 20 min stable baseline. Sample traces before and after tetanus are shown on the right. (c) Ih currents recorded from granule cells (induced by voltage steps from −50 to −100 mV) were blocked in presence of 50 μM ZD7288, 100 μM DK-AH 269, or 1 mM cesium.
In summary, our experiments show that mossy fiber LTP was entirely normal in the presence of three different Ih channel blockers. This finding clearly contradicts a recent report demonstrating that ZD7288 and DK-AH269 both block mossy fiber LTP (16). One way to explain this discrepancy is that in our experiments, for some reason, presynaptic Ih channels were not blocked. To verify that this was not the case, we directly monitored Ih from dentate granule cells that give rise to the mossy fibers and demonstrated that all three blockers abolished Ih (Fig. 5c). In conclusion, our results indicate that Ih channels are unnecessary in mossy fiber LTP.
Do Ih Channels Mediate cAMP-Mediated Potentiation at Mossy Fiber Synapses?
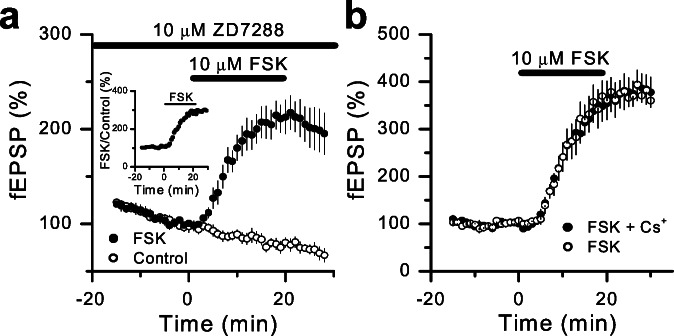
Increasing cAMP enhances mossy fiber synaptic transmission by a presynaptic mechanism that occludes LTP (17). Because Ih channels are heavily modulated by cAMP (1, 7), we also explored whether these channels are required for the cAMP-induced potentiation of mossy fiber synaptic transmission. To test this possibility, the effect of the adenylyl-cyclase activator forskolin (10 μM bath application during 20 min) was analyzed in slices that were incubated for at least 2 hours with 10 μM ZD7288 and also perfused at the same dose. In a separate set of interleaved slices (n = 4), we estimated the rundown of synaptic transmission induced by ZD7288. As shown in Fig. 6a, forskolin-induced potentiation of mossy fiber responses was normal (292% ± 2% of control, measured 20–30 min after bath application) when calculated as the ratio of forskolin-treated vs. nontreated slices (Fig. 6a Inset). In addition, to avoid the rundown induced by ZD7288, we also explored the magnitude of forskolin-induced potentiation in the continuous presence of 1–2 mM Cs+ and found that this potentiation in Cs+ (375% ± 3%, n = 5 slices) was identical (P < 0.001) to control slices (Fig. 6b, 372% ± 3%, n = 3 slices). These experiments strongly suggest that the cAMP-induced potentiation at mossy fiber synapses is independent of presynaptic Ih channels.
Figure 6.
Blocking Ih has no effect on the forskolin-induced enhancement of mossy fiber synaptic responses. (a) Forksolin (10 μM; FSK, filled circles) was bath-applied during 20 min (horizontal bar) prior slice incubation in 10 μM ZD7288 for 2–4 h (5 slices). The rundown effect of ZD7288 application on mossy fiber synaptic transmission was estimated from 4 interleaved slices (Control, open circles). Forskolin-induced potentiation is also shown as the ratio between synaptic responses in forskolin-treated slices and control slices (Inset). (b) Forskolin enhancement of mossy fiber synaptic responses is identical in slices incubated for 1 h in 1–2 mM cesium (filled circles, n = 5) and in control slices (open circles, n = 3).
Role of Ih in Cerebellar LTP.
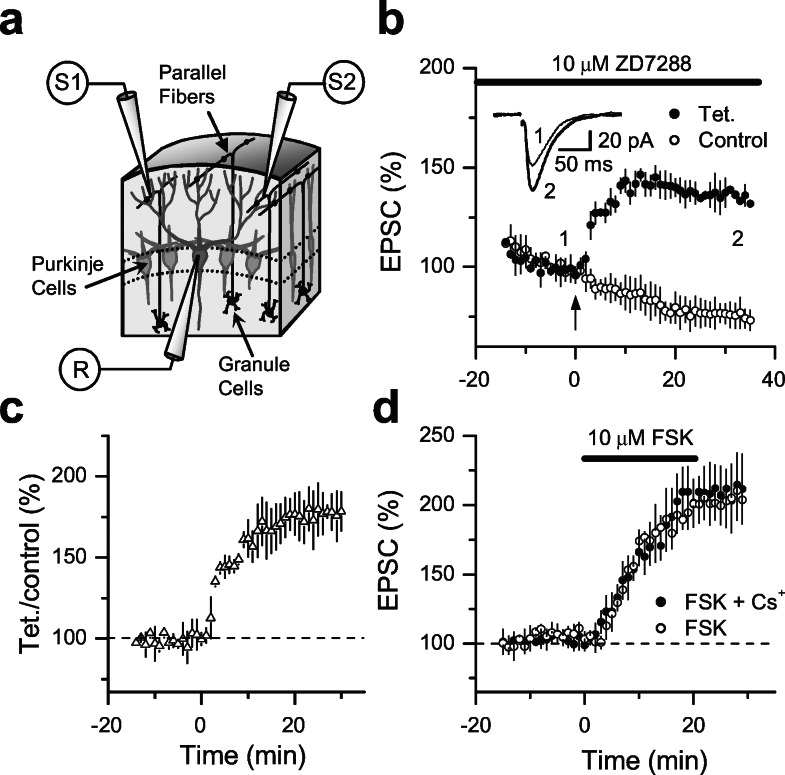
There is significant evidence supporting the idea that LTP observed at the parallel fiber to Purkinje cell in the cerebellum is mechanistically identical to mossy fiber LTP (21, 22); i.e., both synapses express a cAMP/protein kinase A-dependent and presynaptic form of plasticity. To test whether parallel fiber LTP is also independent of Ih channels, we incubated cerebellar slices in 10 μM ZD7288 and monitored two independent sets of parallel fibers (Fig. 7a). Similarly to mossy fiber LTP, we estimated the magnitude of parallel fiber LTP as the ratio of EPSC amplitudes between tetanized and naïve pathways. As illustrated in Fig. 7 b and c, parallel fiber LTP (177% ± 1%, n = 4 Purkinje cells) in the presence of this Ih channel blocker was similar to previously reported LTP (21, 22). Finally, we also tested whether presynaptic Ih channels participate in the cAMP-dependent enhancement of synaptic transmission in these cerebellar synapses. Similar to mossy fiber synapses, forskolin-induced potentiation (Fig. 7d) after Ih channels blockade in 2 mM Cs+ (209 ± 1%, n = 4 Purkinje cells) was indistinguishable from control (203% ± 1%, n = 3). In conclusion, our results indicate that presynaptic Ih channels mediate neither parallel fiber cerebellar LTP nor forskolin-induced potentiation.
Figure 7.
Ih blockade did not affect LTP or forskolin-induced potentiation of transmission at parallel fiber-Purkinje synapses. (a) Experimental paradigm to stimulate and record two independent sets of parallel fibers in the cerebellar cortex. (b) Summary graph of EPSCs recorded in Purkinje cells after at least 2 h of incubation in 10 μM ZD7288 (also applied during the experiment). A single tetanus (100 pulses at 10 Hz) was given at time 0 (arrow) induced normal LTP (n = 4). Average traces from a representative experiment before and 30 min after tetanus are illustrated above. (c) Summary graph of the LTP magnitude that was calculated as the EPSC ratio between the tetanized (Tet.) and nontetanized (Control) synapses for each individual experiment. (d) Forskolin-enhancement of synaptic transmission after at least 1 h of incubation in 2 mM Cs+ (also applied during the experiment, n = 4) is identical to that observed in control (n = 3).
Discussion
In this study, we found no evidence for a role of Ih in excitatory synaptic transmission in the hippocampus and, most importantly, we found that Ih is not involved in mossy fiber or parallel fiber LTP. To our knowledge, only two recent reports have directly tested whether presynaptic Ih channels modulate transmitter release in mammalian brain synapses (4, 5). Southan et al. (4) reported a reduction in spontaneous inhibitory transmitter release after ZD7288 application at the basket cell to Purkinje cell synapse in the cerebellum. However, this effect was blocked by tetrodotoxin, indicating an action of Ih on inhibitory interneurons excitability rather than a direct effect on transmitter release. Cuttle et al. (5), working on the calyx of Held excitatory synapse in the brainstem, found no significant change in transmitter release on activation or blockade of Ih. In agreement with these two studies, we were unable to obtain any evidence for a role of presynaptic Ih channels in excitatory synaptic transmission in the hippocampus. It is still possible, however, that presynaptic Ih channels play a role under different conditions than those we tested in our study. In any case, we predict that this role, if any, would be subtle.
In this study, we have also revealed a novel nonspecific effect of the organic Ih channel blockers, ZD7288 and DK-AH 269. Previous studies have suggested that ZD7288 selectively inhibits Ih without measurable effects on a number of other cell properties (23, 26, 27). Our results indicate, however, that both blockers dramatically depress synaptic transmission, presumably by a presynaptic, Ih-independent mechanism. Interestingly, this effect is not present in the crayfish neuromuscular junction (2, 28), but has been detected at hippocampal mossy fiber synapses (16). It is already well established that extracellular Cs+ at low doses (1–3 mM) blocks Ih, but at this concentration it also blocks some potassium channels. As a result, we must conclude that extra care is required in interpreting data using any of these pharmacological tools to analyze the role of Ih.
One of the most important observations of this paper is that we were unable to replicate recent observations reporting that modulation of presynaptic Ih channels is responsible for hippocampal mossy fiber LTP (16). According to this report, mossy fiber LTP induction and expression would be entirely caused by a global change in dentate granule cell excitability, which includes a persistent depolarization of the mossy fiber terminals and consequent enhancement of transmitter release. This hypothesis challenges recent findings suggesting that mossy fiber LTP expression is caused by a modification of the release machinery, in which two presynaptic proteins, Rab3A and RIM1α, play an essential role (20, 21). Alternatively, based on these previous results, it is possible that presynaptic Ih channels and Rab3A/RIM1α are both required in mossy fiber LTP. However, we present strong evidence that mossy fiber LTP is normal after blockade of Ih. Furthermore, we also found that cerebellar parallel fiber LTP, a form of potentiation that is mechanistically similar to mossy fiber LTP (21, 22), is also normal after blockade of Ih. Thus, we conclude that presynaptic forms of LTP require a persistent modification in the release machinery but are entirely independent of Ih.
One difference between our study and the previous one (16) is that because of the dramatic depression in basal synaptic transmission observed after ZD7288 and DK-AH 269 bath application, we explored mossy fiber LTP by using a two pathways paradigm (Fig. 4a) during which the magnitude of LTP is estimated as the ratio between synaptic responses recorded at tetanized vs. naïve pathways. An alternative approach to minimize the consequences of the synaptic depression induced by the organic Ih blockers is to incubate slices in low doses of these blockers (i.e., 1 μM ZD7288). The disadvantage of this manipulation, however, is that because mossy fiber responses are still depressed under these conditions, it is necessary to increase the stimulus strength, and consequently, non-mossy fibers could be recruited and contaminate the synaptic responses. As a result, two of the most singular functional features of the mossy fiber to CA3 synapse, i.e., the NMDA-independent form of LTP elicited by low-frequency tetanization (5–25 Hz) and the forskolin-induced potentiation could be underestimated. To circumvent these problems, we also included in our study the use of Cs+ to block Ih. Because this cation, unlike the organic blockers, does not induce synaptic depression, low-strength stimulation can easily and selectively activate mossy fibers.
Although Ih has been recorded in mammalian presynaptic terminals (4, 5), there is no evidence as to whether Ih channels are expressed at hippocampal mossy fiber terminals. In the hippocampus, most evidence shows relatively weak expression of Ih channels in cells that give rise to the mossy fibers, i.e., dentate granule cells (refs. 14 and 29, but see ref. 30). In agreement with previous findings (16, 31), we also detected Ih in the dentate granule cells (Fig. 4f) with rapid activation kinetics, which resemble those of the HCN1 isoform. Interestingly, if mossy terminals do express primarily HCN1, this composition could explain why forskolin-induced potentiation is unaffected after Ih channel blockade, as those channels have been shown to be much less sensitive to cAMP modulation than other HCN (13, 32, 33). In the crayfish neuromuscular junction, forskolin-induced enhancement of synaptic responses was partially antagonized by ZD7288 and DK-AH 269 (2, 28). As we have found normal forskolin-induced potentiation at two different synapses (Figs. 4 and 5b), one simple explanation for this dissimilar result could be the different features of mammalian and invertebrate synapses.
In conclusion, our results indicate that the presynaptic form of LTP characterized at hippocampal mossy fibers and cerebellar parallel fibers is independent of presynaptic Ih. Therefore, based on previous studies (20, 21), we believe the most likely mechanism of this form of plasticity lies in the regulation of the transmitter release machinery.
Acknowledgments
We thank Reed Carroll, Alberto Pereda, and Robert Malenka for their constructive review of the manuscript. V.C. is an Albert Einstein Scholar.
Abbreviations
- LTP
long-term potentiation
- EPSP
excitatory postsynaptic potential
- fEPSP
field EPSP
- FV
fiber volley
- PPF
paired-pulse facilitation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pape H C. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 2.Beaumont V, Zucker R S. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher G H, Chiappinelli V A. Brain Res. 1992;575:103–112. doi: 10.1016/0006-8993(92)90429-d. [DOI] [PubMed] [Google Scholar]
- 4.Southan A P, Morris N P, Stephens G J, Robertson B. J Physiol. 2000;526:91–97. doi: 10.1111/j.1469-7793.2000.t01-1-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuttle M F, Rusznak Z, Wong A Y, Owens S, Forsythe I D. J Physiol. 2001;534:733–744. doi: 10.1111/j.1469-7793.2001.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown H F, DiFrancesco D, Noble S J. Nature (London) 1979;280:235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- 7.DiFrancesco D. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 8.McCormick D A, Pape H C. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magee J C. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magee J C. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 11.Santoro B, Grant S G, Bartsch D, Kandel E R. Proc Natl Acad Sci USA. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauss R, Seifert R, Kaupp U B. Nature (London) 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. Nature (London) 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 14.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky G P, Tibbs G R, Siegelbaum S A. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pape H C, McCormick D A. Nature (London) 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 16.Mellor J, Nicoll R A, Schmitz D. Science. 2002;295:143–147. doi: 10.1126/science.1064285. [DOI] [PubMed] [Google Scholar]
- 17.Weisskopf M G, Castillo P E, Zalutsky R A, Nicoll R A. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 18.Villacres E C, Wong S T, Chavkin C, Storm D R. J Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y Y, Kandel E R, Varshavsky L, Brandon E P, Qi M, Idzerda R L, McKnight G S, Bourtchouladze R. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 20.Castillo P E, Janz R, Sudhof T C, Tzounopoulos T, Malenka R C, Nicoll R A. Nature (London) 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- 21.Castillo P E, Schoch S, Schmitz F, Sudhof T C, Malenka R C. Nature (London) 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 22.Salin P A, Malenka R C, Nicoll R A. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 23.Harris N C, Constanti A. J Neurophysiol. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- 24.Raes A, Van de Vijver G, Goethals M, van Bogaert P P. Br J Pharmacol. 1998;125:741–750. doi: 10.1038/sj.bjp.0702153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell J V, Adams P R. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 26.Williams S R, Turner J P, Hughes S W, Crunelli V. J Physiol. 1997;505:727–747. doi: 10.1111/j.1469-7793.1997.727ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasparini S, DiFrancesco D. Pflugers Arch. 1997;435:99–106. doi: 10.1007/s004240050488. [DOI] [PubMed] [Google Scholar]
- 28.Beaumont V, Zhong N, Froemke R C, Ball R W, Zucker R S. Neuron. 2002;33:601–613. doi: 10.1016/s0896-6273(02)00581-0. [DOI] [PubMed] [Google Scholar]
- 29.Moosmang S, Biel M, Hofmann F, Ludwig A. Biol Chem. 1999;380:975–980. doi: 10.1515/BC.1999.121. [DOI] [PubMed] [Google Scholar]
- 30.Monteggia L M, Eisch A J, Tang M D, Kaczmarek L K, Nestler E J. Brain Res Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 31.Brauer A U, Savaskan N E, Kole M H, Plaschke M, Monteggia L M, Nestler E J, Simburger E, Deisz R A, Ninnemann O, Nitsch R. FASEB J. 2001;15:2689–2701. doi: 10.1096/fj.01-0235com. [DOI] [PubMed] [Google Scholar]
- 32.Santoro B, Liu D T, Yao H, Bartsch D, Kandel E R, Siegelbaum S A, Tibbs G R. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Chen S, Siegelbaum S A. J Gen Physiol. 2001;118:237–250. doi: 10.1085/jgp.118.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]