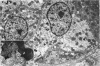Abstract
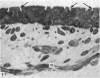
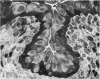
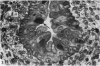
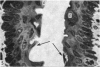
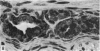
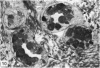
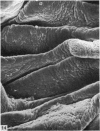
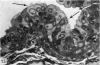
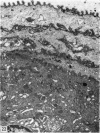
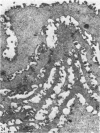
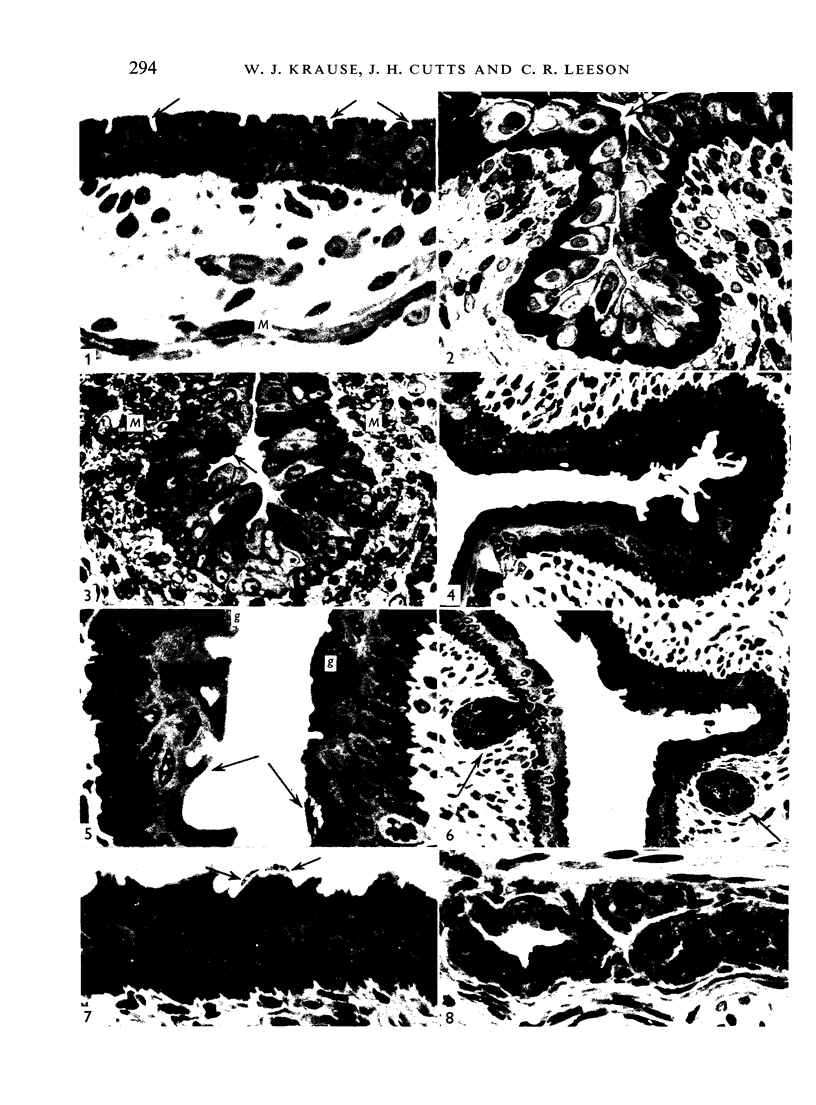
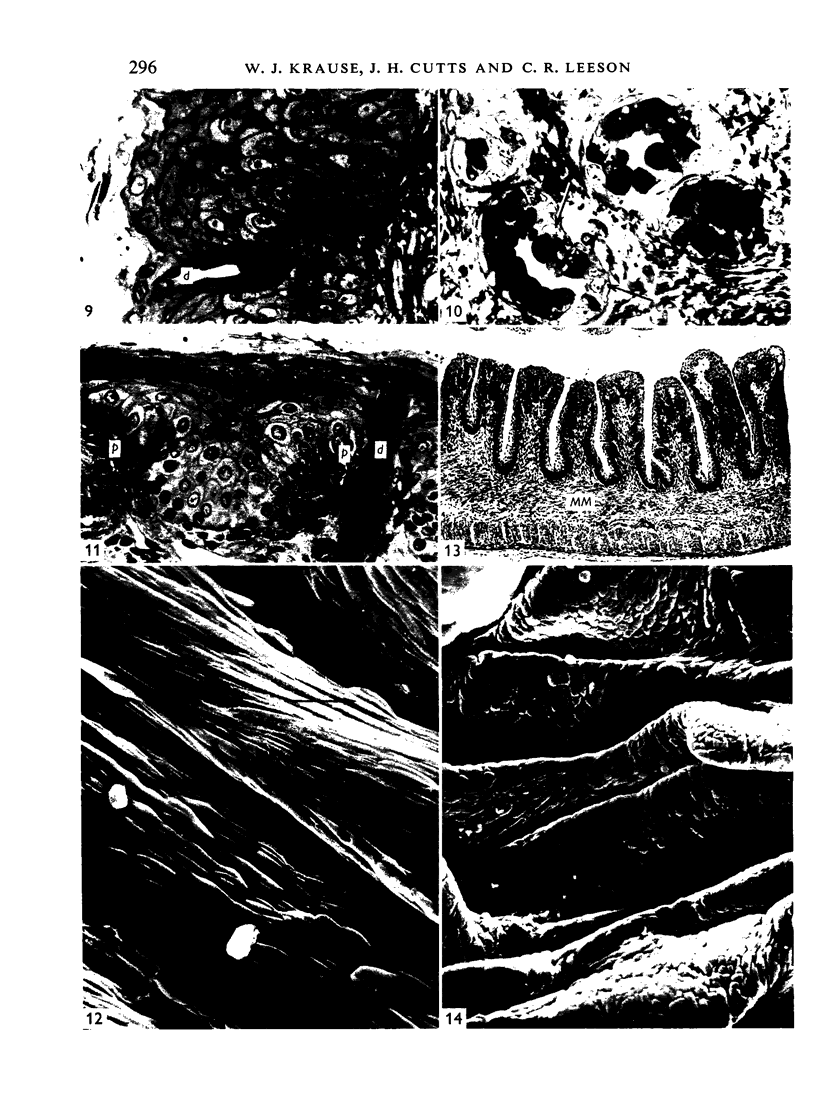
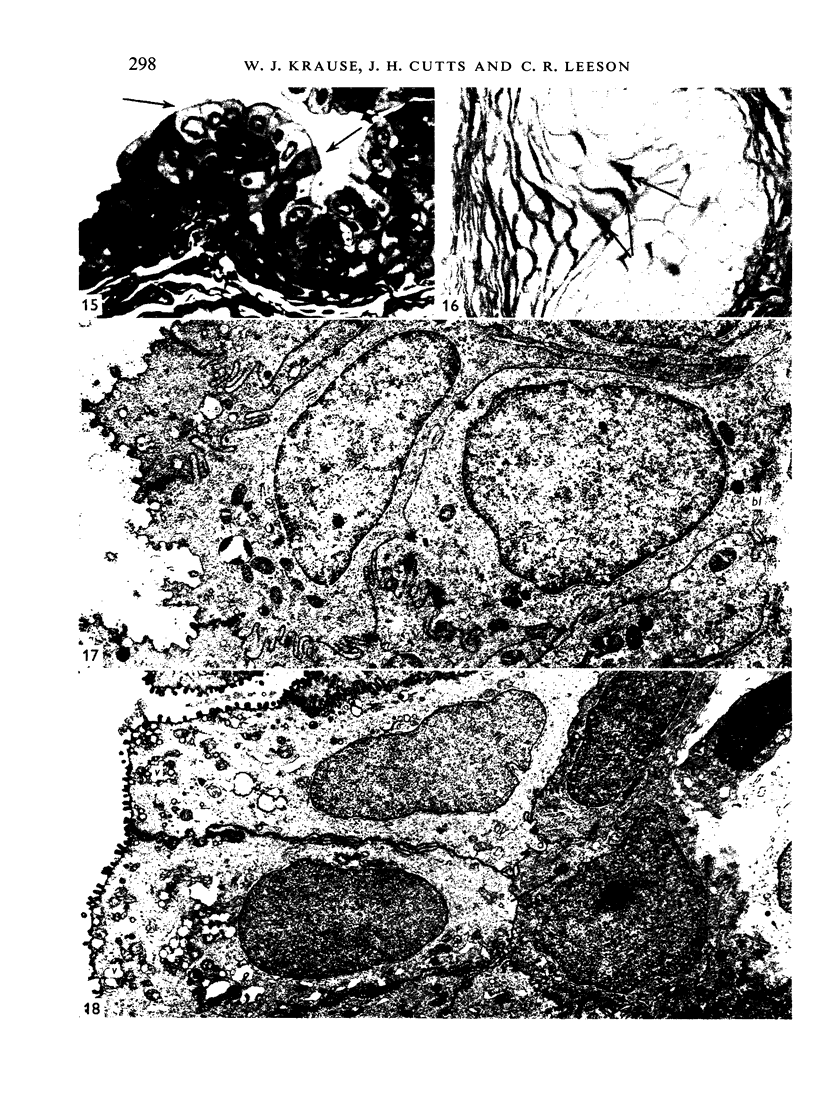
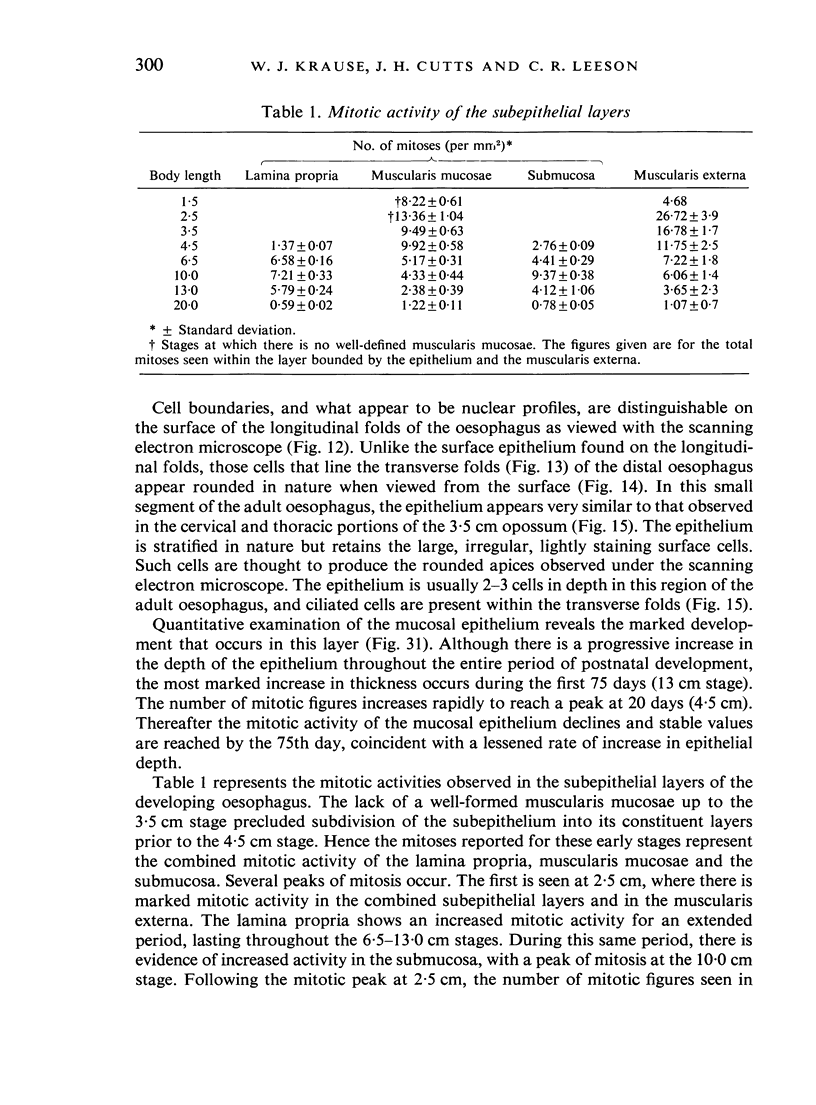
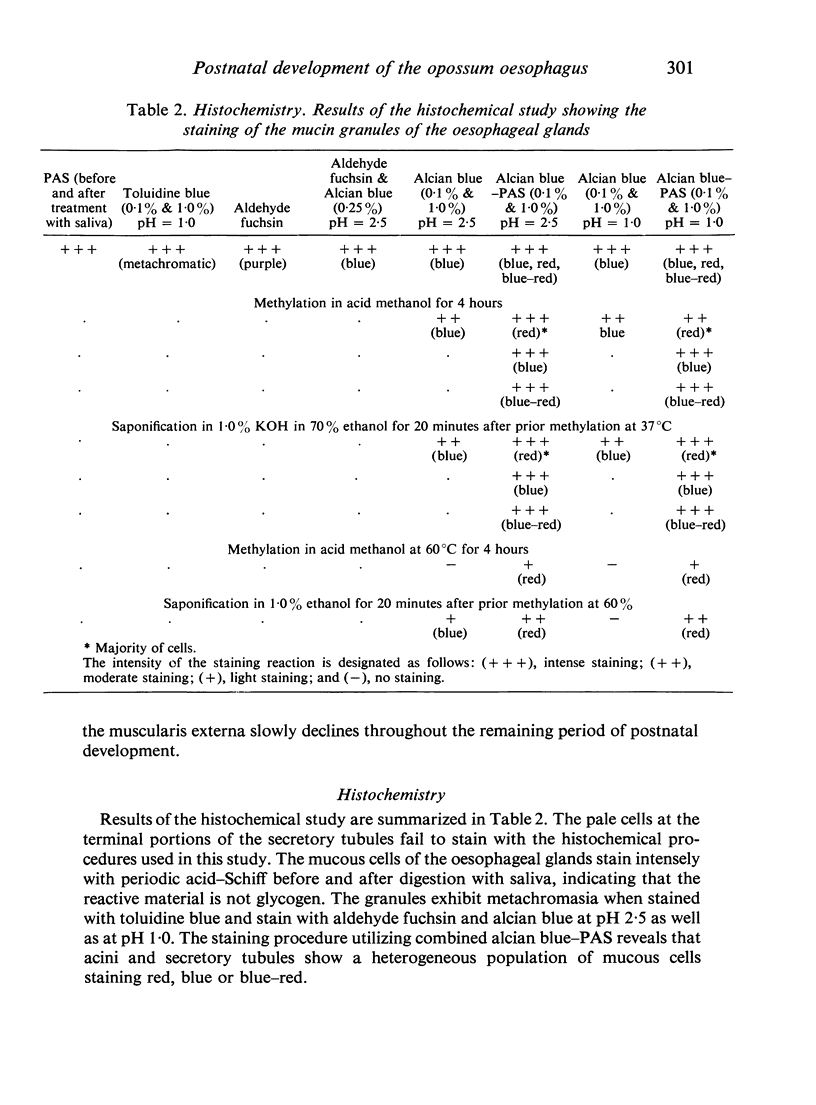
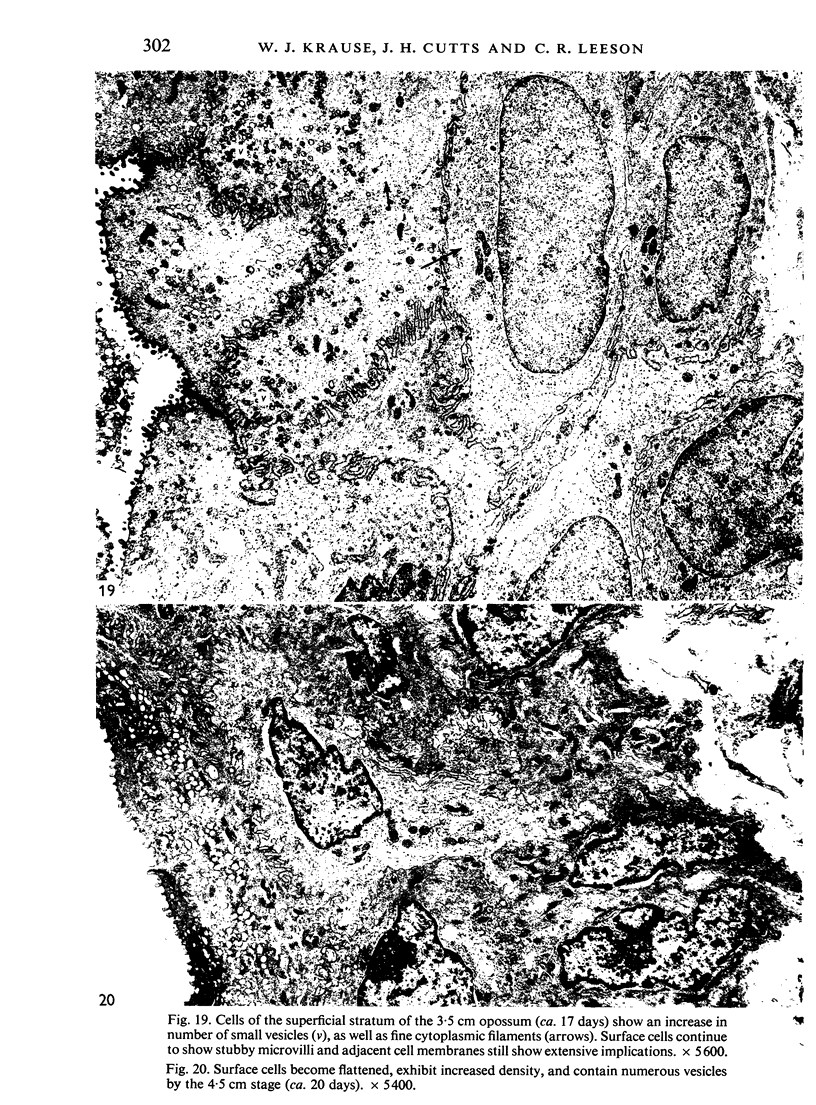
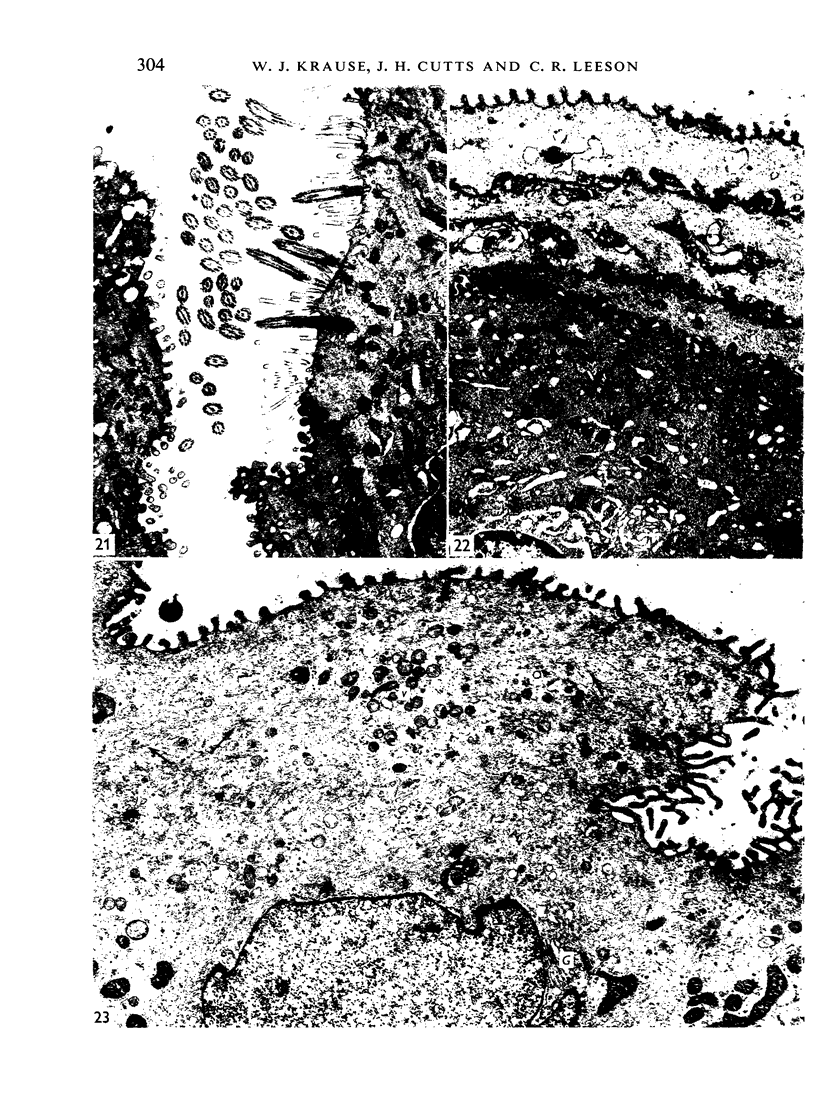
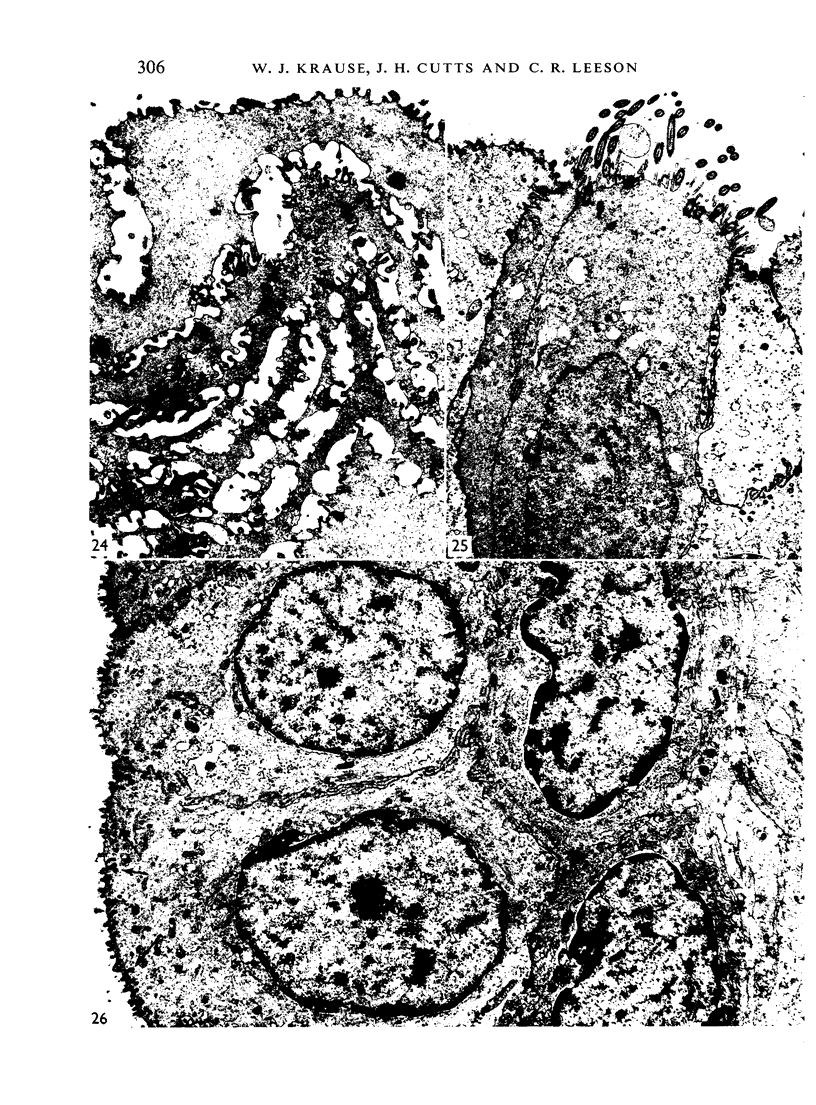
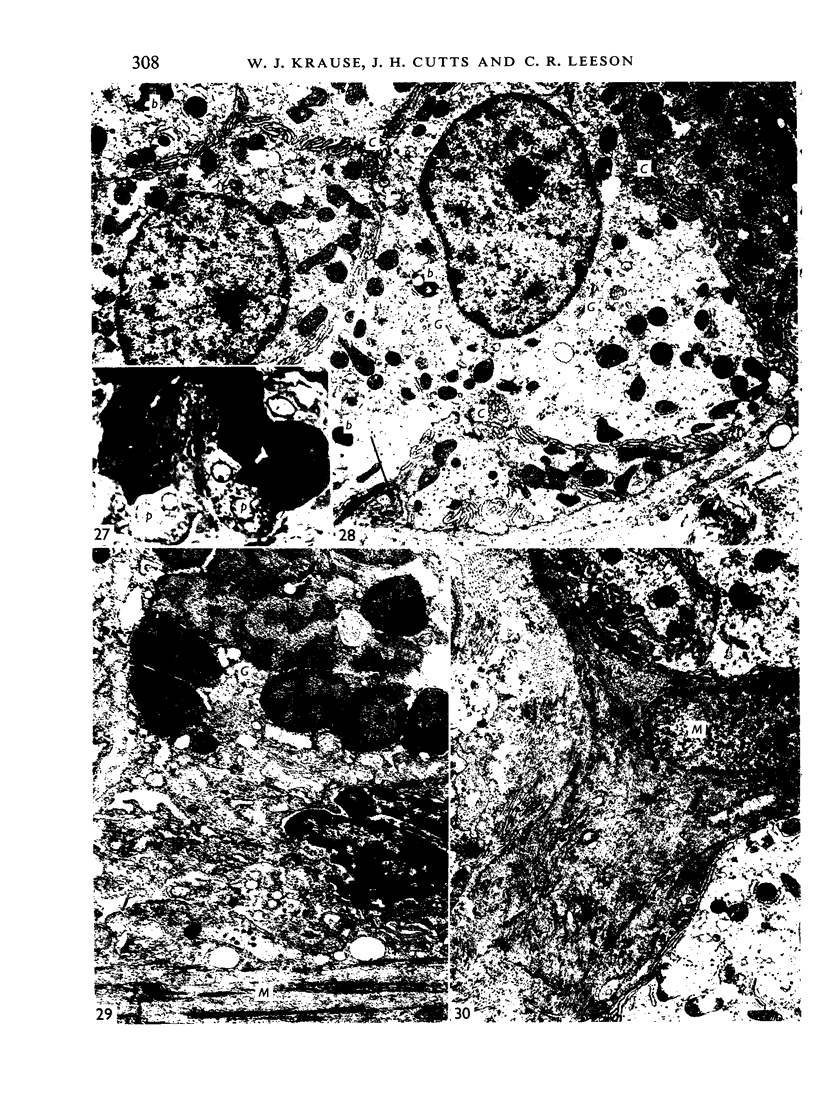
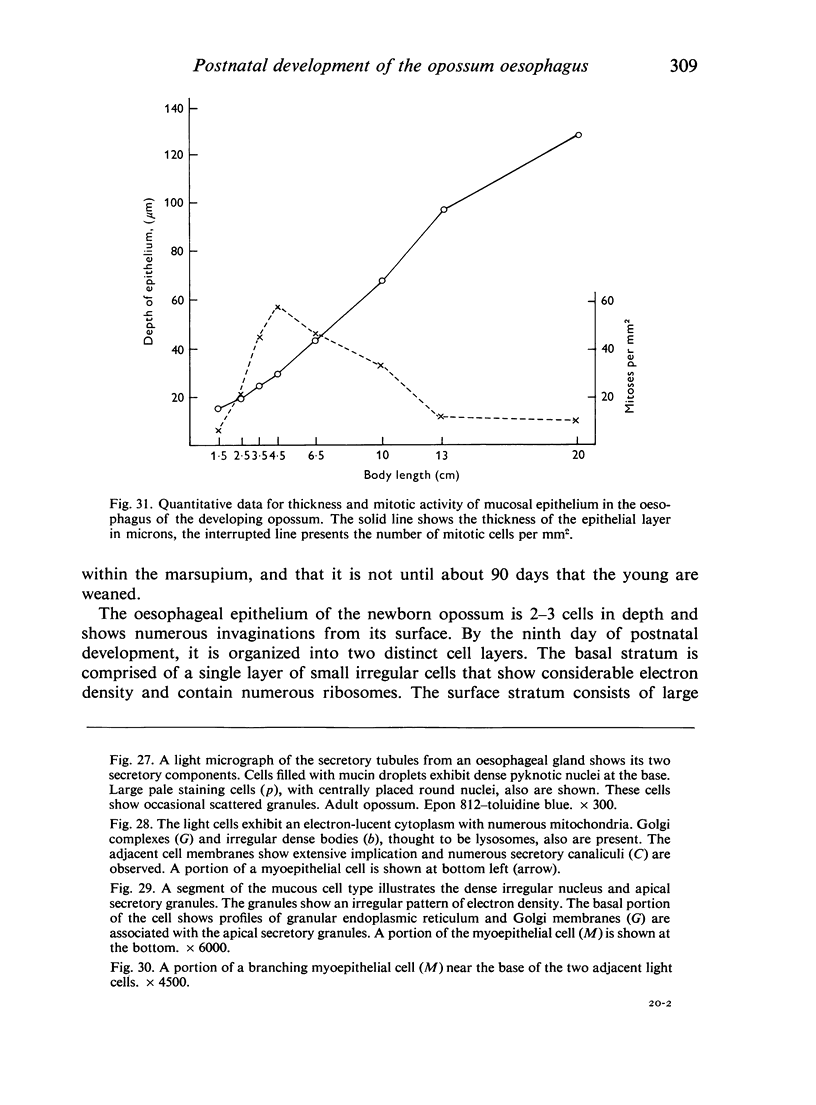
The oesophageal epithelium of the newborn opossum generally is two to three cells in depth and in some regions appears pseudostratified. By the 9th postnatal day the epithelium shows two distinct strata. Ciliated cells and occasional goblet cells also are observed within the epithelium during this stage and in subsequent stages. Cilia persist in the oesophagus of the adult opossum, but are restricted to the depths of the transverse folds found in the distal part of the organ. The epithelium covering the transverse folds of the adult likewise has an immature appearance. By 4-5 cm (ca. 20 days), the epithelium has assumed a more mature appearance and is of greater depth. This and later stages show three basic strata: a germinal layer, a spinous layer and, adjacent to the lumen, a flattened layer of cells that retain their nuclei. The epithelium throughout the postnatal period and in the adult does not undergo complete keratinization. The oesophageal glands begin as outgrowths from the epithelium just prior to 4-5 cm (ca. 20 days). The glands continue their development throughout the remainder of the postnatal period. The secretory units of the oesophageal glands of the the major portion of the secretory elements, and a light, rounded cell type which is less numerous and which occupies the terminal portions of the secretory units. Secretory material of the former appears complex, consisting of both neutral and acid glycoproteins. The secretory product of the light cell type is unknown at present. Both cell types are encompassed by myoepithelial cells. The relationship of the mitotic sequences to the observations made by microscopic examination of the developing oesophagus is discussed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAS P. K., SANYAL A. K., SINHA P. S. STUDIES ON CILIARY MOVEMENTS: I. MECHANISM OF CILIARY MOVEMENT IN FROG'S OESOPHAGUS. Arch Int Pharmacodyn Ther. 1964 Aug 1;150:348–355. [PubMed] [Google Scholar]
- Hinsch G. W. Ultrastructural differentiation of the epithelium and mucous glands of the esophagus in the chick embryo. J Morphol. 1967 Oct;123(2):121–131. doi: 10.1002/jmor.1051230203. [DOI] [PubMed] [Google Scholar]
- IVEY W. D., EDGAR S. A. The histogenesis of the esophagus and crop of the chicken, turkey, guinea fowl and pigeon, with special reference to ciliated epithelium. Anat Rec. 1952 Oct;114(2):189–211. doi: 10.1002/ar.1091140207. [DOI] [PubMed] [Google Scholar]
- JOHNS B. A. E. Developmental changes in the oesophageal epithelium in man. J Anat. 1952 Oct;86(4):431–442. [PMC free article] [PubMed] [Google Scholar]
- Krause W. J., Leeson C. R. Postnatal development of the respiratory system of the opossum. II. Electron microscopy of the epithelium and pleura. Acta Anat (Basel) 1975;92(1):28–44. doi: 10.1159/000144426. [DOI] [PubMed] [Google Scholar]
- Kudo S. Electron microscopic observations on avian esophageal epithelium. Arch Histol Jpn. 1971 Mar;33(1):1–30. doi: 10.1679/aohc1950.33.1. [DOI] [PubMed] [Google Scholar]
- Lambert R., Pansu D., Berard A., Vitani C., Dechelette M. A. Histochemical studies on human mucous secreting glands in the soft palate, uvula and esophagus. Digestion. 1973;8(2):110–119. doi: 10.1159/000197306. [DOI] [PubMed] [Google Scholar]
- Mottet N. K. Mucin biosynthesis by chick and human oesophagus during ontogenetic metaplasia. J Anat. 1970 Jul;107(Pt 1):49–66. [PMC free article] [PubMed] [Google Scholar]
- NORRIS J. L. The normal histology of the esophageal and gastric mucosae of the frog. Rana pipiens. J Exp Zool. 1959 Jun;141:155–173. doi: 10.1002/jez.1401410108. [DOI] [PubMed] [Google Scholar]
- Parakkal P. F. An electron microscopic study of esophageal epithelium in the newborn and adult mouse. Am J Anat. 1967 Sep;121(2):175–195. doi: 10.1002/aja.1001210202. [DOI] [PubMed] [Google Scholar]
- Rowden G. "Membrane-coating" granules of mouse oesophageal and gastric epithelium. J Invest Dermatol. 1966 Oct;47(4):359–362. [PubMed] [Google Scholar]
- Sevcenko G., Brichová H., Vacek Z. Some observations on the ultrastructure of differentiation of the oesophageal epithelium in mammals. Folia Morphol (Praha) 1972;20(1):79–81. [PubMed] [Google Scholar]
- Sevcenko G., Vacek Z. A contribution to the histogenesis of the oesophageal epithelium in mammals. Folia Morphol (Praha) 1973;21(3):261–264. [PubMed] [Google Scholar]
- Sorokin S. P. On the cytology and cytochemistry of the opossum's bronchial glands. Am J Anat. 1965 Nov;117(3):311–337. doi: 10.1002/aja.1001170302. [DOI] [PubMed] [Google Scholar]
- WISLOCKI G. B., FAWCETT D. W., DEMPSEY E. W. Staining of stratified squamous epithelium of mucous membranes and skin of man and monkey by the periodic acid-Schiff method. Anat Rec. 1951 Jul;110(3):359–375. doi: 10.1002/ar.1091100307. [DOI] [PubMed] [Google Scholar]
- Wakuri H., Muto K. I. The fine structure of the cattle esophageal gland with a special reference to the myoepithelial cells. Kitasato Arch Exp Med. 1972 Mar;45(1):45–50. [PubMed] [Google Scholar]