Abstract
The estuarine genus Pfiesteria has received considerable attention since it was first identified and proposed to be the causative agent of fish kills along the mid-Atlantic coast in 1992. The presumption has been that the mechanism of fish death is by release of one or more toxins by the dinoflagellate. In this report, we challenge the notion that Pfiesteria species produce ichthyotoxins. Specifically, we show that (i) simple centrifugation, with and without ultrasonication, is sufficient to “detoxify” water of actively fish-killing cultures of Pfiesteria shumwayae, (ii) organic extracts of lyophilized cultures are not toxic to fish, (iii) degenerate primers that amplify PKS genes from several polyketide-producing dinoflagellates failed to yield a product with P. shumwayae DNA or cDNA, and (iv) degenerate primers for NRPS genes failed to amplify any NRPS genes but (unexpectedly) yielded a band (among several) that corresponded to known or putative PKSs and fatty acid synthases. We conclude that P. shumwayae is able to kill fish by means other than releasing a toxin into bulk water. Alternative explanations of the effects attributed to Pfiesteria are suggested.
Members of the estuarine dinoflagellate genus Pfiesteria have been linked to numerous fish kills in the estuaries of the mid-Atlantic coast of the U.S. in the past decade. Since first being identified and reported as the causative agent of fish kills in 1992 (1), there have been many follow-up investigations. An online literature search reveals over 100 articles with the keyword “Pfiesteria” published since 1995. These include reports of ichthyotoxicity and cytotoxicity (1–7) ascribed to the dinoflagellate as well as links between exposure to Pfiesteria and human health effects (6, 8–19). An entire issue of the journal Environmental Health Perspectives [(2001) Environ. Health Perspect., Suppl. 5, 109,633–808] was devoted exclusively to the Pfiesteria phenomenon and summarizes the state of affairs as of October 2000. Pfiesteria has also received considerable attention in the lay press, sometimes in a spectacularly sensationalized fashion [e.g., “The Coming Plague” (20)]. The emotion surrounding the Pfiesteria phenomenon, including accusations of data suppression and questionable ethics, has even sparked debate in anthropology journals (21–24).
Many authors either presume or explicitly state that Pfiesteria species are toxicogenic (i.e., toxin-producing) dinoflagellates, and that a “small-molecule” toxin is responsible for the observed effects on fish and mammals. This is a reasonable position, because a structurally diverse array of toxins are produced by many dinoflagellates and related microalgae, commonly referred to as harmful algal blooms (25–28). Based on the presumed toxicogenicity, we set out to isolate and characterize the putative toxin using bioassay-guided fractionation. Furthermore, to characterize possible secondary metabolites of Pfiesteria structurally, we used molecular genetic techniques to search for evidence of polyketide synthase (PKS) and nonribosomal peptide (NRPS) genes such as those involved in the biosynthesis of typical secondary metabolites of algae. We report the results of our investigations herein.
Materials and Methods
Dinoflagellate Cultures.
A clonal isolate of Pfiesteria shumwayae [Center for Culture of Marine Phytoplankton (CCMP) 2089] was maintained in cultures as described below. The identity of P. shumwayae was confirmed additionally by PCR using species-specific primers for regions of the ribosomal RNA gene complex (29).
Algal prey.
CCMP 2089 was cultured clonally on algal prey, Rhodomonas salina (CCMP 1319), in York River water (estuarine water adjusted to 12 psu with distilled water, autoclaved, and 0.22-μm-filtered) with bacterial growth minimized by the addition of penicillin and streptomycin (final concentrations 2,500 units/liter and 2,500 μg/liter, respectively).
Tilapia prey.
CCMP 2089 was cultured in 38-liter aquaria containing artificial seawater (ASW) and tilapia (Oreochromis niloticus) in a Biosafety Level 3 containment facility. Water-quality parameters in tanks including pH, temperature, salinity, ammonia, nitrites, and dissolved oxygen were monitored weekly. Water was changed (≈60–75% aquarium volume using ASW) after fish mortalities in tanks ceased and ammonia levels were high (>30 ppm). Cell densities were determined daily during fish mortalities by fixing 50-ml aliquots of culture in Lugol's iodine, centrifuging at 4,000 × g for 15 min, decanting, and counting cells with a hemacytometer (Neubauer, Brightline, Horsham, PA).
Cell-Free Fractions.
Raw tank water consisting of a culture of P. shumwayae that had been killing tilapia (O. niloticus, 20–50% daily mortality) for 8 months before the assay was treated as follows.
Supernatant.
Tank water was centrifuged (4,500 rpm, 1 h) with and without prior sonication (Brinkman Polytron PT 10/35 at 27,000 rpm) to obtain cell-free aqueous lysates and supernatants, respectively.
Solvent extracts.
Raw tank water was lyophilized and extracted sequentially with dichloromethane and methanol (MeOH) to obtain soluble extracts of cellular material. For assays, organic extracts were evaporated to dryness and redissolved in volumes of water equivalent to the original volume of the culture material to achieve concentrations comparable to whole cultures.
Ichthyotoxicity Controls.
To ensure that our experiments were done with toxic cultures, ichthyotoxicity of CCMP 2089 was assessed using either adult Gambusia holbrooki or larval Cyprinodon variegatus. G. holbrooki were collected from the wild and maintained in 20-gallon aquaria filled with ground water. They were tested in 15-ml volumes of ground water (with or without solvent extracts) in 150-ml beakers (two fish per beaker) supplied with aeration. Mortality was assessed up to 14 days.
To evaluate toxicity of cell-free supernatants and lysates, C. variegatus were placed in six-well plates (3–5 fish per well). ASW alone was used as a negative control. To verify ichthyotoxicity of live dinospores, C. variegatus were placed in 10 ml of ASW medium containing dinoflagellates (diluted 1:1 in ASW to give a final concentration of 500 dinospores per ml). In both cases, mortality was monitored for 7 days, after which the water levels and water quality deteriorated.
Preparation of DNA and cDNA for PCR.
Genomic DNA and cDNA were prepared from samples of clonal cultures of CCMP 2089 grown on algal prey as described above. For genomic DNA, cells from 100-ml samples were collected by vacuum filtration onto 3-μm Nuclepore (Costar) filters, and DNA was prepared using the DNeasy tissue kit (Qiagen, Valencia, CA). For cDNA, total RNA was isolated from CCMP 2089 obtained from a 125-ml sample of the algal-grown culture and a 125-ml sample of the algal-grown culture that was exposed to C. variegatus for 18 h before RNA extraction. For comparison, cells from a dense culture (60 ml) of the algal food, R. salina, were collected and processed identically for RNA extraction and cDNA preparation. Cells were collected on 3-μm, RNase-free Nuclepore (Costar) filters by vacuum filtration. RNA was extracted from cells by denaturation with TRIzol followed by chloroform/isoamyl alcohol (25:1) extraction and overnight precipitation of RNA at −20°C with an equal volume of isopropanol. RNA was subsequently redissolved in nuclease-free water (25 μl), quantified spectrophotometrically, and shown by formaldehyde gel electrophoresis to be of high quality (30).
The RNA was reverse-transcribed by Moloney murine leukemia virus reverse transcriptase and random hexamers. Aliquots (5 μl) of RNA were transferred to RNase-free Eppendorf tubes and incubated for 15 min at 65°C with random hexamers (2 μl × 50 ng/μl) in 17 μl of nuclease-free water. To each, 3.5 μl of 5 mM dNTPs, 7 μl of 5× reverse transcription (RT) buffer, and 0.5 μl of Moloney murine leukemia virus reverse transcriptase were added, and reactions were incubated at 42°C for 2 h. cDNA was stored at −20°C for subsequent PCR.
PCR and RT-PCR Evaluation of PKS and NRPS Genes.
The presence and expression of PKS and NRPS genes were evaluated by PCR of total genomic DNA and total cDNA, respectively, with primers (synthesized by Invitrogen Life Technologies, Carlsbad, CA) specific to these genes (31–34). PCR of PKSs and NRPSs used the following primer sequences, based on the ketosynthase domain and adenylation domain of PKSs and NRPSs, respectively: PKSI, forward/reverse, 5′-MGIGARGCIYTICARATGGAYCCICARMG-3′/5′-GGRTCNCCIARYTGIGTICCIGTICCRTGIGC-3′; PKSII, forward/reverse, 5′-TSGCSTGCTTCGAYGCSATC-3′/5′-TGGAANCCGAABCCGCT-3′; NRPSI, forward/reverse, 5′-TWYCGIACIGGIGAYYKIGKICG-3′/5′-AWIGARKSICCICCIRRSIMRAARAA-3′; and NRPSII, forward/reverse, 5′-GCNGCNGGNGCITAYGTICC-3′/5′CCNCKDATYTTNACYTG-3′. Reactions (25 μl) consisted of 50 mM KCl, 10 mM Tris⋅HCl, 1.5 mM MgCl2, 200 μM dNTPs, 1.5 units of Taq polymerase and reverse and forward primers (2.4 pM), and 0.5 or 2 μl of template for genomic DNA and cDNA, respectively. Cycling conditions, based on a touchdown method, were: 94°C for 2 min; 10 cycles of 94°C for 30 s, 65°C (decreasing 1°C per cycle) for 90 s, 72°C for 2 min; 25–35 cycles of 94°C for 30 s, 55°C for 60 s, and 72°C for 2 min; and a final extension at 72°C for 10 min. As a positive control, type I PKS sequences were amplified with the PKSI primer from the plasmids PL29-PKS and AK112 containing known PKS sequences from Prorocentrum lima and Aphidinium klebsii, respectively.
In addition to specific primers, PCR with primers (Invitrogen Life Technologies) specific to the 18S ribosomal subunit or the internal transcribed spacer (ITS) region of the ribosomal RNA gene complex were used to confirm the presence of eukaryotic, and specifically P. shumwayae, DNA. The universal 18S primers, 16s-A/16s-B (5′-GGTTGATCCTGCCAGTAGTCATATGCCTG-3′/5′-GATCCTTCCGCAGGTTCACCTACGGAAACC-3′) were derived from Rowan and Powers (35). The species-specific primers PfBSSU/16s-B and PfBSSU/PfBITSR201L were developed based on comparing the SSU gene, or SSU gene and ITS region sequences, of P. shumwayae to sequences for these regions from Pfiesteria piscicida and several Pfiesteria-like organisms (29). PfBSSU has been described previously (29); the sequence of PfBSSU/ITSR201L is as follows: 5′-CCAGCTTCTGGATTTTGTCGC-3′/5′-GCGATGAGGAAGAGAAAAATGACG-3′. Reactions for universal 18S primers were conducted identically to PKS-specific primers. Reactions with species-specific primers required the addition of 0.4 mg/ml nonacetylated BSA as a stabilizer. Conditions for amplification with the PfBSSU/16s-B amd PfBSSU/PfBITSR201L primer sets were as follows: 94°C for 4 min; 40 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 90 s; and 72°C for 5 min. PCR products were analyzed by gel electrophoresis (30).
Results
Fish-Killing Activity of Dinoflagellate Cultures, Supernatant, Lysate, and Extracts.
Relatively high densities (>1,000 zoospores per ml) of P. shumwayae that had been maintained on a supply of R. salina in culture or O. niloticus in aquaria were found to cause mortality of larval fish in less than 24 h (Fig. 1). P. shumwayae raised on R. salina and that had not been previously exposed to fish demonstrated 100% mortality of larval fish in 24–48 h compared with 72 h for aquarium cultures that had been killing O. niloticus in aquaria before assay with C. variegatus.
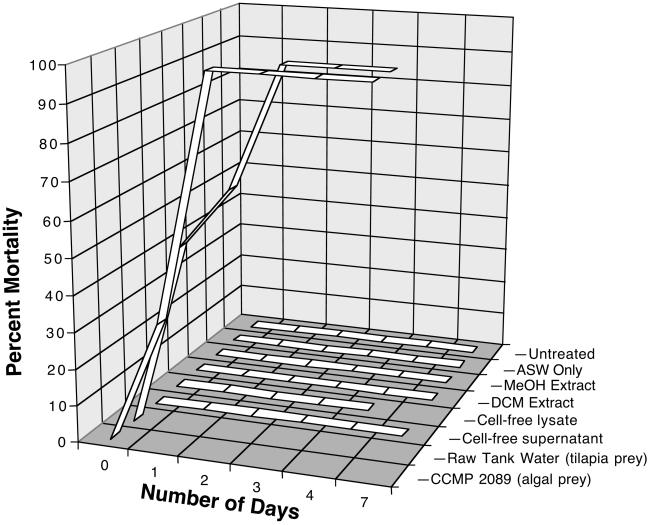
Fig 1.
Fish-killing activity of P. shumwayae grown on algae and tilapia, plus supernatant and lysate, were assayed with C. variegatus (n = three per well × two wells per treatment). Cell-free lysates and supernatants were from raw tank water cultured on tilapia prey and centrifuged with or without ultrasonication. For lysate, no data were collected after 4 days, because the water in the wells evaporated. MeOH and dichloromethane (DCM) extracts of lyophilized raw tank water were tested against G. holbrooki (n = two fish per beaker × two beakers per treatment); no mortality was observed in this case up to 14 days (last 7 days not shown). “Untreated” and “ASW only” controls refer to fish tested in ground water (for G. holbrooki) or ASW (for C. variegatus), respectively.
However, cell-free supernatants and lysates (prepared by sonication), failed to kill larval C. variegatus in ichthyotoxicity assays lasting up to 7 days. Likewise, organic extracts (dichloromethane and MeOH) of lyophilized zoospores, after reconstitution in ASW, were not ichthyotoxic when tested with G. holbrooki for 14 days at concentrations equivalent to or greater than the concentrations present in whole culture. Furthermore, no obvious behavioral changes were observed in fish in the cell-free fractions as compared with fish tested in the whole cultures that showed characteristic responses to an irritant, such as hyperactivity, “wavering,” and flick responses of the fins.
PCR and RT-PCR with PKS- and NRPS-Specific Primers.
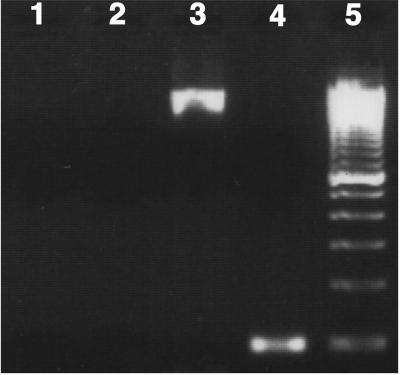
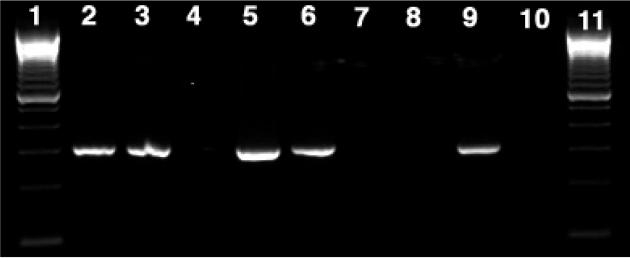
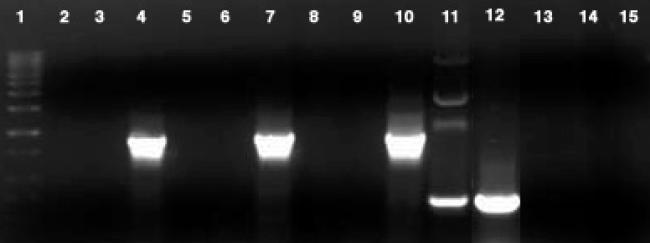
We used primers specific for type I and II PKS and NRPS genes (31, 33, 34, 36) to investigate the possible presence and expression of these genes by P. shumwayae using PCR and RT-PCR. Amplification of 1.8-kbp and 100- or 400-bp products with universal 18S (35) and P. shumwayae-specific primers (29), respectively, confirmed the presence of P. shumwayae DNA and cDNA (Figs. 2–4). Neither PKS-specific primer set amplified DNA from either total genomic DNA or cDNA isolated from P. shumwayae (Figs. 2 and 3). However, amplification of the ketosynthase region (700 bp) of the PKS (type I) sequence from plasmids PL29-PKS and AK112 confirmed the effectiveness of these primers (Fig. 3).
Fig 2.
Gel (2% agarose) showing a lack of PCR amplification with genomic DNA from P. shumwayae with primers specific to ketosynthase domain of type I and II PKSs (PKSI and PKSII, lanes 1 and 2, respectively). Shown is positive amplification of 1.8-kb (lane 3) and 100-bp (lane 4) products, respectively, with “universal” 18S primer (35) and P. shumwayae-specific primers (PfBSSU/16s-B) confirm the presence of P. shumwayae DNA. Lane 5 is a 100-bp ladder.
Fig 4.
Gel (2% agarose) of RT-PCR products showing amplification with P. shumwayae-specific primer. Amplification of the expected 400-bp sequence was observed with cDNA from P. shumwayae (CCMP 2089) as well as the same culture exposed to larval C. variegatus 18 h before RNA extraction using the primers PfBSU/PfBITSR201L (lanes 2 and 5), confirming the presence of P. shumwayae cDNA. No amplification was seen (lane 8) with the algal food, R. salina. Positive amplification was also observed for all positive controls of P. shumwayae genomic DNA (lanes 3, 6, and 9). No amplification was observed for any of the negative controls of no template (lanes 4, 7, and 10). Lanes 1 and 11 are 100-bp DNA ladders.
Fig 3.
Gel (1% agarose) of RT-PCR products showing lack of expression of type I or II PKS genes by P. shumwayae. No amplification was observed with cDNA from P. shumwayae using type I PKSI (lane 2) or type II PKSII (lane 3) primer. Expression, likewise, is not seen with primers PKSI and PKSII (lanes 5 and 6, respectively) for the same culture exposed to larval fish (C. variegatus) 18 h before RNA extraction or with negative control of the algal food, R. salina, only (lanes 8 and 9, respectively). Positive amplification was observed with universal eukaryotic 18S primers (35) for CCMP 2089 (lane 4), CCMP 2089 exposed to C. variegatus (lane 7), and R. salina (lane 10). Amplification of the type I PKS sequences was observed for positive control plasmids PL29 (lane 11) and AK112 (lane 12). Negative controls of no template (lanes 13, 14, and 15, respectively) showed no amplification with PKSI, PKSII, or 18S primers. Lane 1 is a 1-kb DNA ladder.
Neither NRPS-specific primer set yielded a product of the expected size (600 or 1,000 bp; see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org) from either P. shumwayae genomic DNA or cDNA. However, both NRPS primer sets yielded several bands of various sizes from the genomic PCR and RT-PCR (see Fig. 5, which is published as supporting information on the PNAS web site). Several of the bands from NRPSII were cloned, and the inserts were sequenced. Analysis by BLAST (37) revealed (i) no homologies to any genes in the GenBank database (several bands of various sizes), (ii) homology to succinate dehydrogenases (495-bp band), or (iii) homology to the N-terminal portion of the ketosynthase domain of known and putative PKSs or fatty acid synthases (401-bp band). For details on the cloning, sequencing, and PCR studies with nested primers of this band, see Supporting Text.
Discussion
In general, demonstration of toxicogenicity requires that chemical constituents capable of causing the attributed effect(s) can be isolated from the suspected source when a given toxicosis is observed and subsequently shown to induce the same effect in vitro. Specifically therefore, in terms of the production of an exogenous ichthyotoxin by Pfiesteria, it must be shown that a compound can be separated from the medium in which fish are dying or dinoflagellate biomass itself and subsequently shown to induce mortality in fish.
The proposed “gold standard” for detecting ichthyotoxic Pfiesteria is a standardized fish bioassay (2, 38), although DNA probes have been used also to indicate the presence of Pfiesteria but not toxicity (39). In our hands, clonal cultures of P. shumwayae grown on either Rhodomonas algae or in aquaria with fish (O. niloticus) produced rapid mortality (Fig. 1) at rates comparable to those of the allegedly toxicogenic “TOX-A” strain of P. shumwayae reported by the Burkholder group (2, 38). Interestingly, and in contrast to previous reports (2), prior exposure to fish is not required since P. shumwayae grown on R. salina algae produced rapid mortality (Fig. 1).
If fish were being killed or even stunned by a toxin released by the dinoflagellate, that toxin should be present in the bulk water of the tank in which the fish are dying. However, we find that centrifugation of water from actively killing cultures of Pfiesteria renders the medium nontoxic. Conceivably, a toxin might be released by the dinoflagellate after physical contact with a fish. If this were true, then the toxin would have to be stored in the cell. However, lysis of the cells by ultrasonication followed by removal of cellular matter by centrifugation produced a solution that was completely nontoxic. In other words, removal of cells or cellular solids detoxifies the medium.
The only way a toxin that is present in the cultures could be removed from solution through centrifugation of cellular matter is by adsorption onto the solid cellular matter. However, methanolic and dichloromethane extracts of the lyophilized cell mass failed to produce any mortality after reconstitution in water. These observations, by themselves, refute the hypothesis that there is a toxin in the cultures killing the fish. Conceivably, the toxin could be unstable, such that it decomposes during the centrifugations and sonications described above. This seems unlikely, since it would be difficult to reconcile the presence of a labile toxin with the massive fish kills ascribed to these species. Others have also tried to find a toxin (2–7, 40), but the only isolate characterized thus far is a phthalate artifact presumed to originate in an ASW preparation (41). Fish in control aquaria without Pfiesteria show no mortality; thus, ASW is not toxic.
As far as we are aware, all known ichthyotoxins produced by dinoflagellates are polyketides (27, 28, 42–45), which are biosynthesized by a family of homologous enzymes known as PKSs (45). Polyketide toxins include the brevetoxins, the ciguatoxins, maitotoxin, the recently elucidated ichthyotoxin prymnesin (46), pectenotoxin, prorocentrolides, zooxanthellotoxins, okadaic acid, luteophanols, and amphidonols (45).
The presence of a PKS gene in Pfiesteria would be an indication that a toxin indeed might be produced, but fractionation bioassays had simply missed it. To that end, we tested the Pfiesteria genome for the presence of PKS genes. Neither PKS primer set yielded a product from either genomic DNA (Fig. 2) or total cDNA (Fig. 3). Apparently, the primers lack sufficient homology to PKS genes in Pfiesteria, although the type I PKS primer set amplifies products with significant homologies to known and putative PKSs from the DNA of polyketide toxin-producing dinoflagellates such as Karenia brevis (brevetoxins), P. lima (okadaic acid), A. klebsii (amphidinolides), and Symbiodinium (zooxanthellatoxins) (refs. 31 and 32; further details will be published elsewhere).
Given this homology in PKSs, the production of a 401-bp product having homology to known and putative PKS or fatty acid synthase genes, using the NRPSII primer set, was very surprising! Inspection of the sequence revealed that the forward NRPSII primer primed both the forward and reverse directions. Control experiments confirmed that this amplification product originated in P. shumwayae and was not found in genomic or cDNAs of the cultured algal prey, R. salina, or ASW that had been incubated with fish in the absence of CCMP 2089 (see Supporting Text). In fact, one of the highest scoring sequences in the BLAST analysis of the 401-bp product was a fatty acid synthase homologue (GenBank accession no. AF082993) from Cryptosporidium parvum (47). C. parvum belongs to the Coccidia, a sister clade to the Dinophyceae within the Alveolata (48), suggesting that this gene does indeed originate from P. shumwayae and not from bacteria associated with either the cultures or fish. The function of the AF082993 gene is unclear. Although described as a fatty acid synthase homologue (47), it contains a loading domain found only in other PKSs such as FK506 and rapamycin. Dinoflagellates are prodigious producers of polyunsaturated fatty acids (PUFAs). Recently, type I PKS-like enzymes have been demonstrated to be involved in the production of PUFAs in both marine prokaryotes and lower marine eukaryotes (49). Careful inspection of these enzymes indicates that they are unique among type I PKSs in both their domain organization and their amino acid sequence. Furthermore, amplification of the ketosynthase domain of these PKSs using our PKSI primer set would be unlikely (see Supporting Text). Thus we conclude that P. shumwayae does express PKS-encoding genes. However, these genes may be involved in PUFA biosynthesis rather than toxin production. Sequencing of the full-length gene and further characterization will be needed before we can understand the function of the encoded enzyme.
While these experiments do not eliminate polyketides as possible toxins produced by Pfiesteria, they strongly suggest that if Pfiesteria is capable of producing this class of secondary metabolites, the polyketide is either not excreted into the bulk medium and not released by cell lysis or is nontoxic. Taken together with the lack of ichthyotoxicity in centrifuged medium or solvent extracts, the conclusion is virtually inescapable that P. shumwayae does not produce an exogenous ichthyotoxin. Nevertheless, cultures of P. shumwayae (CCMP 2089) kill fish at rates that classify these cultures in Burkholder's TOX-A category (1, 2, 40). This paradox can be resolved by invoking other mechanisms.
P. shumwayae zoospores can attach to dermal cells with a peduncle and feed via myzocytosis (29). In tilapia, this causes a loss of mucus coat and scales as well as mild petecchial hemorrhage, thereby precipitating epidermal erosion and bacterial colonization (29). Interestingly, lesions such as those ascribed to Pfiesteria have been recently shown to be caused by a fungal pathogen without prior exposure to other stressors (50). The fungus is highly pathogenic, particularly to menhaden exhibiting a prior portal of entry. Although numerous reports have demonstrated mortality and morbidity of tilapia exposed to Pfiesteria (1–7, 35), all have used live cultures of the dinoflagellates.
The taxonomy of the Pfiesteria genus is becoming increasingly complex and unclear. Currently, two species, P. piscicida (51) and P. shumwayae (40), are recognized. As originally described (1, 3), P. piscicida allegedly had up to 24 life-cycle stages. Using a variety of techniques, Litaker et al. have challenged this assertion (52). Using species-specific hybridization probes, no evidence could be found for a chrysophyte-like cyst stage or the amoeba stages originally proposed. Some authors (3, 7, 53, 54) describe a more intricate “complex” of Pfiesteria-like organisms, often occurring in association with organisms such as fungi, bacteria, and similar dinoflagellates (53, 55). Moreover, most work on Pfiesteria is conducted using nonaxenic cultures that undoubtedly contain innumerable microorganisms unrelated to Pfiesteria. Further complicating matters, some authors assert a variety of “functional types” of Pfiesteria having different abilities to kill fish and produce a toxin (2, 7). Accordingly, it might be a suggested that the lack of toxicogenicity observed here is specific to the species and isolate used; however, we have observed a similar lack of ichthyotoxicity for several isolates of P. shumwayae, P. piscicida, and other Pfiesteria-like organisms (unpublished observations made by the present authors in Miami, Wilmington, NC, and Gloucester Point, VA; see also ref. 29)
It may be that at least some of the effects attributed to Pfiesteria are due to other algae. Recently, one raphidophyte species, Chattonella cf. verruculosa, recovered from Rehoboth Bay, DE, during a massive fish-kill event in August, 2000 was found to produce the potent ichthyotoxin brevetoxin (56). It is possible that large fish kills are not the result of a single organism but ensue from a combination of ichthyotoxins such as a Chattonella-derived brevetoxin, seasonal hypoxia (57), and predation by Pfiesteria as well as subsequent or concurrent fungal invasion (50). Parenthetically, it is noteworthy that brevetoxin is a potent neurotoxin (58–64), so some of the neurological problems reported in humans and associated with Pfiesteria blooms (65–68) could be the result of brevetoxin insult from a previously unidentified raphidophyte.
In conclusion, we confirm that P. shumwayae can kill fish within 24 h. However, we find no evidence of ichthyotoxins from P. shumwayae and no evidence of genes encoding for PKSs typical of those found in toxin-producing dinoflagellates. We therefore conclude that P. shumwayae is not toxicogenic.
Supplementary Material
Acknowledgments
We thank T. Shedd and the U.S. Army Center for Environmental Health Research for the lease of the BSL3 facility at Virginia Institute of Marine Science (VIMS). We also thank C. Squyars, A. Miller, V. Foster, L. Ott, L. Walker, N. Stokes, and K. Hudson for technical assistance at VIMS (VIMS contribution no. 2480). This work was supported by Environmental Protection Agency and National Oceanic and Atmospheric Administration ECOHAB Grants R826655, R828225-01-0, and R826791-01-0 and National Institute of Environmental Health Sciences Grants T32-ES07320 and P30-ES05705. Work at the University of North Carolina was funded in part by a subcontract on P01-ES09563 awarded to the University of Maryland.
Abbreviations
PKS, polyketide synthase
NRPS, nonribosomal peptide synthase
CCMP, Center for Culture of Marine Phytoplankton
ASW, artificial seawater
RT, reverse transcription
ITS, internal transcribed spacer
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF525290).
References
- 1.Burkholder J. M., Noga, E. J., Hobbs, C. H. & Glasgow, H. B., Jr. (1992) Nature (London) 358, 407-410. [DOI] [PubMed] [Google Scholar]
- 2.Burkholder J. M., Glasgow, H. B., Jr. & Deamer-Melia, N. (2001) Phycologia 40, 186-214. [Google Scholar]
- 3.Burkholder J. M. & Glasgow, H. B., Jr. (1997) Limnol. Oceanogr. 42, 1052-1075. [Google Scholar]
- 4.Fairey E. R., Edmunds, J. S., Deamer-Melia, N. J., Glasgow, H. B., Jr., Johnson, F. M., Moeller, P. D., Burkholder, J. M. & Ramsdell, J. S. (1999) Environ. Health Perspect. 107, 711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasgow H. B., Burkholder, J. M., Schmechel, D. E., Tester, P. A. & Rublee, P. A. (1995) J. Toxicol. Environ. Health 46, 501-522. [DOI] [PubMed] [Google Scholar]
- 6.Kimm-Brinson K. L., Moeller, P. D., Barbier, M., Glasgow, H. B., Jr., Burkholder, J. M. & Ramsdell, J. S. (2001) Environ. Health Perspect. 109, 457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall H. G., Gordon, A. S., Seaborn, D. W., Dyer, B., Dunstan, W. M. & Seaborn, A. M. (2000) J. Exp. Mar. Biol. Ecol. 255, 51-74. [DOI] [PubMed] [Google Scholar]
- 8.Shoemaker R. C. & Hudnell, H. K. (2001) Environ. Health Perspect. 109, 539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudnell H. K., House, D., Schmid, J., Koltai, D., Stopford, W., Wilkins, J., Savitz, D. A., Swinker, M. & Music, S. (2001) J. Toxicol. Environ. Health 109, 457-462. [DOI] [PubMed] [Google Scholar]
- 10.El-Nabawi A., Quesenberry, M., Saito, K., Silbergeld, E., Vasta, G. & Eldefrawi, A. (2000) Toxicol. Appl. Pharmacol. 169, 84-93. [DOI] [PubMed] [Google Scholar]
- 11.Levin E. D., Rezvani, A. H., Christopher, N. C., Glasgow, H. B., Jr., Deamer-Melia, N. J., Burkholder, J. M., Moser, V. C. & Jensen, K. (2000) Neurotoxicol. Teratol. 22, 533-540. [DOI] [PubMed] [Google Scholar]
- 12.Levin E. D., Simon, B. B., Schmechel, D. E., Glasgow, H. B., Jr., Deamer-Melia, N. J., Burkholder, J. M., Moser, V. C., Jensen, K. & Harry, G. J. (1999) Neurotoxicol. Teratol. 21, 215-221. [DOI] [PubMed] [Google Scholar]
- 13.Grattan L. M., Oldach, D., Perl, T. M., Lowitt, M. H., Matuszak, D. L., Dickson, C., Parrot, C., Shoemaker, R. C., Kauffman, C. L., Wasserman, M. P., Hebel, J. R., Charache, P. & Morris, J. G., Jr. (1998) Lancet 352, 532-539. [DOI] [PubMed] [Google Scholar]
- 14.Bever C. T., Grattan, L. & Morris, J. G., Jr. (1998) Md. Med. J. 47, 120-123. [PubMed] [Google Scholar]
- 15.Shoemaker R. C. (1998) Md. Med. J. 47, 64-66. [PubMed] [Google Scholar]
- 16.Matuszak D. L., Sanders, M., Taylor, J. L. & Wasserman, M. P. (1997) Md. Med. J. 46, 515-520. [PubMed] [Google Scholar]
- 17.Rullan J. V. & Jenkins, S. R. (1998) Va. Med. Q. 125, 78-80. [PubMed] [Google Scholar]
- 18.Shoemaker R. C. (1997) Md. Med. J. 46, 521-523. [PubMed] [Google Scholar]
- 19.Levin E. D., Schmechel, D. E., Burkholder, J. M., Deamer-Melia, N. J., Moser, V. C. & Harry, G. J. (1997) Environ. Health Perspect. 105, 1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise J., (2001) MAXIM, October, 75–184.
- 21.Griffith D. (1999) Hum. Organ. 58, 119-127. [Google Scholar]
- 22.Burkholder J. M. & Glasgow, H. B., Jr. (1999) Hum. Organ. 58, 443-454. [Google Scholar]
- 23.Lewitus A. J., Rublee, P. A., Mallin, M. A. & Shumway, S. E. (1999) Hum. Organ. 58, 455-458. [Google Scholar]
- 24.Oldach D. (1999) Hum. Organ. 58, 459-462. [Google Scholar]
- 25.Fenical W., Baden, D., Burg, M., de Goyet, C. d. V., Grimes, D. J., Katz, M., Marcus, N., Pomponi, S., Rhines, P., Tester, P. & Vena, J., (1999) From Monsoons to Microbes: Understanding the Ocean's Role in Human Health (Natl. Acad. Press, Washington, DC).
- 26.Botana L. M., (2000) Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection (Dekker, New York).
- 27.Yasumoto T. & Murata, M. (1993) Chem. Rev. 93, 1897-1909. [Google Scholar]
- 28.Shimizu Y. (1993) Chem. Rev. 93, 1685-1698. [Google Scholar]
- 29.Vogelbein W. K., Shields, J. D., Haas, L. W., Reece, K. S. & Zwerner, D. E. (2001) Environ. Health Perspect. 109, 687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J., Fritsch, E. F. & Maniatis, T., (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 31.Metsä-Ketelä M., Salo, V., Halo, L., Hautala, A., Hakala, P. M. & Ylihonko, K. (1999) FEMS Microbiol. Lett. 180, 1-6. [DOI] [PubMed] [Google Scholar]
- 32.Brown D. W., Adams, T. H. & Keller, N. P. (1996) Proc. Natl. Acad. Sci. USA 93, 14873-14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marahiel M. A., Stachelhause, T. & Mootz, H. D. (1997) Chem. Rev. 97, 2651-2673. [DOI] [PubMed] [Google Scholar]
- 34.Neilan B. A., Dittmann, E., Rouhiainen, L., Bass, R. A., Schaub, V., Sivonen, K. & Börner, T. (1999) J. Bacteriol. 181, 4089-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowan R. & Powers, D. A. (1992) Proc. Natl. Acad. Sci. USA 89, 3639-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown D. W., Yu, J. H., Kelkar, H. S., Fernandes, M., Nesbitt, T. C., Keller, N. P., Adams, T. H. & Leonard, T. J. (1996) Proc. Natl. Acad. Sci. USA 93, 1418-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altschul S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. (1990) J. Mol. Biol. 215, 403-410. [DOI] [PubMed] [Google Scholar]
- 38.Glasgow H. B., Burkholder, J. M., Mallin, M. A., Dearner-Melia, N. J. & Reed, R. E. (2001) Environ. Health Perspect. 109, 715-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melo A. C., Moeller, P. D. R., Glasgow, H. B., Jr., Burkholder, J. M. & Ramsdell, J. S. (2001) Environ. Health Perspect. 109, Suppl. 5, 731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasgow H. B., Burkholder, J. M., Morton, S. L. & Springer, J. (2001) Phycologia 40, 234-245. [Google Scholar]
- 41.Moeller P. D. R., Morton, S. L., Mitchell, B. A., Sivertsen, S. K., Fairey, E. R., Mikulski, T. M., Glasgow, H. B., Jr., Deamer-Melia, N. J., Burkholder, J. M. & Ramsdell, J. S. (2001) Environ. Health Perspect. 109, 739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall S. & Strichartz, G., (1990) Marine Toxins: Origin, Structure, and Molecular Pharmacology (Am. Chem. Soc., Washington, DC).
- 43.Yasumoto T., Oshima, Y. & Fukuyo, Y., (1996) Harmful and Toxic Algal Blooms (Intergovernmental Oceongraphic Commission of UNESCO, New York).
- 44.Cooksey K. E., (1998) Molecular Approaches to the Study of the Ocean (Chapman & Hall, London).
- 45.Rein K. & Borrone, J. (1999) Comp. Biochem. Physiol. 124, 117-131. [DOI] [PubMed] [Google Scholar]
- 46.Igarashi T., Satake, M. & Yasumoto, T. (1999) J. Am. Chem. Soc. 121, 8499-8511. [Google Scholar]
- 47.Zhu G., Marchewka, M. J., Woods, K. M., Upton, S. J. & Keithly, J. S. (2000) Mol. Biochem. Parasitol. 105, 253-260. [DOI] [PubMed] [Google Scholar]
- 48.Cavalier-Smith T. (1993) Microbiol. Rev. 57, 953-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metz J. G., Roessler, P., Facciotti, D., Levering, C., Dittrich, F., Lassner, M., Valentine, R., Lardizabal, K., Domergue, F., Yamada, A., Yazawa, K., Knauf, V. & Browse, J. (2001) Science 293, 290-293. [DOI] [PubMed] [Google Scholar]
- 50.Kiryu Y., Shields, J. D., Vogelbein, W. K., Zwerner, D. E., Kator, H. & Blazer, V. S. (2002) J. Aquat. Anim. Health 14, 11-24. [Google Scholar]
- 51.Steidinger K. A., Burkholder, J. M., Glasgow, H. B., Jr., Ruby, E., Garrett, J., Noga, E. J. & Smith, S. A. (1996) J. Phycol. 32, 157-164. [Google Scholar]
- 52.Litaker R. W., Vandersea, M. W., Kibler, S. R., Madden, V. J., Noga, E. J. & Tester, P. A. (2002) J. Phycol. 38, 442-463. [Google Scholar]
- 53.Dykstra M. J. & Kane, A. S. (2000) J. Aquat. Anim. Health 12, 18-25. [DOI] [PubMed] [Google Scholar]
- 54.Lewitus A. J., Glasgow, H. B., Jr. & Burkholder, J. M. (1999) J. Phycol. 35, 303-312. [Google Scholar]
- 55.Alavi M., Miller, T., Erlandson, K., Schneider, R. & Belas, R. (2001) Environ. Microbiol. 3, 380-396. [DOI] [PubMed] [Google Scholar]
- 56.Bourdelais A. J., Tomas, C. R., Naar, J., Kubanek, J. & Baden, D. G. (2002) Environ. Health Perspect. 110, 465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paerl H. W., Bales, J. D., Ausley, L. W., Buzzelli, C. P., Crowder, L. B., Eby, L. A., Fear, J. M., Go, M., Peierls, B. L., Richardson, T. L. & Ramus, J. S. (2001) Proc. Natl. Acad. Sci. 98, 5655-5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baden D. G., Fleming, L. E. & Bean, J. A. (1995) in Handbook of Clinical Neurology: Intoxications of the Nervous System, Part III, ed. de Wolff, F. A. (Elsevier, Amsterdam), Vol. 21, pp. 141–175. [Google Scholar]
- 59.Gawley R. E., Rein, K. S., Kinoshita, M. & Baden, D. G. (1992) Toxicon 30, 780-785. [DOI] [PubMed] [Google Scholar]
- 60.Gawley R. E., Rein, K. S., Jeglitsch, G., Adams, D. J., Theodorakis, E. A., Tiebes, J., Nicolaou, K. C. & Baden, D. G. (1995) Chem. Biol. 2, 533-541. [DOI] [PubMed] [Google Scholar]
- 61.Jeglitsch G., Rein, K. S., Baden, D. G. & Adams, D. J. (1998) J. Pharmacol. Exp. Ther. 284, 516-525. [PubMed] [Google Scholar]
- 62.Poli M. A., Mende, T. J. & Baden, D. G. (1986) Mol. Pharmacol. 30, 129-135. [PubMed] [Google Scholar]
- 63.Purkerson S. L., Baden, D. G. & Fieber, L. A. (1999) Neurotoxicology 20, 909-920. [PubMed] [Google Scholar]
- 64.Rein K. S., Lynn, B., Gawley, R. E. & Baden, D. G. (1994) J. Org. Chem. 59, 2107-2113. [Google Scholar]
- 65.Moe C. L., Turf, E., Oldach, D., Bell, P., Hutton, S., Savitz, D., Koltai, D., Turf, M., Ingsrisawang, L., Hart, R., et al. (2001) Environ. Health Perspect. 109, 781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris J. G. (2001) Environ. Health Perspect. 109, 787-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoemaker R. C. (2001) Environ. Health Perspect. 109, 791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Backer L. C., Niskar, A. S., Rubin, C., Blindauer, K., Christianson, D., Naeher, L. & Rogers, H. S. (2001) Environ. Health Perspect. 109, 797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.