Abstract
We recently discovered the existence of the oxytocin/oxytocin receptor (OT/OTR) system in the heart. Activation of cardiac OTR stimulates the release of atrial natriuretic peptide (ANP), which is involved in regulation of blood pressure and cell growth. Having observed elevated OT levels in the fetal and newborn heart at a stage of intense cardiomyocyte hyperplasia, we hypothesized a role for OT in cardiomyocyte differentiation. We used mouse P19 embryonic stem cells to substantiate this potential role. P19 cells give rise to the formation of cell derivatives of all germ layers. Treatment of P19 cell aggregates with dimethyl sulfoxide (DMSO) induces differentiation to cardiomyocytes. In this work, P19 cells were allowed to aggregate from day 0 to day 4 in the presence of 0.5% DMSO, 10−7 M OT and/or 10−7 M OT antagonist (OTA), and then cultured in the absence of these factors until day 14. OT alone stimulated the production of beating cell colonies in all 24 independently growing cultures by day 8 of the differentiation protocol, whereas the same result was obtained in cells induced by DMSO only after 12 days. Cells induced with OT exhibited increased ANP mRNA, had abundant mitochondria (i.e., they strongly absorbed rhodamine 123), and expressed sarcomeric myosin heavy chain and dihydropyridine receptor-α1, confirming a cardiomyocyte phenotype. In addition, OT as well as DMSO increased OTR protein and OTR mRNA, and OTA completely inhibited the formation of cardiomyocytes in OT- and DMSO-supplemented cultures. These results suggest that the OT/OTR system plays an important role in cardiogenesis by promoting cardiomyocyte differentiation.
Oxytocin (OT), a nonapeptide largely expressed in the hypothalamus, has long been recognized as a female reproductive hormone necessary for uterine contraction during parturition, timing and amplification of labor, milk ejection during lactation, and ovulation (1). However, the last decades have shed new light on OT functions. It has been shown that both sexes have equivalent concentrations of OT in the hypophysis and plasma as well as a similar number of oxytocinergic neurons in the hypothalamus (2), and both sexes respond to the same stimuli for OT release (3, 4). It also appears that reproductive functions and maternal behavior are preserved in OT−/− mutant mice (5). Both OT−/− males and females are fertile, and females are capable of parturition although they lack the milk ejection reflex (5, 6). These observations indicate that OT is not essential for reproduction, and data now underline the involvement of OT in sexual behavior, cognition, memory, tolerance, adaptation, food and water intake, and cardiovascular functions (1, 7, 8).
Recently, a role has been suggested for OT as a growth and cellular differentiation factor. The antiproliferative effect of OT, mediated by OT receptors (OTRs), has been documented in breast cancer cells (9) and other tumors (10–12). In contrast to its effect on tumoral cells, a mitogenic action of OT has also been described. OT stimulates the proliferation of thymocytes (13, 14) and mitotic activity in the prostate epithelium (15), vascular endothelium (16), and trophoblasts (17). OT has also been reported to enhance myoepithelial cell differentiation and proliferation in the mouse mammary gland (18). The possibility that OT has trophic effects on the embryo has not been investigated intensively. However, OT has been shown to have an influence on the developing heart: OT administered in excess to the fetus may impair cardiac growth in humans and rats (19, 20), and OTR suppression by specific OT antagonists (OTAs) in the early stage of chicken egg development leads to cardiac malformation in the embryos.‖ It is not known whether the trophic effects of OT on the heart are direct or indirect.
OT's indirect actions could be related to its cardiovascular functions observed in adult rats (7, 21–23). Indeed, we uncovered the entire OT/OTR system in the rat heart, and showed that cardiac OTR activation is coupled to the release of atrial natriuretic peptide (ANP), a potent diuretic, natriuretic, and vasorelaxant hormone that is also involved in cell growth regulation (7, 8). A role for ANP in cardiomyogenesis has even been suggested by Cameron et al. (24). In support of a potential action of OT on cardiac development, a maximal OT protein level was seen in the heart at day 21 of gestation and postnatal days 1–4, when cardiac myocytes are at a stage of intense hyperplasia.**
The P19 mouse embryonal carcinoma cell line is an established model of cell differentiation. Developmentally, pluripotent P19 cells give rise to the formation of cell derivatives of all three germ layers (25, 26) and appear to differentiate by the same mechanisms as normal embryonic stem cells (25, 27). When cultured in the presence of 10−6 M retinoic acid, a physiologically relevant morphogen, P19 cells efficiently (≥95%) differentiate to neurons (25, 28, 29). The solvent dimethyl sulfoxide (DMSO) induces cardiac differentiation, albeit not as efficiently (≤15%) (25, 30). DMSO has been shown to activate essential cardiogenic transcription factors, such as GATA-4 and Nkx-2.5 (30, 31). However, the mechanisms responsible for triggering these genes in the embryo are still unknown, as is the mode of action of DMSO with respect to the cardiomyogenic program in P19 cells.
Our discovery of a functional OT/OTR system in the heart, in conjunction with the fact that it is expressed at higher levels in developing than in adult hearts, led us to consider OT as a potential naturally occurring cardiomorphogen. In this respect, we investigated whether OT induces differentiation of P19 cells into a cardiomyocyte phenotype.
Materials and Methods
Culture and Differentiation of P19 Cells.
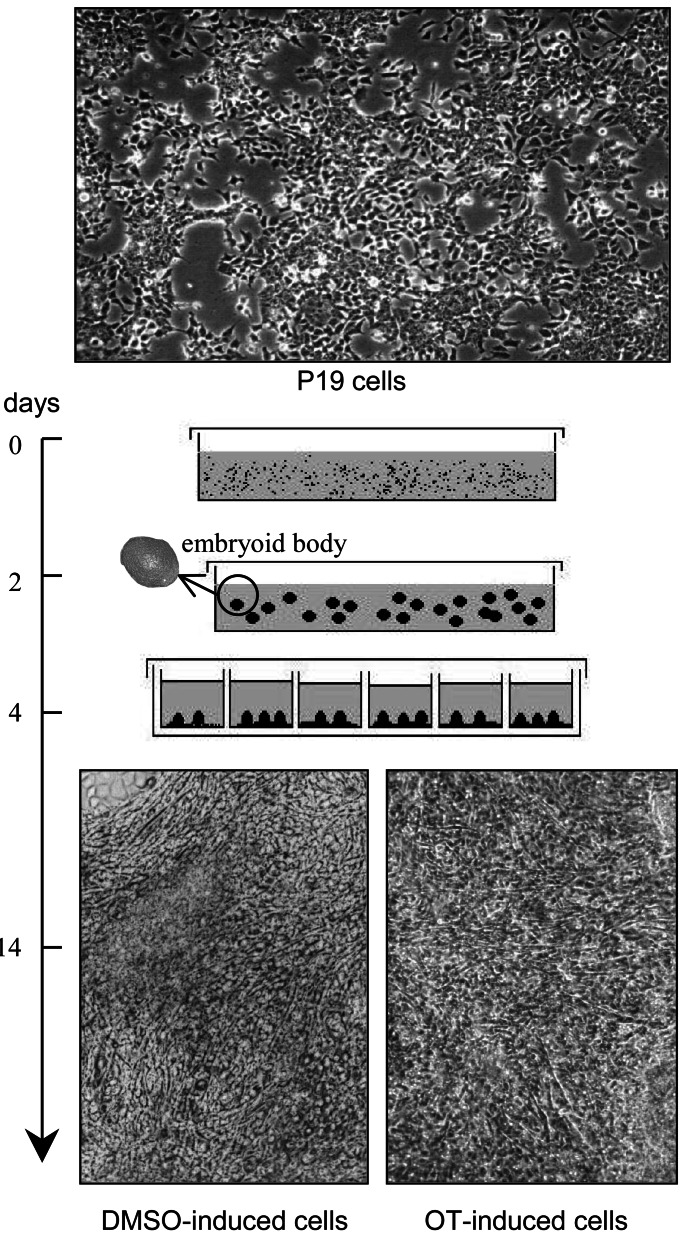
P19 cells were propagated and differentiated according to the procedures of Rudnicki and McBurney (26), with minor modifications. Undifferentiated cells were propagated in α-modified Eagle's MEM (GIBCO-BRL Burlington, ON, Canada) supplemented with 2.5% heat-inactivated FBS, 7.5% heat-inactivated donor bovine serum (Cansera International, Rexdale, ON, Canada), and the antibiotics (GIBCO/BRL) penicillin G (50 units/ml) and streptomycin (50 μg/ml). The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and passaged every 2 days. The general protocol used for differentiation of P19 cells is depicted in Fig. 1.
Figure 1.
Time schedule of the differentiation of P19 cells to cardiomyocytes. P19 cells were cultivated as aggregates from day 0 to day 4 in the presence of DMSO (0.5%) or OT (10−7 M) as the agent inducing cellular differentiation. At day 4, aggregates (embryoid bodies) were transferred to tissue culture dishes or multiwell plates and grown in the absence of the agent. Micrographs (×100) show undifferentiated cells and day 14 cardiomyocyte derivatives obtained after DMSO or OT treatment.
Differentiation was routinely induced with DMSO. Briefly, 0.25 × 106 cells were allowed to aggregate for 4 days in nonadhesive bacteriological grade Petri dishes (6-cm diameter) containing 5 ml of complete medium, in the presence of 0.5% DMSO (Sigma). At day 2 of aggregation, the inducing culture medium was replenished. At day 4, aggregates were transferred to tissue culture grade vessels (10-cm diameter dishes or 24/48-well plates) and cultured in complete medium in the absence of differentiation-inducing agent. Aggregation was also done in the absence of DMSO, and in the presence of 10−7 M OT and/or 10−7 OTA ([d(CH2)51,Tyr(Me)2,Thr-4,Orn-8,Tyr-NH29] vasotocin), both from Peninsula Laboratories. The cell populations were analyzed at days 10–14 of the entire differentiation protocol, at a time cardiac cells normally beat synchronously.
Cell Morphology, Staining, and Immunocytochemistry.
Examinations were done under a Zeiss inverted microscope (Zeiss IM, Carl Zeiss, Jena, Germany) equipped with phase-contrast objectives, filters for rhodamine and fluorescein fluorescence, an MC 100 camera, and a photoautomat unit. Micrographs were taken with Kodak Technical Pan film (for cell morphology) or with Kodak T-Max 400 or Elite-II 100 film (for fluorescence).
For morphological examination, cells were grown directly onto the plastic surface of tissue culture vessels. For staining with rhodamine 123 (Sigma), day-4 aggregates were distributed in 24-well culture plates and grown until day 8. Then, dye was added to the culture medium at a final concentration of 1 μg/ml for 45 min, and afterward, the cells were washed extensively with PBS and cultured for 48 h in the absence of the dye. Dye retained by cells in each well was measured by a fluorescence microplate reader (SPECTRA Max Gemini, Molecular Devices, Sunnyvale, CA) at 505 nm for excitation and 534 nm for emission.
For immunocytofluorescence studies, cells were grown onto glass coverslips coated with 0.1% gelatin. They were then fixed by 20-min incubation in PBS containing 4% paraformaldehyde, rinsed in PBS, and stored at 4°C in this buffer until used. All subsequent steps of permeabilization, washing, and incubation with antibodies were performed at room temperature. Fixed cells were permeabilized for 10 min in PBS containing 0.005% saponin, blocked for 60 min in PBS/BSA/saponin (PBS containing 1% BSA and 0.005% saponin), and incubated for 45 min with the primary antibody diluted 1/50 and for 45 min with a fluorescein-conjugated swine anti-goat IgG antibody (Biosource International, Camarillo, CA) diluted 1/1,000. PBS/BSA/saponin was used for washing between incubations and antibodies were diluted in the same buffer but containing 1.5% normal swine serum (Jackson ImmunoResearch). Coverslips were mounted in PBS containing 50% glycerol and were immediately examined under the microscope. The primary antibodies were all from Santa Cruz Biotechnology and produced in goat: antibody C-20 against OTR, antibody K-16 against sarcomeric myosin heavy chain (MHC), and antibody N-19 against dihydropyridine receptor-α1 (DHPR-α1).
Analysis by Reverse Transcription–PCR (RT-PCR).
Total cellular RNA was extracted with Trizol Reagent (Invitrogen Life Technologies, Burlington, ON, Canada), and poly(A)+ mRNA was affinity purified from 200 μg of total RNA onto Oligotex mRNA columns (Qiagen, Mississauga, ON, Canada), according to the manufacturers' instructions. First-strand cDNA was synthesized in a final volume of 40 μl containing first-strand buffer, 3 μg of cellular RNA, 4 μl of hexanucleotide primers (Amersham Pharmacia-Pharmacia, Baie d'Urfé, QC, Canada), and avian myeloblastosis virus reverse transcriptase (12 units/μg RNA; Invitrogen). First-strand cDNA (5 μl) was then used for PCR amplification with OTR, ANP, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) exon-specific oligonucleotide primers in a Robocycler Gradient 40 thermocycler (Stratagene). Sequences of mouse OTR and ANP genes have been described (1, 32). Conditions for RT-PCR analysis of mouse OTR were adapted from Wagner et al. (6, 7). For all PCR studies the number of cycles used was within the linear range of amplification. The OTR sense and antisense primers were, respectively, the 22-bp 5′-AAGATGACCTTCATCATTGTTC-3′ and the 23-bp 5′-CGACTCAGGACGAAGGTGGAGGA-3′. Amplification was performed over 32 cycles, each involving 1 min at 94°C, 1.5 min at 62°C, and 1.5 min at 72°C, and was terminated by a 5-min final extension at 72°C. The ANP antisense and sense primers were, respectively, the 24-bp 5′-GTCAATCCTACCCCCGAAGCAGCT-3′ and the 20-bp 5′-CAGCATGGGCTCCTTCTCCA-3′. Amplification was performed over 25–30 cycles, each involving 1 min at 94°C, 1 min at 65°C, and 3 min at 72°C, and was terminated by a 5-min final extension at 72°C. The amplification of GAPDH mRNA, a constitutively and ubiquitously expressed gene, served as an internal standard for RT-PCR analysis. The 23-bp antisense primer 5′-CAGTGATGGCATCCACTGTGGTC-3′ and the 23-bp sense primer 5′-AAGGTCGGTGTCAACCCATTTGGCCGT-3′ were used. Amplification was performed over 23 cycles, each involving 1 min at 94°C, 1.5 min at 59°C, and 2 min at 72°C.
Western Blot Analysis.
Cells were collected by scraping, homogenized in sucrose buffer (20 mM Hepes/Tris, pH 7.4, containing 250 mM sucrose and 20 μg/ml of the protease inhibitor phenylmethylsulfonyl fluoride), then centrifuged at 3,000 × g for 10 min at 4°C to remove debris. The supernatants were centrifuged at 100,000 × g for 45 min at 4°C, and the pellets were resuspended in sucrose buffer for analysis of protein content by a modified Bradford assay (28). Aliquots (20 μg of protein) were subjected to polyacrylamide gel electrophoresis in the presence of SDS (SDS/PAGE) under reducing conditions (33) followed by electrotransfer onto pure nitrocellulose membrane (Hybond-C; Amersham Pharmacia-Pharmacia). Molecular size was calibration with Broad Standard Solution (Bio-Rad; Mississauga, ON, Canada). The nitrocellulose blots were blocked overnight with 5% nonfat milk in Tris-buffered saline (TBS: 20 mM Tris⋅HCl, pH 8.0/140 mM NaCl/1% BSA/0.1% Tween-20), then probed with goat C20 antibody (anti-OTR; 1/1,000) for 2 h at room temperature. Antibody incubations and washes were performed in TBS throughout. Detection was realized by enhanced chemiluminescence with an Amersham Pharmacia-Pharmacia ECL kit and an appropriate peroxidase-conjugated secondary antibody (27). Autoluminograms were developed in an AFP Imaging Minimed 190 X-Ray Film Processor (AFP, Elmsford, NY).
Statistics.
Results are reported as the mean values ± SEM. Treatments were compared by unpaired Student's t test.
Results
When the time schedule depicted in Fig. 1 was used, treatment of
P19 cell aggregates with 10−7 M OT induced the
formation of rhythmically beating cells resembling primary
cardiomyocytes isolated from the heart of newborn animals. A similar
phenotypic change was already reported for treatment with 0.5–1% DMSO
(25, 26, 28, 30). We observed that aggregates treated with OT or DMSO
had a mean diameter  that of their untreated
counterparts (data not shown), a finding that could reflect the
antimitotic activity of OT and DMSO.
that of their untreated
counterparts (data not shown), a finding that could reflect the
antimitotic activity of OT and DMSO.
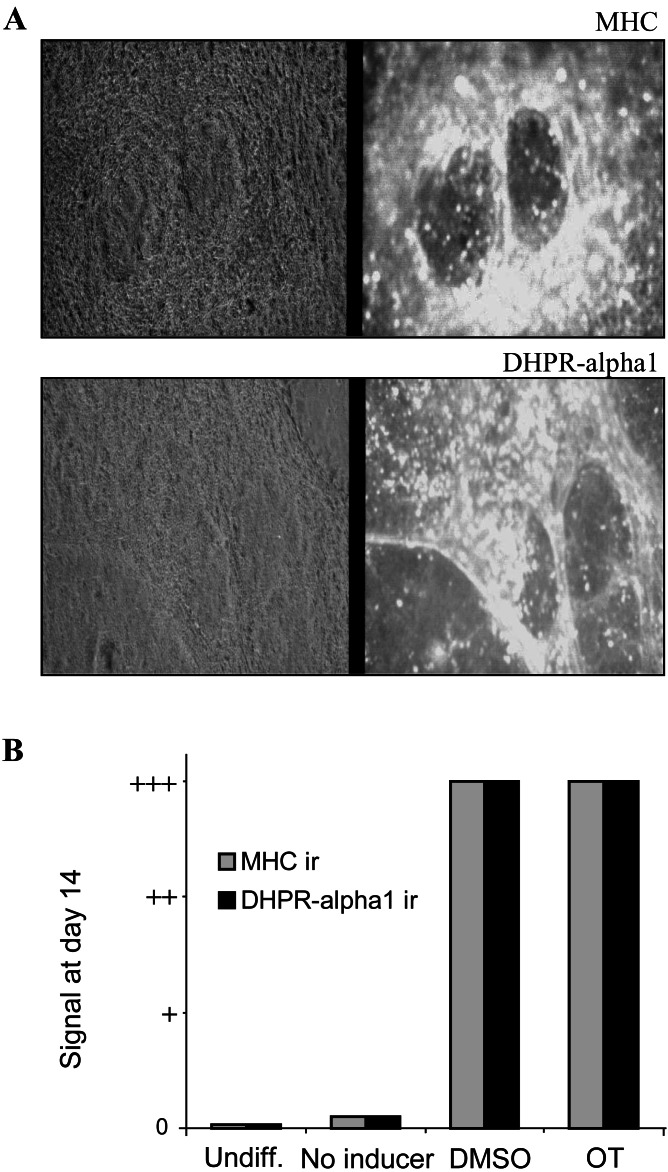
We examined whether treatment of cell aggregates with OT induced the expression of the cardiac muscle markers sarcomeric MHC and DHPR-α1. Sarcomeric MHC is expressed in contractile muscle cells, as is DHPR-α1, a component of intracellular junctions critical for the coupling of excitation and contraction (25, 30, 34). As presented in Fig. 2B, undifferentiated cells were negative for MHC, as reported (25, 26, 30), and for DHPR-α1. However as with DMSO, OT induced the appearance of numerous, intense, immunoreactive foci in cell populations (Fig. 2). In both cases, there were cell subpopulations that did not respond positively (Fig. 2A) and seemed to be mainly undifferentiated cells according to morphological criteria. We and others have shown that undifferentiated cells remain in DMSO-treated P19 cultures by probing for stage-specific embryonic antigen 1, an established marker of the undifferentiated state (25, 26, 28). Cell aggregates not exposed to OT or DMSO were not positive for MHC and DHPR-α1, although they sometimes showed very rare and small immunoreactive foci (Fig. 2B, no inducer). This occasional staining could be because of spontaneous differentiation events triggered by high cell densities such as those encountered in aggregates (25, 26).
Figure 2.
OT induces myocyte immunological markers in P19 cells. P19 cell aggregates were treated from day 0 to day 4 with DMSO, OT, or no differentiation agent and were stained on day 14 with anti-MHC or anti-DHPR-α1 antibodies. (A) Micrographs (×100) show day 14 cells exposed to OT treatment. Normal light (Left) and fluorescence (Right) pictures are presented side by side. (B) Immunoreactivity (ir) signals were obtained for undifferentiated cells grown in monolayers (Undiff.), nontreated cell aggregates (No inducer), and cell aggregates treated with DMSO or OT. Immunoreactive foci were absent (0), very rare (slightly above 0), or abundant (++ and +++). Results are representative of three independent experiments. Although not presented, aggregates were also treated for 6 days with OT. There was no difference with the 4-day treatment.
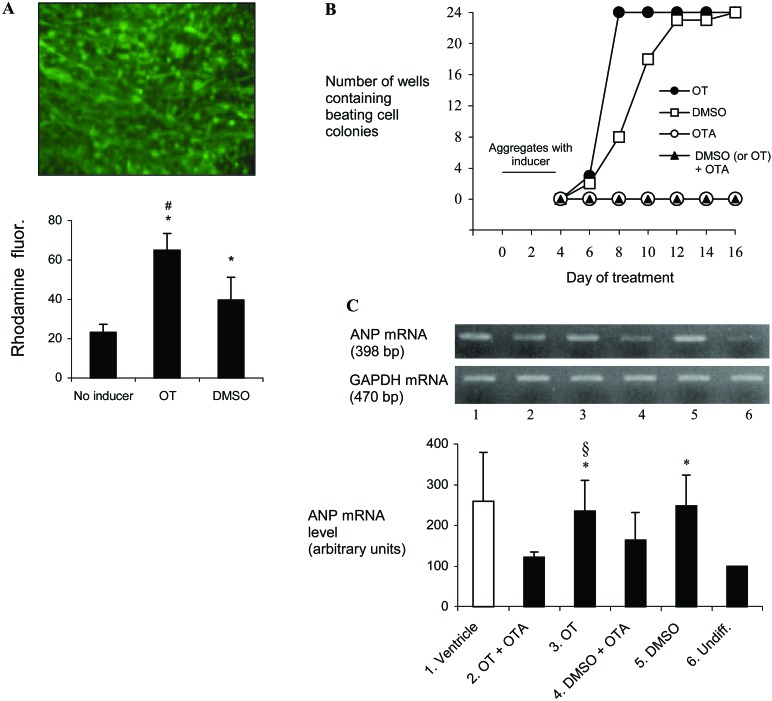
We also compared the cardiogenic potency of OT and DMSO. First, potency was simply quantitated by rhodamine 123 retention in cells, taking advantage of the fact that this dye, which penetrates all cell types, is retained for much longer periods (days instead of hours) in cardiac cells than in other cell types (35). To meet their energy requirements for muscular contraction, cardiomyocytes have indeed abundant mitochondria, the cell organelles that accumulate rhodamine 123. Fig. 3A shows that exposure of the cell aggregates to OT and DMSO significantly increased cellular retention of the dye by 2- to 3-fold compared with noninduced aggregates (P < 0.001), and this increase at day 10 of differentiation was even significantly higher after OT than DMSO treatment (P < 0.001). Because P19-derived cardiomyocytes beat in culture, we also compared the time course of appearance of beating cells after treatment of aggregates with DMSO or OT. We found that OT stimulated the production of beating cell colonies in all 24 independently growing cultures by day 8, whereas the same result was obtained in cells induced by DMSO only by day 12 (Fig. 3B). The cardiogenic action of OT was specific and receptor-mediated, because no beating cells were seen when 10−7 M OTA was used in place of OT or in combination with OT (Fig. 3B). Interestingly, OTA also abolished the cardiogenic action of DMSO (Fig. 3B). Finally, cardiogenic potency was evaluated by means of ANP expression because this peptide is abundantly produced by cardiomyocytes. The results showed that at day 14 of differentiation ANP mRNA level was significantly up-regulated in OT-treated P19 aggregates as compared with undifferentiated cells (P < 0.05), and this up-regulation was at similar level after DMSO treatment (Fig. 3C). As for cell beating, OTA prevented OT-induced up-regulation of ANP expression (Fig. 3C, P < 0.05). Although the effect of OTA on DMSO-induced ANP expression was not statistically significant, the inhibitory tendency was observed in all experiments (Fig. 3C). The inhibitory action of OTA on DMSO cardiomyogenic properties was thus more evident by the beating than by the ANP criterion. Altogether, rhodamine 123 absorption, and the time-course of formation of beating cells and abundance of ANP mRNA pointed to a potent cardiomyogenic effect of OT. In addition, the cardiomyogenic action of OT and even that of DMSO appear to involve OTR.
Figure 3.
Comparison of the cardiomyogenic effect of OT and DMSO. (A) Retention of rhodamine 123 in noninduced and induced P19 cultures. P19 cells were cultured as aggregates for 4 days in the absence (No inducer) or the presence of OT or DMSO, using one Petri dish per treatment. At day 4, aggregates of each Petri dish were evenly distributed in wells of a 24-well tissue culture plate. At day 8, the cells were incubated for 45 min in the presence of 1 μg/ml of the dye, washed extensively, and cultured in complete medium without dye for 48 h. The photograph shows rhodamine 123 retention by cells induced by OT at day 10 of culture. The retained dye was fluorimetrically quantified for each well, and the results are reported as the means ± SEM of 24 determinations. The * indicates a highly significant difference with No inducer, and #, a highly significant difference between OT and DMSO treatments (P < 0.001). (B) Time course of appearance of beating cell colonies upon treatment with different agents. Aggregates of 1 Petri dish treated for 4 days with the indicated agent(s) were evenly distributed in wells of a 24-well tissue culture plate. Then each plate was examined at 2-day intervals for the number of wells containing beating cell colonies. The results are representative of three independent differentiation experiments. (C) RT-PCR analysis of ANP gene transcript in undifferentiated and induced cultures. Cell aggregates were exposed to OT or DMSO in the absence or presence of OTA from day 0 to day 4, and RNA was extracted at day 14 of the differentiation protocol. ANP transcript was also evaluated in undifferentiated cells grown in monolayers (Undiff.). Mouse heart ventricle mRNA was used as a positive control. Levels of ANP mRNA were adjusted by dividing by corresponding GAPDH mRNA and then expressed as the percentage of the Undiff. value. Results are reported as the means ± SEM of five independent studies. The * indicates a significant difference with Undiff. and §, a significant difference between OT and OT + OTA treatments (P < 0.05).
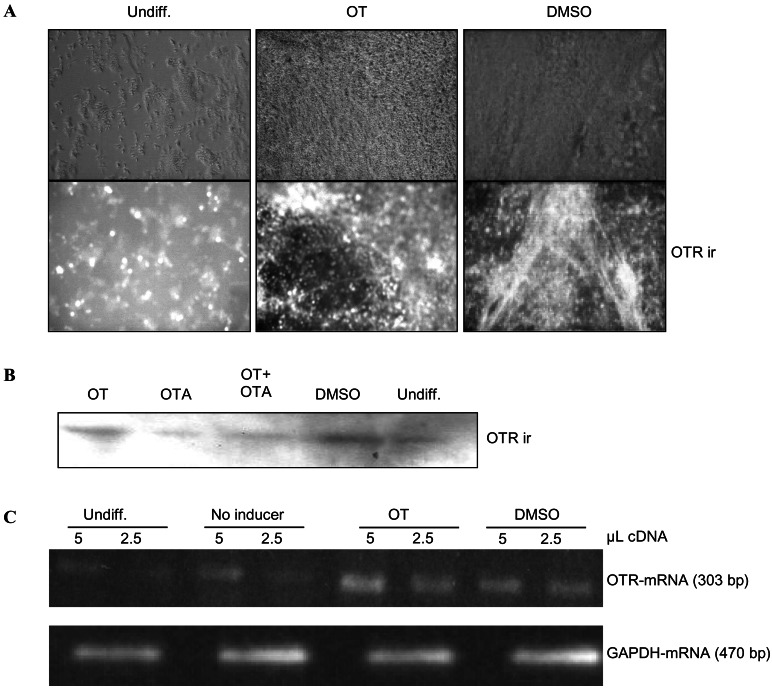
To further investigate the involvement of OTR in cardiomyogenesis, we examined OTR expression in P19 cells. OTR protein (Fig. 4 A and B) and mRNA (Fig. 4C) were present at low levels in undifferentiated cells, indicating that these cells can respond minimally to OT. OTR expression remained at low levels in aggregates not exposed to OT or DMSO (Fig. 4C, No inducer). In contrast, intense OTR immunoreactive foci were observed in cell populations after OT or DMSO treatment (Fig. 4A). These findings corresponded to the results of Western blotting (Fig. 4B) and RT-PCR analysis of OTR (Fig. 4C), both indicating increased OTR expression. In accordance with the absence of a cardiomyogenic effect of OTA and the inhibitory action of OTA on OT-induced cardiac differentiation, OTA did not up-regulate OTR expression by itself and inhibited OT-induced OTR up-regulation (Fig. 4B). Thus, the OTR-dependent cardiogenic effect of OT and DMSO seems to involve up-regulation of OTR expression.
Figure 4.
OT and DMSO increase OTR expression in P19 cells. P19 cells were cultured as aggregates for 4 days in the absence (No inducer) or presence of DMSO (0.5%), OT (10−7 M), and/or OTA (10−7 M) and then plated in tissue culture dishes, where they grew in the absence of the agent. At day 14 of differentiation, the cells were examined for OTR expression in the form of immunoreactivity (ir), together with undifferentiated (Undiff.) cells grown in monolayers. The results are representative of three independent differentiation experiments. (A) Immunocytochemistry. (B) Immunoblotting (20 μg of protein per lane). (C) RT-PCR.
Discussion
This report shows that OT added to the culture medium of P19 stem cell aggregates induced cardiomyogenic differentiation, which was demonstrated by monitoring the expression of MHC, DHPR-α1, and ANP cardiac markers, retention of a mitochondria-specific dye, and the appearance of beating cell colonies. The cardiogenic effect of OT was specific and mediated by OTR, since it was abolished by OTA. OT also up-regulated OTR expression. These results suggest a new role for the OT/OTR system in heart genesis and development.
The P19 cell line is an excellent cell differentiation model that mimics the events of early cardioembryogenesis. Differentiation of P19 cells to cardiomyocytes by aggregation and exposure to DMSO was shown to be associated with induction of the cardiac-specific subtype of endothelin receptors (36). In addition, brain natriuretic peptide and ANP were observed in newly formed striated muscle structures upon DMSO treatment and not in undifferentiated P19 cells and their neuronal derivatives (37). In this work, DMSO- and OT-induced ANP transcript levels reached about 5–10% of th level found in the adult mouse atrium—the richest site of ANP synthesis. Several transcription factors having an essential role in cardiogenesis are up-regulated in DMSO-induced P19 cells. This was shown to be the case for the zinc-finger-containing GATA-4, the homeobox gene Nkx2–5, and the myocyte enhancer factor 2C (30, 31, 38), and the overexpression of either factor in P19 cells was sufficient to induce cardiac differentiation in the absence of DMSO (30, 39, 40). Little is known about the molecular mechanisms underlying the activation of these genes, but DMSO was found to increase intracellular Ca2+ levels and was suspected to affect a pathway that has an extracellular component, possibly serum-borne (25, 41, 42). Interestingly, our data indicate that OTRs are up-regulated to a similar extent by OT and DMSO, and other studies have reported that OTR function modulates intracellular Ca2+ concentration in some cell types (1). It is thus tempting to suggest that OT could be a serum-borne factor that is active in DMSO-induced differentiation. Alternatively, the hormone could be produced by P19 cells or their derivatives, since OT synthesis was demonstrated in cardiomyocyte cultures from newborn rats (7), and OT levels were significantly higher in the cardiac chambers of fetuses and 1-day-old neonates than in adult animals**. Hartman et al. (43) also reported OT levels that are high in the plasma of young animals and decrease during development.
One of the mechanisms by which OT and DMSO trigger cardiac differentiation involves OTR, since both agents up-regulated the expression of this receptor, and OTA abolished their cardiomyogenic action as well as prevented OT-stimulating effect on OTR expression. Homologous regulation of OTR expression by OT itself was observed in the brain and in astroglial cell cultures (44, 45). It is noteworthy that, like DMSO, retinoic acid, used at low levels (10−8 to 10−9 M), induces cardiac differentiation of P19 cells (25, 26). This observation could have some relevance to the OT/OTR system, because retinoic acid was shown to up-regulate OT expression in the fetal heart.** Further investigations are required to clarify the relationships that could exist among OT/OTR, DMSO, and retinoic acid in the P19 cell model.
Several studies have proposed a role for OT as a growth and differentiation/maturation factor in a gestational/perinatal context. In the mother, OT is required for postpartum alveolar proliferation, and it induces differentiation and proliferation of myoepithelial cells of the mammary gland necessary for milk ejection (1, 18). The OT/OTR system is expressed in human cumulus/luteal cells surrounding oocytes, and weak OTR gene expression is even observed in oocytes (46). Moreover, when fertilized mouse oocytes are cultured with OT in vitro, they develop into the blastocyst stage at a higher rate than their unstimulated counterparts (46). Spontaneous myometrial contractures are known to occur during pregnancy in sheep, and controlled contractures induced by application of OT pulses to pregnant ewes have been shown to accelerate fetal cardiovascular function (47).
All these studies thus strongly suggest involvement of the maternal and embryonal OT/OTR systems in development of the embryo, and our work points to a particular involvement of OT in the priming of cardiogenesis. We think that OT could also assist the maturation of newly differentiated cardiomyocytes by stimulating their fusion, since beating cells derived from OT-induced P19 cells formed fiber-like structures. Such a fusogenic action was recently reported for OT on skeletal myoblasts in vitro (48). Our results may find application in regenerative therapies that consider the replacement of cardiac tissue lost after injury. In this context, OT could be used as a trophic factor to assist the compensatory division of myocytes shown to occur in infarcted organs (49), or to prime the cardiomyogenesis of a variety of progenitor/stem cells to be grafted in the injured heart (50, 51).
In conclusion, our study indicates that OT primes the cardiac differentiation of embryonic stem cells, and its action is mediated by OTR and a transduction pathway(s) that has yet to be defined. These results suggest that the OT/OTR system plays an important role in heart development.
Acknowledgments
We acknowledge the excellent secretarial assistance of Mrs. Antoinette Paolitto. This work was supported by grants of the Canadian Institutes of Health Research (MT-11674 to J.G. and MT-15049 to M.J. and J.G.), a grant from the Université du Québec à Montréal (to J.P.), and National Institutes of Health Grant MH-51853 (to S.M.M.).
Abbreviations
- OT
oxytocin
- OTR
OT receptor
- OTA
OT antagonist
- ANP
atrial natriuretic peptide
- MHC
myosin heavy chain
- DHPR-α1
dihydropyridine receptor-α1
- RT-PCR
reverse transcription–PCR
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
Footnotes
Widmer, H., Durroux, T., Kempf, H., Mouillac, B., Gasc, J. M. & Barberis, C., World Congress on Neurohypophysial Hormones, Aug. 28 to Sept. 12, 1999, Edinburgh, Scotland, p. 94 (abstr.).
Gutkowska, J., Bhat, P., Wang, D., Mukaddam-Daher, S., McCann, S. M. & Jankowski, M., 19th Scientific Meeting of the International Society of Hypertension, June 23–27, 2002, Prague, in press (abstr.)
References
- 1.Gimpl G, Fahrenholz F. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 2.Ashton N, Balment R J. Acta Endocrinol (Copenhagen) 1991;124:91–97. doi: 10.1530/acta.0.1240091. [DOI] [PubMed] [Google Scholar]
- 3.Stoneham M D, Everitt B J, Hansen S, Lightman S L, Todd K. J Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- 4.Verbalis J G, Mangione M P, Stricker E M. Endocrinology. 1991;128:1317–1322. doi: 10.1210/endo-128-3-1317. [DOI] [PubMed] [Google Scholar]
- 5.Nishimori K, Young L J, Guo Q, Wang Z, Insel T R, Matzuk M M. Proc Natl Acad Sci USA. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner K U, Young W S, III, Liu X, Ginns E I, Li M, Furth P A, Hennighausen L. Genes Funct. 1997;1:233–244. doi: 10.1046/j.1365-4624.1997.00024.x. [DOI] [PubMed] [Google Scholar]
- 7.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg H H, McCann S M. Proc Natl Acad Sci USA. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski M, Hajjar F, Al Kawas S, Mukaddam-Daher S, Hoffman G, McCann S M, Gutkowska J. Proc Natl Acad Sci USA. 1998;95:14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Int J Cancer. 1997;72:340–344. doi: 10.1002/(sici)1097-0215(19970717)72:2<340::aid-ijc23>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Cassoni P, Sapino A, Stella A, Fortunati N, Bussolati G. Int J Cancer. 1998;77:695–700. doi: 10.1002/(sici)1097-0215(19980831)77:5<695::aid-ijc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Cassoni P, Fulcheri E, Carcangiu M L, Stella A, Deaglio S, Bussolati G. J Pathol. 2000;190:470–477. doi: 10.1002/(SICI)1096-9896(200003)190:4<470::AID-PATH550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Copland J A, Ives K L, Simmons D J, Soloff M S. Endocrinology. 1999;140:4371–4374. doi: 10.1210/endo.140.9.7130. [DOI] [PubMed] [Google Scholar]
- 13.Martens H, Kecha O, Charlet-Renard C, Defresne M P, Geenen V. Neuroendocrinology. 1998;67:282–289. doi: 10.1159/000054324. [DOI] [PubMed] [Google Scholar]
- 14.Geenen V, Kecha O, Brilot F, Charlet-Renard C, Martens H. Neuroimmunomodulation. 1999;6:115–125. doi: 10.1159/000026371. [DOI] [PubMed] [Google Scholar]
- 15.Plecas B, Popovic A, Jovovic D, Hristic M. J Endocrinol Invest. 1992;15:249–253. doi: 10.1007/BF03348721. [DOI] [PubMed] [Google Scholar]
- 16.Thibonnier M, Conarty D M, Preston J A, Plesnicher C L, Dweik R A, Erzurum S C. Endocrinology. 1999;140:1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- 17.Cassoni P, Sapino A, Munaron L, Deaglio S, Chini B, Graziani A, Ahmed A, Bussolati G. Endocrinology. 2001;142:1130–1136. doi: 10.1210/endo.142.3.8047. [DOI] [PubMed] [Google Scholar]
- 18.Sapino A, Macri L, Tonda L, Bussolati G. Endocrinology. 1993;133:838–842. doi: 10.1210/endo.133.2.8344220. [DOI] [PubMed] [Google Scholar]
- 19.Chard T, Boyd N R, Forsling M L, McNeilly A S, Landon J. J Endocrinol. 1970;48:223–234. doi: 10.1677/joe.0.0480223. [DOI] [PubMed] [Google Scholar]
- 20.Schriefer J A, Lewis P R, Miller J W. Biol Reprod. 1982;27:362–368. doi: 10.1095/biolreprod27.2.362. [DOI] [PubMed] [Google Scholar]
- 21.Haanwinckel M A, Elias L K, Favaretto A L, Gutkowska J, McCann S M, Antunes-Rodrigues J. Proc Natl Acad Sci USA. 1995;92:7902–7906. doi: 10.1073/pnas.92.17.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favaretto A L, Ballejo G O, Albuquerque-Araujo W I, Gutkowska J, Antunes-Rodrigues J, McCann S M. Peptides. 1997;18:1377–1381. doi: 10.1016/s0196-9781(97)00209-x. [DOI] [PubMed] [Google Scholar]
- 23.Mukaddam-Daher S, Yin Y L, Roy J, Gutkowska J, Cardinal R. Hypertension. 2001;38:292–296. doi: 10.1161/01.hyp.38.2.292. [DOI] [PubMed] [Google Scholar]
- 24.Cameron V A, Aitken G D, Ellmers L J, Kennedy M A, Espiner E A. Endocrinology. 1996;137:817–824. doi: 10.1210/endo.137.3.8603590. [DOI] [PubMed] [Google Scholar]
- 25.McBurney M W. Int J Dev Biol. 1993;37:135–140. [PubMed] [Google Scholar]
- 26.Rudnicki M A, McBurney M W. In: Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. Robertson E J, editor. Oxford: IRL Press; 1987. pp. 19–49. [Google Scholar]
- 27.Laplante I, Paquin J, Beliveau R. Brain Res Dev Brain Res. 2001;129:157–168. doi: 10.1016/s0165-3806(01)00197-3. [DOI] [PubMed] [Google Scholar]
- 28.Jeannotte R, Paquin J, Petit-Turcotte C, Day R. DNA Cell Biol. 1997;16:1175–1187. doi: 10.1089/dna.1997.16.1175. [DOI] [PubMed] [Google Scholar]
- 29.Cadet N, Paquin J. Dev Brain Res. 2000;120:211–221. doi: 10.1016/s0165-3806(00)00011-0. [DOI] [PubMed] [Google Scholar]
- 30.Skerjanc I S. Trends Cardiovasc Med. 1999;9:139–143. doi: 10.1016/s1050-1738(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava D, Olson E N. Nature (London) 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 32.Seidman C E, Bloch K D, Smith J A, Seidman J G. Science. 1984;226:1206–1209. doi: 10.1126/science.6542248. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Flucher B E, Franzini-Armstrong C. Proc Natl Acad Sci USA. 1996;93:8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summerhayes I C, Lampidis T J, Bernal S D, Nadakavukaren J J, Nadakavukaren K K, Shepherd E L, Chen L B. Proc Natl Acad Sci USA. 1982;79:5292–5296. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monge J C, Stewart D J, Cernacek P. J Biol Chem. 1995;270:15385–15390. doi: 10.1074/jbc.270.25.15385. [DOI] [PubMed] [Google Scholar]
- 37.Boer P H. Biochem Biophys Res Commun. 1994;199:954–961. doi: 10.1006/bbrc.1994.1322. [DOI] [PubMed] [Google Scholar]
- 38.Mohun T, Sparrow D. Curr Opin Genet Dev. 1997;7:628–633. doi: 10.1016/s0959-437x(97)80010-x. [DOI] [PubMed] [Google Scholar]
- 39.Grepin C, Nemer G, Nemer M. Development (Cambridge, UK) 1997;124:2387–2395. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- 40.Skerjanc I S, Petropoulos H, Ridgeway A G, Wilton S. J Biol Chem. 1998;273:34904–34910. doi: 10.1074/jbc.273.52.34904. [DOI] [PubMed] [Google Scholar]
- 41.Morley P, Whitfield J F. J Cell Physiol. 1993;156:219–225. doi: 10.1002/jcp.1041560202. [DOI] [PubMed] [Google Scholar]
- 42.Wilton S, Skerjanc I. In Vitro Cell Dev Biol Anim. 1999;35:175–177. doi: 10.1007/s11626-999-0023-7. [DOI] [PubMed] [Google Scholar]
- 43.Hartman R D, Rosella-Dampman L M, Emmert S E, Summy-Long J Y. Endocrinology. 1986;119:1–11. doi: 10.1210/endo-119-1-1. [DOI] [PubMed] [Google Scholar]
- 44.Insel T R, Winslow J T, Witt D M. Endocrinology. 1992;130:2602–2608. doi: 10.1210/endo.130.5.1315251. [DOI] [PubMed] [Google Scholar]
- 45.Di Scala-Guenot D, Strosser M T. Am J Physiol. 1995;268:C413–C418. doi: 10.1152/ajpcell.1995.268.2.C413. [DOI] [PubMed] [Google Scholar]
- 46.Furuya K, Mizumoto Y, Makimura N, Mitsui C, Murakami M, Tokuoka S, Ishikawa N, Nagata I, Kimura T, Ivell R. Adv Exp Med Biol. 1995;395:523–528. [PubMed] [Google Scholar]
- 47.Shinozuka N, Yen A, Nathanielsz P W. Am J Physiol. 2000;278:H41–H49. doi: 10.1152/ajpheart.2000.278.1.H41. [DOI] [PubMed] [Google Scholar]
- 48.Breton C, Haenggeli C, Barberis C, Heitz F, Bader C R, Bernheim L, Tribollet E. J Clin Endocrinol Metab. 2002;87:1415–1418. doi: 10.1210/jcem.87.3.8537. [DOI] [PubMed] [Google Scholar]
- 49.Beltrami A P, Urbanek K, Kajstura J, Yan S M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami C A, et al. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 50.Condorelli G, Borello U, De Angelis L, Latronico M, Sirabella D, Coletta M, Galli R, Balconi G, Follenzi A, Frati G, et al. Proc Natl Acad Sci USA. 2001;98:10733–10738. doi: 10.1073/pnas.191217898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson K A, Majka S M, Wang H, Pocius J, Hartley C J, Majesky M W, Entman M L, Michael L H, Hirschi K K, Goodell M A. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]