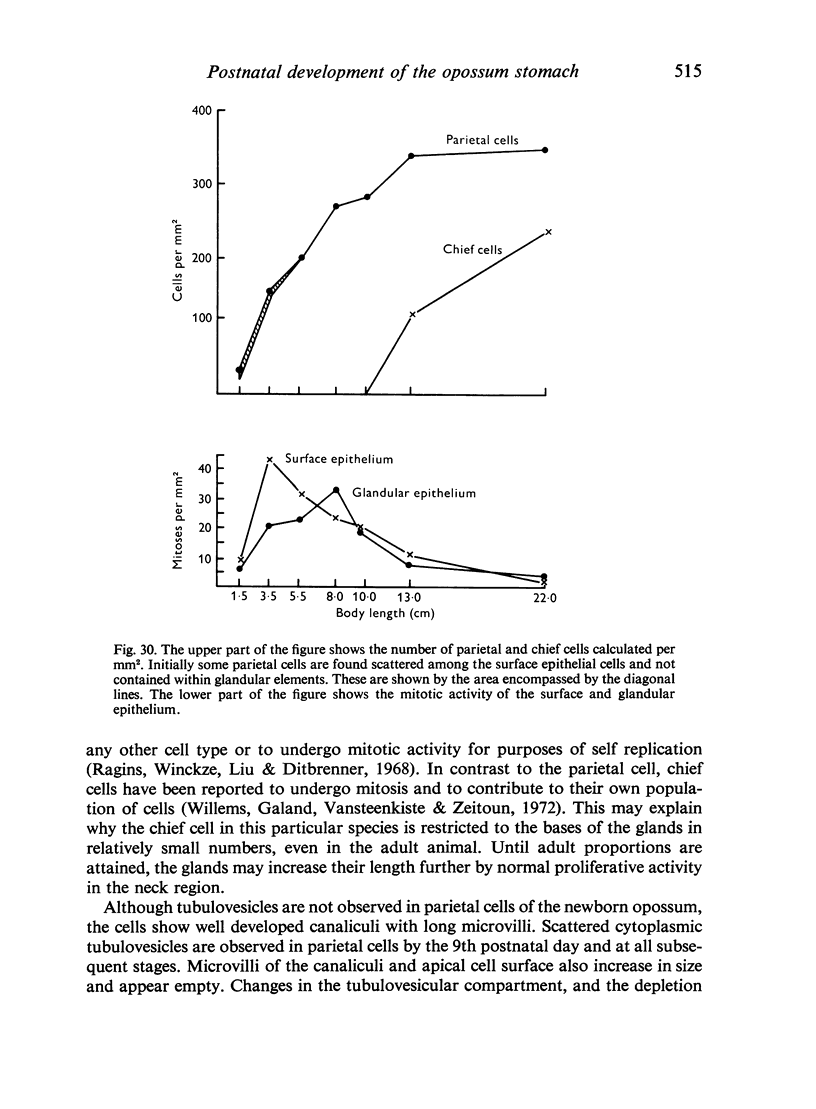
Abstract
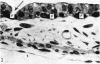
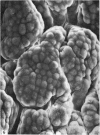
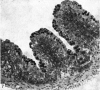
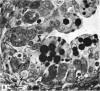
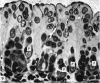
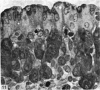
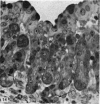
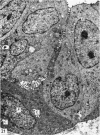
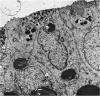
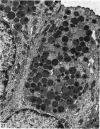
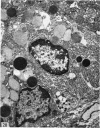
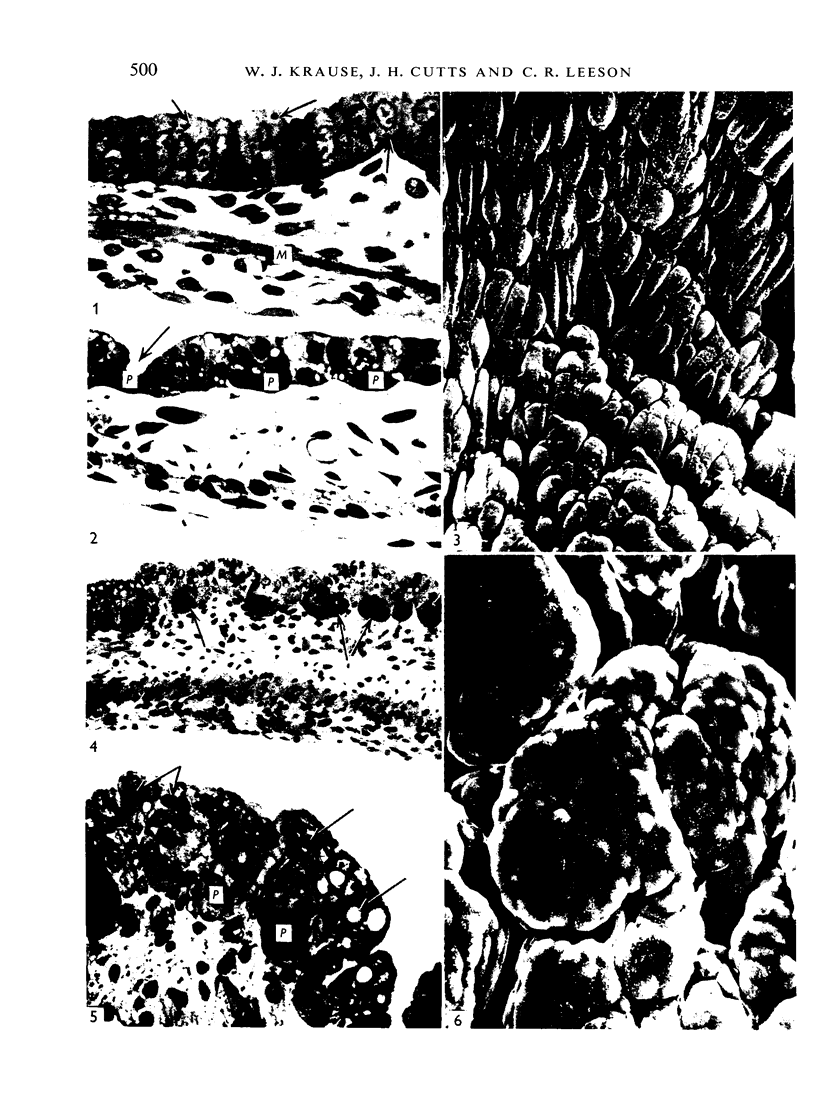
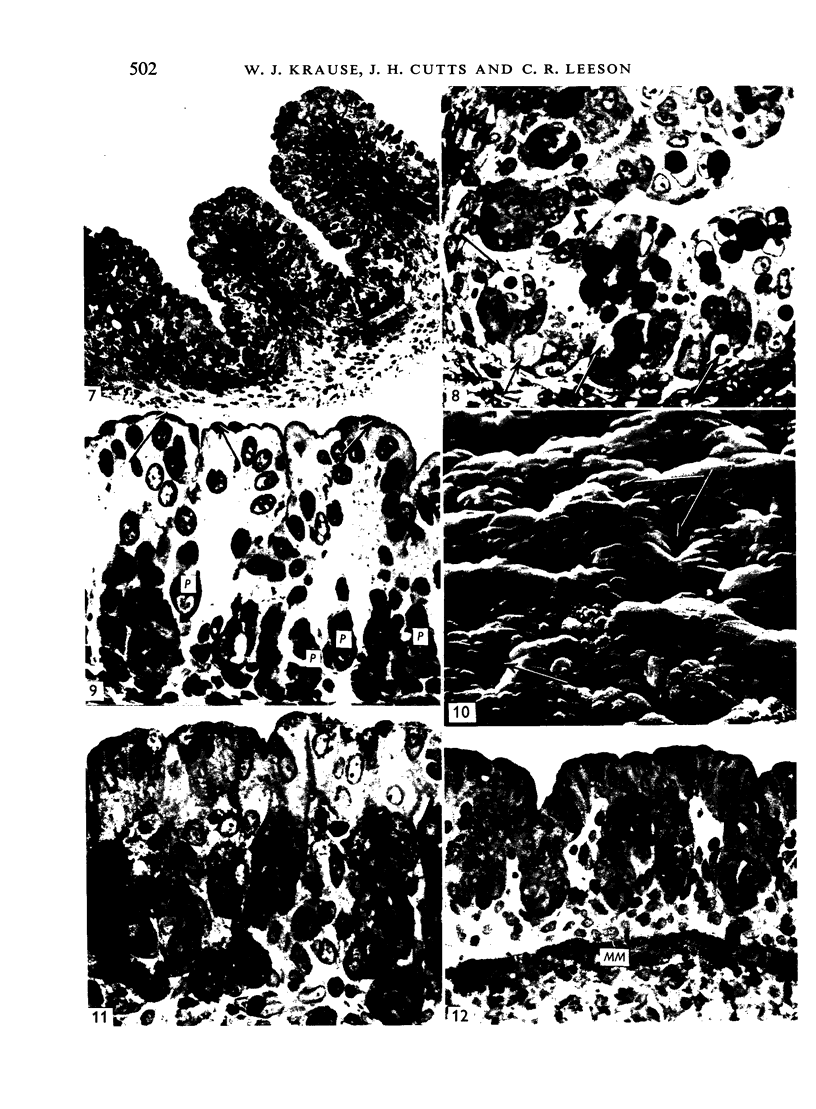
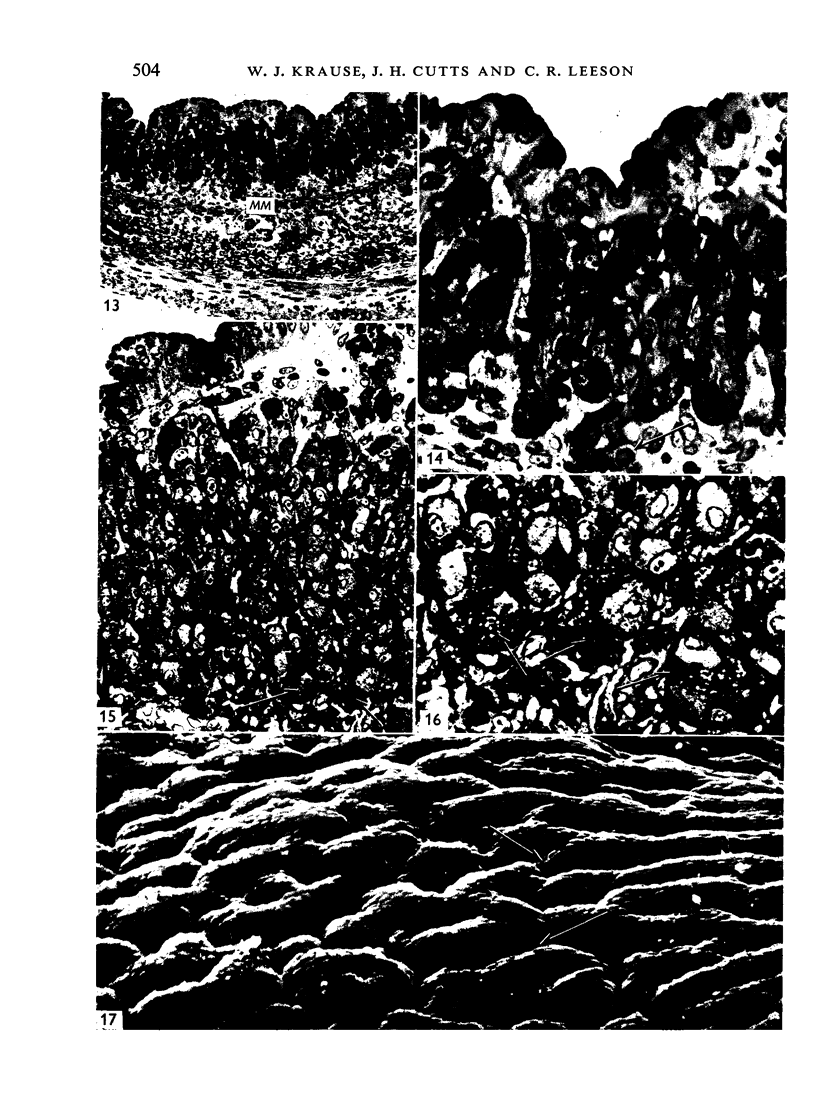
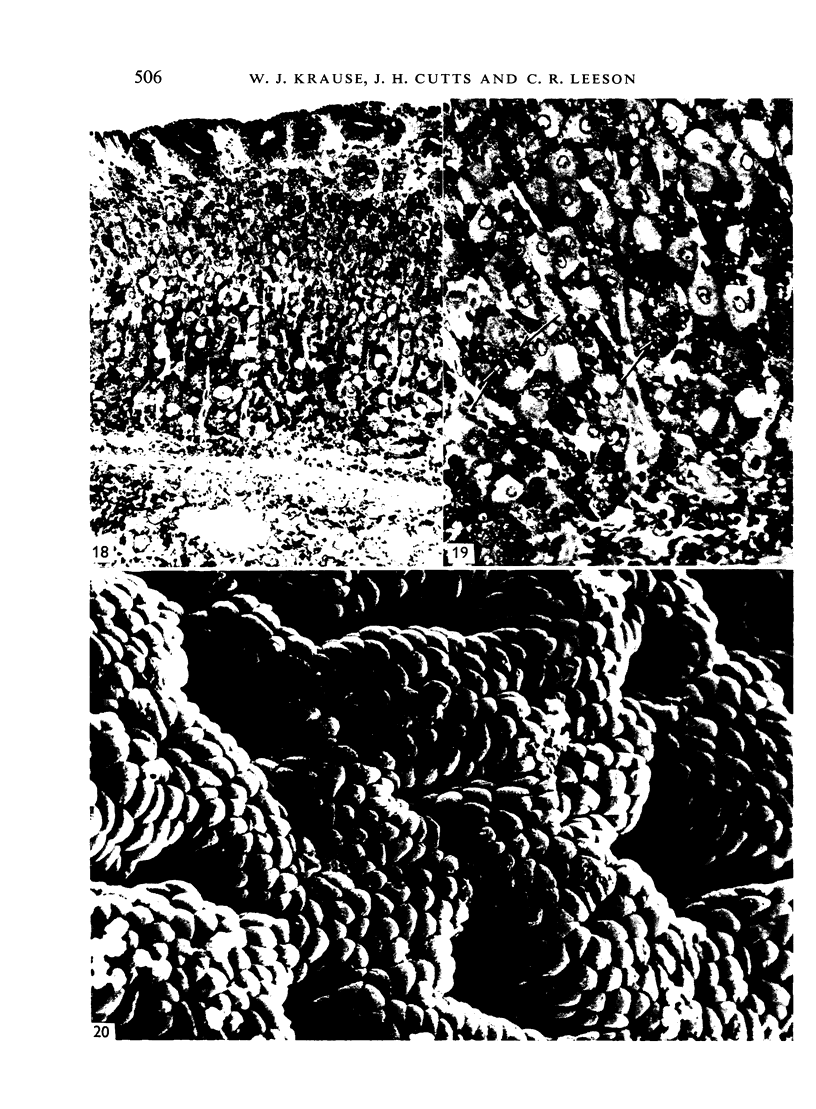
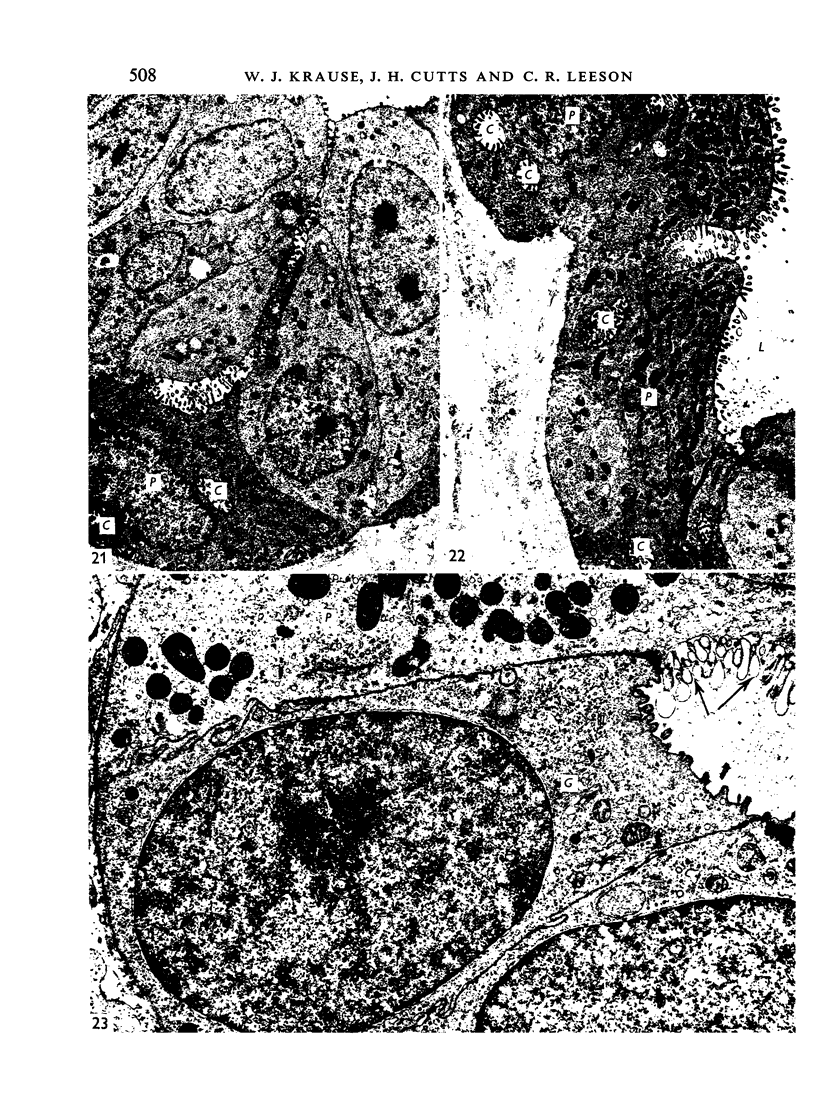
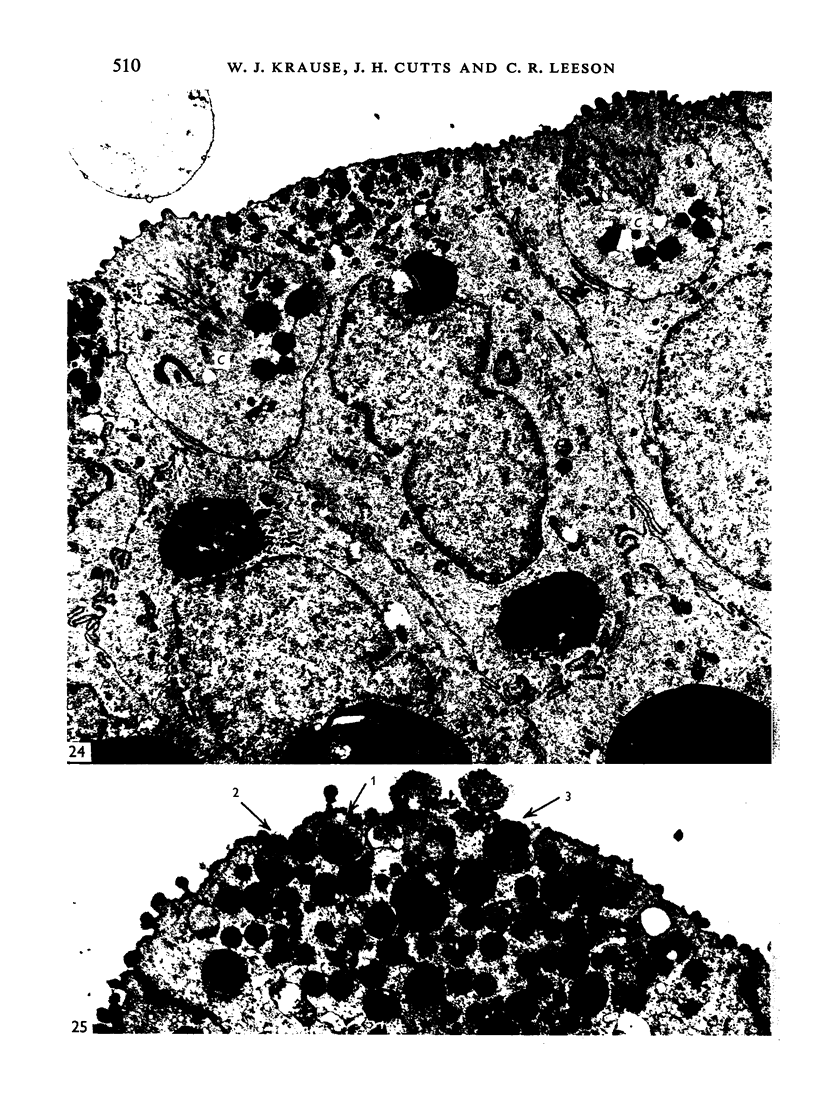
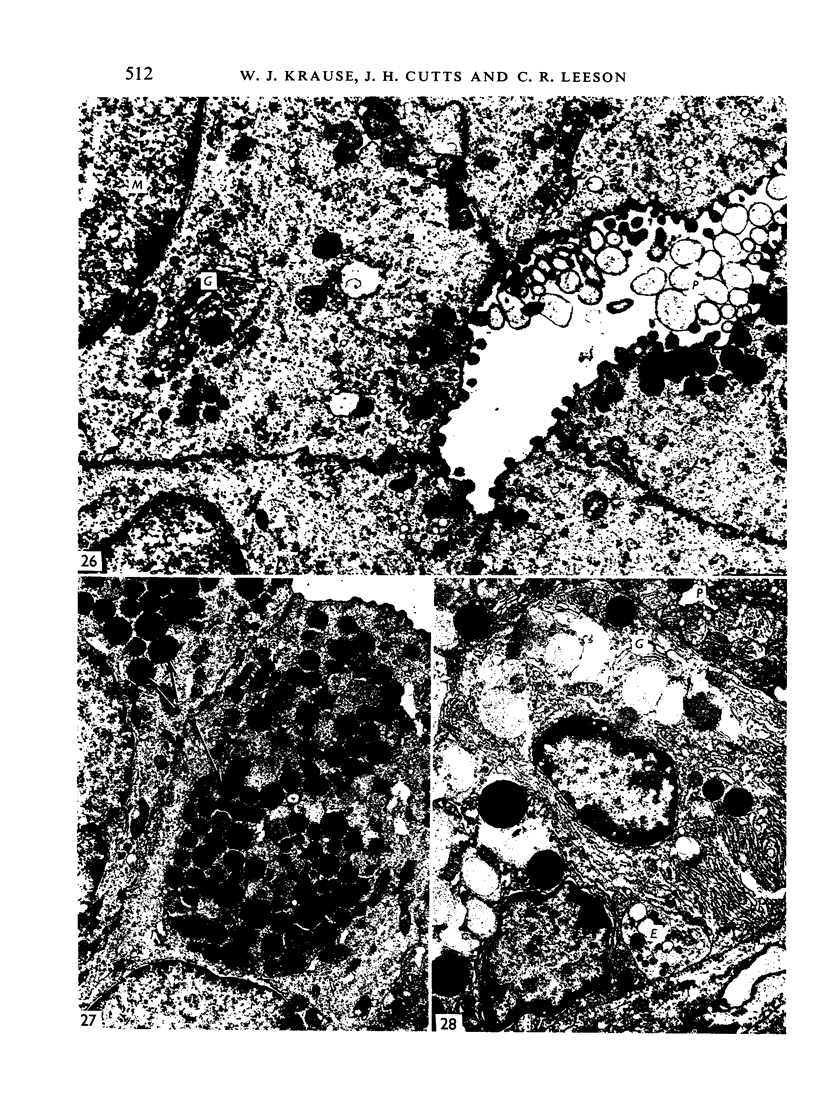
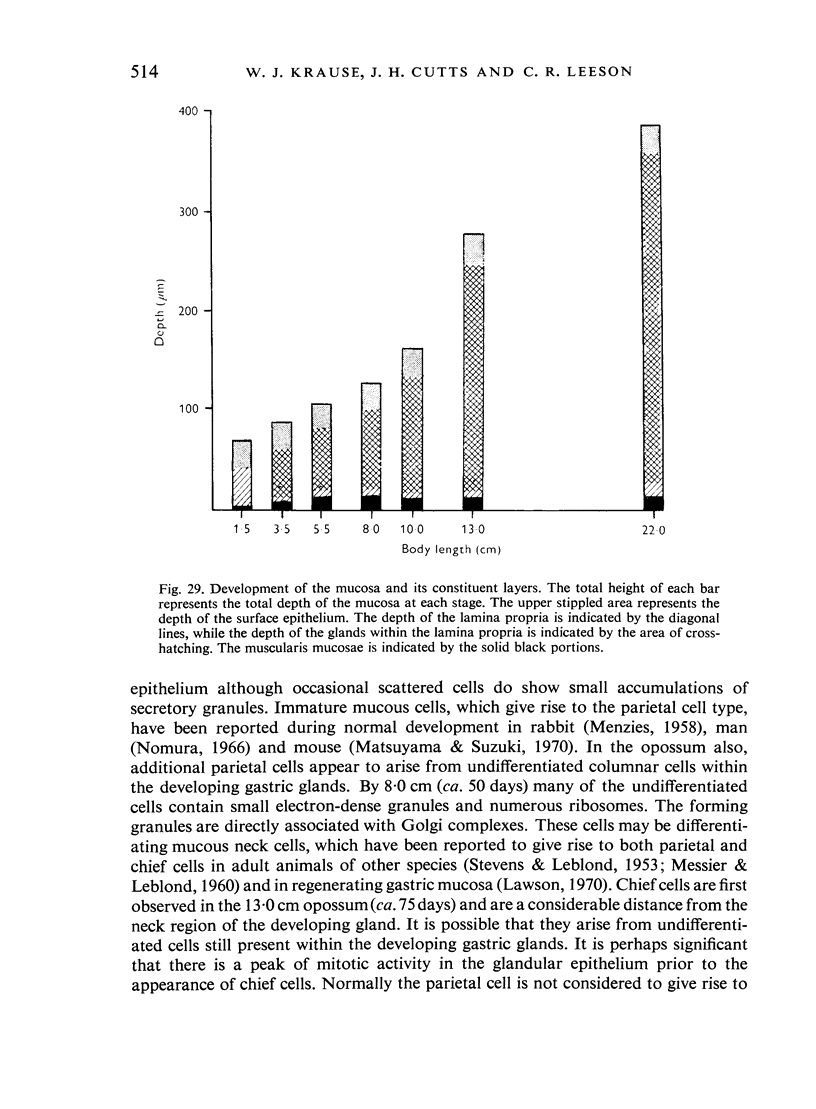
The postnatal development of the gastric mucosa in the opossum has been traced with the light, transmission and scanning electron microscopes. The formation of fovea and gastric glands occurs simultaneously during the postnatal period. During the first 60 postnatal days the developing gastric glands are composed of undifferentiated cells, parietal cells and scattered endocrine cells. Chief cells are not present until just before weaning (13 cm, i.e. ca. 75 days). Juvenile and adult animals show only a small population of chief cells, and these are confined to the bases of the gastric glands. The pH of stomach contents ranges from 6-0 to 6-5 until the time of appearance of solid food within the stomach, when it drops to 2-0-2-5. The surface cells lining the gastric lumen contain a considerable amount of what appears to be lipid during the first 3 weeks after birth, and this may indicate that the gastric mucosa is involved in the absorption of lipid during this period. The mode of lipid absorption appears to be different from that described for the intestinal tract of several other species.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capella C., Solcia E. The endocrine cells of the pig gastrointestinal mucosa and pancreas. Arch Histol Jpn. 1972 Nov;35(1):1–29. doi: 10.1679/aohc1950.35.1. [DOI] [PubMed] [Google Scholar]
- Capella C., Solcia E., Vassallo G. Identification of six types of endocrine cells in the gastrointestinal mucosa of the rabbit. Arch Histol Jpn. 1969 Aug;30(5):479–495. doi: 10.1679/aohc1950.30.479. [DOI] [PubMed] [Google Scholar]
- Cathcart R. S., 3rd, Fitts C. T., McAlhany J. C., Spicer S. S. Histochemical changes in gastric mucosubstances in patients with acute and chronic ulcer disease. Ann Surg. 1974 Jul;180(1):1–8. doi: 10.1097/00000658-197407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. B., Brause B., Holt P. R. Lipolysis and absorption of fat in the rat stomach. Gastroenterology. 1969 Feb;56(2):214–222. [PubMed] [Google Scholar]
- DEANE H. W. SOME ELECTRON MICROSCOPIC OBSERVATIONS ON THE LAMINA PROPRIA OF THE GUT, WITH COMMENTS ON THE CLOSE ASSOCIATION OF MACROPHAGES, PLASMA CELLS, AND EOSINOPHILS. Anat Rec. 1964 Jul;149:453–473. doi: 10.1002/ar.1091490315. [DOI] [PubMed] [Google Scholar]
- Egelrud T., Olivecrona T., Helander H. Sttudies on gastric absorption of lipids in the suckling rat. Scand J Gastroenterol. 1971;6(4):329–333. doi: 10.3109/00365527109181129. [DOI] [PubMed] [Google Scholar]
- Helander H. F., Olivecrona T. Lipolysis and lipid absorption in the stomach of the suckling rat. Gastroenterology. 1970 Jul;59(1):22–35. [PubMed] [Google Scholar]
- Helander H. F. Ultrastructure and function of gastric mucoid and zymogen cells in the rat during development. Gastroenterology. 1969 Jan;56(1):53–70. [PubMed] [Google Scholar]
- Helander H. F. Ultrastructure and function of gastric parietal cells in the rat during development. Gastroenterology. 1969 Jan;56(1):35–52. [PubMed] [Google Scholar]
- Ito S., Schofield G. C. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974 Nov;63(2 Pt 1):364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. R., Young B. A. Undifferentiated cells in gastric mucosa. J Anat. 1968 Mar;102(Pt 3):541–551. [PMC free article] [PubMed] [Google Scholar]
- Krause W. J., Leeson C. R. The gastric mucosa of two monotremes: the duck-billed platypus and echidna. J Morphol. 1974 Mar;142(3):285–299. doi: 10.1002/jmor.1051420305. [DOI] [PubMed] [Google Scholar]
- Krause W. J. Light and electron microscopic studies on the gastrointestinal tract of the suckling echidna (Tachyglossus aculeatus). Anat Rec. 1972 Apr;172(4):603–621. doi: 10.1002/ar.1091720402. [DOI] [PubMed] [Google Scholar]
- Lawson H. H. The origin of chief and parietal cells in regenerating gastric mucosa. Br J Surg. 1970 Feb;57(2):139–141. doi: 10.1002/bjs.1800570214. [DOI] [PubMed] [Google Scholar]
- Lev R. The mucin histochemistry of normal and neoplastic gastric mucosa. Lab Invest. 1965 Dec;14(12):2080–2100. [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil, and chief cells in stomach grafts. Science. 1970 Jul 24;169(3943):385–387. doi: 10.1126/science.169.3943.385. [DOI] [PubMed] [Google Scholar]
- Nabeyama A., Leblond C. P. "Caveolated cells" characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat. 1974 Jun;140(2):147–165. doi: 10.1002/aja.1001400203. [DOI] [PubMed] [Google Scholar]
- Ragins H., Wincze F., Liu S. M., Dittbrenner M. Theorigin and survival of gastric parietal cells in the mouse. Anat Rec. 1968 Sep;162(1):99–110. doi: 10.1002/ar.1091620109. [DOI] [PubMed] [Google Scholar]
- Rodewald R. Selective antibody transport in the proximal small intestine of the neonatal rat. J Cell Biol. 1970 Jun;45(3):635–640. doi: 10.1083/jcb.45.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin W. Endocrine cells in the normal human stomach. A fine structural study. Gastroenterology. 1972 Nov;63(5):784–800. [PubMed] [Google Scholar]
- SALENIUS P. On the ontogenesis of the human gastric epithelial cells. A histologic and histochemical study. Acta Anat Suppl (Basel) 1962;50(46):1–76. [PubMed] [Google Scholar]
- STEVENS C. E., LEBLOND C. P. Renewal of the mucous cells in the gastric mucosa of the rat. Anat Rec. 1953 Feb;115(2):231–245. doi: 10.1002/ar.1091150206. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Leppi T. J., Henson J. G. Sulfate-containing mucosubstances of dog gastric mucosa. Lab Invest. 1967 May;16(5):795–802. [PubMed] [Google Scholar]
- Spicer S. S., Sun D. C. Gastric pepsin, mucus and clinical secretory studies. II. The role of the mucous barrier in the defense of the stomach vs. peptic ulceration. Carbohydrate histochemistry of gastric epithelial secretions in dog. Ann N Y Acad Sci. 1967 Jan 26;140(2):762–783. doi: 10.1111/j.1749-6632.1967.tb51002.x. [DOI] [PubMed] [Google Scholar]
- Vassallo G., Capella C., Solcia E. Endocrine cells of the human gastric mucosa. Z Zellforsch Mikrosk Anat. 1971;118(1):49–67. doi: 10.1007/BF00331766. [DOI] [PubMed] [Google Scholar]
- Vassallo G., Solcia E., Capella C. Light and electron microscopic identification of several types of endocrine cells in the gastrointestinal mucosa of the cat. Z Zellforsch Mikrosk Anat. 1969;98(3):333–356. doi: 10.1007/BF00346679. [DOI] [PubMed] [Google Scholar]
- Willems G., Galand P., Vansteenkiste Y., Zeitoun P. Cell population kinetics of zymogen and parietal cells in the stomach of mice. Z Zellforsch Mikrosk Anat. 1972;134(4):505–518. doi: 10.1007/BF00307670. [DOI] [PubMed] [Google Scholar]