Abstract
Cellulose synthase (CesA) proteins are components of CesA complexes (rosettes) and are thought to catalyze the chain elongation step in glucan polymerization. Little is understood about rosette assembly, including how CesAs interact with each other or with other components within the complexes. The first conserved region at the N terminus of plant CesA proteins contains two putative zinc fingers that show high homology to the RING-finger motif. We show that this domain in GhCesA1 can bind two atoms of Zn2+, as predicted by its structure. Analysis in the yeast two-hybrid system indicates that the N-terminal portions of cotton fiber GhCesA1 and GhCesA2 containing these domains can interact to form homo- or heterodimers. Although Zn2+ binding occurs only when the protein is in the reduced form, biochemical analyses show that under oxidative conditions, the GhCesA1 zinc-finger domain and also the full-length protein dimerize via intermolecular disulfide bonds, indicating CesA dimerization can be regulated by redox state. We also provide evidence that the herbicide CGA 325′615 (Syngenta, Basel), which inhibits synthesis of crystalline cellulose and leads to a disruption of rosette architecture, may affect the oxidative state of the zinc-finger domain that is necessary for rosette stability. Taken together, these results support a model in which at least part of the process of rosette assembly and function may involve oxidative dimerization between CesA subunits.
Cellulose is a highly abundant biopolymer consisting of microfibrils of β-1,4 glucan chains. In plants, these chains are synthesized and assembled into microfibrils at the plasma membrane from 6-fold symmetric complexes referred to as rosettes (1). The catalytic subunit responsible for glucan chain elongation is believed to be a plasma membrane glycosyltransferase named CesA (cellulose synthase). CesA genes were first discovered in cotton (2) and comprise a fairly large gene family of at least 10 members in Arabidopsis and maize, some groups of which are expressed during synthesis of primary wall cellulose, whereas others are expressed at the time of secondary wall synthesis (3, 4). Their importance in cellulose synthesis was further confirmed by mutations in CesA genes in Arabidopsis that lead to reduced cellulose content (5–9) by recent work showing that cotton GhCesA-1 expressed in yeast can initiate chain elongation using a sterol glucoside as primer (10) and by immunogold labeling of rosettes using antibodies directed against CesA (11). Rosette assembly has also been shown to be impaired in certain cellulose-deficient mutants (5, 12). Furthermore, the herbicide CGA 325′615 (Syngenta, Basel), which inhibits synthesis of crystalline cellulose and promotes accumulation of noncrystalline cellulose, also leads to rosette disruption (13).
One challenge for the future is to understand the mode of assembly of the rosette structures, including how CesA subunits might interact with each other and/or with other suspected proteins important for the process, such as the membrane-associated 1,4-β-glucan endohydrolase (also call the Korrigan cellulase; refs. 14, 15) or sucrose synthase (16). Recent work has also suggested that more than one distinct type of CesA may be required to function together to assure proper rosette assembly and function (7, 17, 18). All CesAs possess conserved motifs surrounding three conserved Asp (D) residues and eight putative transmembrane helices (1). According to current topological models, the three Asp residues localize close to each other and, together with a conserved QXXRW motif, form the active site on the cytoplasmic face of the plasma membrane, whereas the eight transmembrane helices create a channel in which the glucan chain is secreted. One notable characteristic of all of the CesA proteins is the presence of two zinc-finger domains located within the cytoplasmic N-terminal region of the proteins. (For sequences, see Fig. 8, which is published as supporting information on the PNAS web site, www.pnas.org). Except for a few members of the CslD family, the closest ancestors to the CesA genes and some of which may function in cellulose synthesis in tip-growing cells, these domains are notably lacking in the other members of the CesA superfamily (3, 19). Zinc-finger motifs contain four conserved cysteine residues (X3CX2–4CX12–15CX2C) that are arranged in tandem and can often possess DNA-binding properties. The cotton GhCesA1 and GhCesA2 zinc fingers (hereafter referred to as the Zn domain) show high similarity to several soybean putative transcription factors (20) and also to RING-finger domains. The RING-finger domain of the type C3HC4 has been defined as a series of conserved Cys and His residues (CX2CX9–39CX1–3HX2–3C/HX2CX4–48CX2C) that binds two zinc atoms in a unique “cross-brace” arrangement located at the N-terminal portion (reviewed in refs. 21, 22). Localization of the CesA Zn domain within the N terminus of CesA proteins at the cytoplasmic face of the plasma membrane suggests a likely possibility that the Zn domain is involved in protein–protein interactions, perhaps between CesA subunits themselves. Depending on the specific proteins, zinc- or RING-finger domains may interact and dimerize either via interactions in the state where the cysteine residues are reduced and zinc is inserted or, on oxidation, via specific disulfide linkages. Thus, control of the redox state of such domains can be important for determining whether dimerization and change in functional state of the interacting proteins can occur (23–27). In this regard, production of H2O2 strongly increases during secondary wall formation in cotton fibers and coincides with increases in CesA mRNA and protein expression and onset of rapid secondary wall cellulose synthesis (28). Furthermore, secondary cell wall development can be inhibited by depletion of endogenous H2O2 and promoted by addition of exogenous H2O2, indicating that redox state may function as an important signal in these processes, perhaps, at least in part, by regulating rosette assembly and function. Because RING fingers have also recently shown specific interactions with E2 conjugating enzyme in the process of ubiquitin-mediated protein degradation (21), it may also be possible that the redox state of the Zn domains regulates rates of CesA degradation.
In this study, we show that the GhCesA1 Zn domain binds zinc and exists as a monomer in the reduced state, and that either this domain alone or in full-length CesA protein can form dimers via intermolecular disulfide bridges in the oxidized state. By using isolated recombinant GhCesA-1 and GhCesA-2 domains, we show that these can also interact to form heterodimers. We also provide evidence supporting the concept that the herbicide CGA 325′615 may inhibit synthesis of crystalline cellulose by interfering with redox regulation in vivo.
Materials and Methods
Growth of Plants and Herbicide Treatments Conditions.
Gossypium hirsutum CV Coker 130 was grown in the greenhouse, and bolls were collected at 24 d postanthesis (DPA), as previously described (16). Arabidopsis thaliana (ecotype Columbia) seeds were chilled for 3 d at 4°C in Gamborg's plant nutrient solution supplemented with 2% sucrose in the dark. Seedlings were transferred to room temperature and grown in the dark for an additional 4 d. The herbicide CGA 325′615 (10 nM, from Syngenta, Basel) and H2O2 (as indicated) were added to the seedlings in solution containing 0.1% DMSO, and the seedlings were incubated at room temperature as indicated. Control seedlings were treated with 0.1% DMSO. Seedlings were visualized by using a Zeiss Axiovert 100 microscope.
Zinc-Binding Assays.
A PCR fragment encoding the GhCesA1 N-terminal region from Met-1-Ile-176 was cloned into pGEX-4T-3 (Pharmacia) to give [glutathione S-transferase (GST)-A1N176]. GST-A1N176 and GST were expressed in Escherichia coli and purified according to the manufacturer's protocol. For the Zn2+ blot assay, zinc-containing carbonic anhydrase and ovalbumin (Sigma) were used as positive and negative controls, respectively. Three micrograms of each protein were separated on 10% SDS/PAGE, transferred onto a nitrocellulose membrane, washed briefly in deionized water, and then incubated for 3 hr in 0.1 M Tris⋅HCl, pH 6.8/50 mM NaCl. The membrane was then incubated for 1 hr at 25°C with the same buffer containing 80 μCi of 65Zn2+ (Amersham Pharmacia, 1 Ci/g), washed three times with this buffer, dried, and exposed overnight to x-ray film.
Yeast Two-Hybrid System.
GhCesA1 and GhCesA2 N-terminal regions (Met-1-Ile-88 and Met-1-Arg-111, respectively) were PCR cloned into p-GAL4BD vector (Stratagene) to give pCesA1N-GAL4BD and pCesA2N-GAL4BD, respectively. CesA1 and CesA2 zinc domains (Met-1-Val-66 and Met-1-Tyr-79, respectively) were also PCR cloned into p-GAL4AD to give pCesA1ZD-GAL4AD and pCesA2ZD-GAL4AD, respectively. Interactions between the plasmids were tested by introducing the indicated pairs of constructs into YRG-2 yeast cells (Stratagene). Transformants were plated on synthetic minimal medium that lacked the amino acids Leu, Trp, and His and supplemented with 5 mM 3-amino-1,2,4-triazol. Yeast transformation and β-galactosidase activity were carried out according to the instructions of Stratagene.
Pull-Down Assay.
GhCesA1 and GhCesA2 N-terminal regions (Met-1-Arg-115 and Met-1-Arg-111, respectively) were PCR cloned into a GST expression vector pGEX-4T (Pharmacia) and named GST-A1N115 and GST-A2N111, respectively. The recombinant proteins were expressed in E. coli BL21, affinity purified according to the instructions of Pharmacia, and dialyzed against 137 mM NaCl/2.7 mM KCl/4.3 mM Na2HPO4/1.4 mM KH2PO4, pH 7.3. The GhCesA1 zinc domain (H-A1ZD; Met-1 Val-66) was PCR cloned into the His-tag expression vector pQE30 (Qiagen, Chatsworth, CA), expressed in E. coli BL21, affinity purified according to the instructions of Qiagen and dialyzed against 20 mM Hepes–NaOH, pH 7.4. Protein concentrations were determined by using Coomassie Plus Protein Assay Reagent (Pierce) with BSA as standard.
For pull-down experiments, H-A1ZD (7.5 μM) was incubated in the presence of 2 μM GST-A1N115, GST-A2N111, or GST at 4°C for 4 h in 250 μl of binding buffer (250 mM NaCl/2.7 mM KCl/4.3 mM Na2HPO4/1.4 mM KH2PO4, pH 7.3/5% glycerol). Thirty microliters of glutathione-Sepharose 4B beads (Pharmacia) prewashed with 30 volumes of binding buffer was added to the proteins, and samples were incubated for an additional 45 min at 4°C, centrifuged at 1,420 × g for 5 min at 4°C, and the beads were then washed three times with 750 μl of binding buffer containing 0.2% Nonidet P-40. Proteins were released by boiling the beads 5 min with 30 μl of SDS/PAGE sample buffer. Samples (10 μl) were separated on a 15% SDS/PAGE and detected by silver staining.
Determination of GhCesA Zn Domain Interactions.
Recombinant H-A1ZD (2 μg) was analyzed on native PAGE (15% PAGE containing 6.25% glycerol) using 250 mM Tris⋅HCl, pH 8.8, as gel and running buffer. Gels were run at 20 mA for 6 hr at 4°C, and proteins were visualized by silver staining. The molecular size of H-A1ZD in solution was estimated by separating 75 μg of purified protein on a Superdex 75 HR column (Amersham Pharmacia Biotech), equilibrated with 20 mM Hepes–NaOH, pH 7.4/200 mM NaCl/20 μM ZnCl2 using System Gold HPLC (Beckman Coulter). Flow rate was 0.5 ml/min, and the column was calibrated with standards of lactalbumin (14.2 kDa) and carbonic anhydrase (29 kDa). To estimate the mol size of H-A1ZF in 15% SDS/PAGE, the protein (5 μg) was incubated in the presence of diamide for 20 min at 4°C and then incubated in loading buffer for 10 min at 37°C, separated on SDS/PAGE, and visualized by silver staining. Reduction of H-A1ZD was also monitored with the fluorescent probe monobromobimane (THIOLYTE, Calbiochem). Twenty micrograms of recombinant protein was reduced by 10 mM DTT for 10 min at 4°C and further incubated with 40 nmol monobromobimane for 30 min at room temperature. The reaction was stopped by incubating with SDS/PAGE sample buffer for 10 min at 37°C and separated on 15% SDS/PAGE gels, and fluorescent labeling was visualized as previously described (29).
Expression of GhCesA1 in Yeast.
Full-length GhCesA1 cDNA was cloned into pYes2 (Invitrogen), expressed in Saccharomyces cerevisiae INVSc1 (Invitrogen), and extracted as previously described (10). Total yeast membrane proteins (20 μg per lane) were separated on SDS/PAGE gradient gel (4–12%), electroblotted onto nitrocellulose membranes, and immunodetected with antibodies against the Zn domain of GhCesA1, as previously described (13). This antibody specifically recognizes only the Zn domain of GhCesA1 (and not that of GhCesA2); it reacts well with this domain after blotting from SDS/PAGE and, for the native form, it shows a strong preference for the reduced form of the Zn domain.
Native PAGE of Cotton Fiber Membrane Proteins.
Fibers from five 24-DPA locules (24 DPA) were extracted in 5 ml of ice-cold extraction buffer [50 mM 4-morpholinepropanesulfonic acid (Mops)–NaOH, pH 7.5/5 mM EDTA/0.25M sucrose/protease inhibitor mixture; Boehringer] with or without 10 mM DTT, as indicated. The extract was centrifuged at 2,800 × g for 30 min at 4°C, 15,000 × g for 30 min at 4°C, and 400,000 × g for 60 min at 4°C. The 400,000 × g pellet was resuspended at 4°C in 3 ml of 50 mM Mops–NaOH, pH 7.5/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/protease inhibitor mixture and recentrifuged at 400,000 × g for 60 min at 4°C. Supernatant (20 μg) was separated on 4–12% gradient Tris-glycine native PAGE (NOVEX, San Diego). Gels were soaked in 12 mM Tris-base/96 mM glycine/0.25% SDS before electroblotting.
Results
The GhCesA1 N-Terminal Region Is a Zinc-Binding Domain.
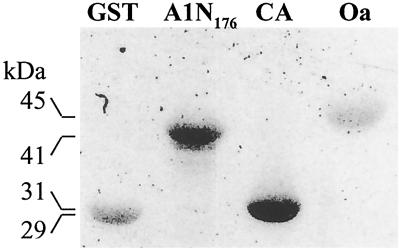
As a first goal to characterize the structure and function of the Zn domains of CesA proteins, we show that purified recombinant GST-A1N176 (GhCesA1; Met-1-Ile-176) binds Zn65 after renaturation following SDS/PAGE separation and blotting onto nitrocellulose. The level of binding is similar to that of carbonic anhydrase, a known zinc-containing protein (Fig. 1). Purified nonzinc proteins GST and ovalbumin showed only weak nonspecific binding. Direct chemical analysis indicated that GST-A1N176 specifically binds about 2 mol of zinc per mol of protein after the estimated nonspecific binding is subtracted (Table 1, which is published as supporting information on the PNAS web site, www.pnas.org).
Fig 1.
Analysis of the zinc-binding capacity of the GhCesA1 zinc domain. Binding of Zn65 to recombinant GST, GST-A1N176, positive binding control carbonic anhydrase (CA), and negative control ovalbumin (Oa).
Interaction of GhCesA1 and GhCesA2 Zn Domains.
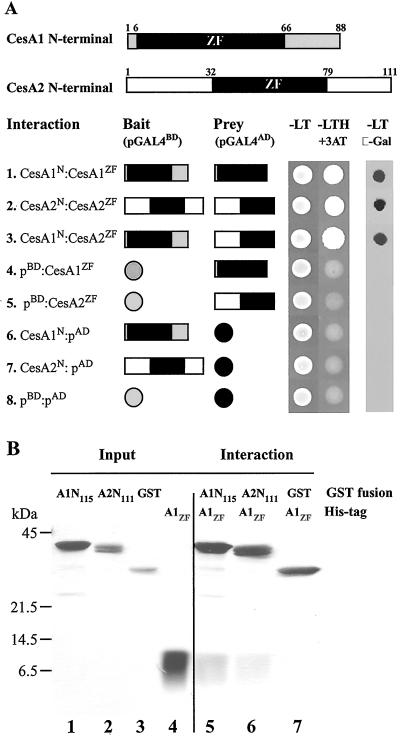
Interaction studies using the yeast two-hybrid system show that the Zn domains of GhCesA1 can interact with themselves to form homodimers or with each other to form heterodimers (Fig. 2A). Controls shown indicate that neither of these domains shows self-activation properties. That the GhCesAs Zn domains cannot form heterodimers with the pGAL4BD domain containing a C2H2-type zinc finger, which itself forms a dimer, and binds to DNA (30) indicates there is a specific interaction of the CesA1 and CesA2 Zn domains.
Fig 2.
Interactions of GhCesA1 and GhCesA2 N-terminal region in yeast and in vitro. (A) Schematic presentation of the GhCesA1 and GhCesA2 N-terminal region (Upper) that contain the Zn domains (black). Yeast transformed with the plasmids containing the GAL-4 binding domain (pGALbd: bait) and the GAL-4 activating domain (pGALad: prey), as indicated, were grown on synthetic complete medium minus leu and trp (-LT) and minus leu, trp, and his and 5 mM 3-amino 1,2,4-triazole (-LTH + 3-amino-1,2,4-triazol). β-Galactosidase activity was assayed on –LT medium. (B) Purified recombinant GhCesA1 N-terminal GST fusion (A1N115), GhCesA2 N-terminal GST fusion (A2N111), and GST (“Input”) incubated with His-tagged GhCesA1 Zn domains (A1zd) were washed and eluted (“Output”) and proteins subjected to 12% SDS/PAGE developed by silver staining.
Pull-down experiments with recombinant proteins were used to confirm these interactions. Purified GST-A1N115, GST-A2N111, or GST (Fig. 2B, lanes 1–3, respectively) was tested for interaction with purified His-tagged CesA1 Zn domain (A1ZD; Fig. 2B, lane 4) by coincubation of pairs followed by incubation and pull-down with glutathione Sepharose beads. As for the yeast two-hybrid system, this domain of CesA1 can interact with itself (Fig. 2B, lane 5) or with the similar domain of CesA2 (Fig. 2B, lane 6), each interacting in an approximate 1:1 molar ratio as judged crudely by silver staining, but only very weakly with GST (Fig. 2B, lane 7).
Dimerization Is Regulated in Vitro by Redox State.
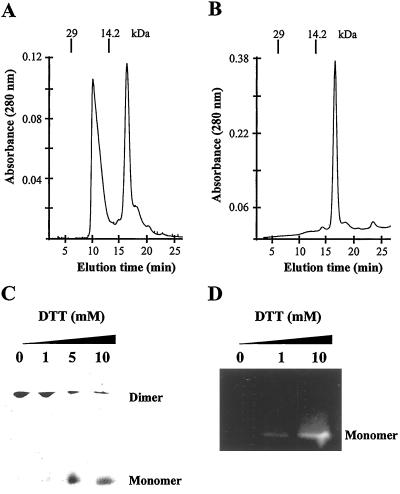
Because zinc-finger motifs can function as protein-binding domains and sometimes undergo dimerization that is redox dependent (27), we tested the effect of redox state on the interactions between these CesA domains. Size-exclusion chromatography of His-tagged GhCesA1 Zn domain (H-A1ZD; Met-1-Val-66) in buffer lacking DTT showed that this protein exists as a mixture of monomer (8.2 kDa) and dimer (16.4 kDa) (Fig. 3A) and shifts completely to the monomeric state in the presence of 5 mM DTT (Fig. 3B).
Fig 3.
Effect of DTT on GhCesA1 Zn domain dimerization. Size-exclusion HPLC of purified recombinant His-tagged GhCesA1 Zn domain (H-A1ZD) in the presence of Zn2+ (20 μM ZnCl2) under nonreduced (A) and (B) reduced conditions (5 mM DTT). The elution time (min) of the marker proteins lactalbumin (14.2 kDa) and carbonic anhydrase (29 kDa) are indicated. (C) H-A1ZD was preincubated with the indicated amounts of DTT for 10 min at 4°C and analyzed on 15% nondenaturing PAGE developed by silver staining. Monomer and dimer are indicated. (D) H-A1ZD, pretreated as described in C was incubated with monobromobimane, subjected to 15% SDS/PAGE, and fluorescent labeling was visualized at 365 nm.
H-A1ZD was further analyzed on nondenaturing PAGE wherein increasing amounts of DTT (5–10 mM) lead to conversion of most dimers to monomers (Fig. 3C). Furthermore, without DTT but in the presence of the strong Zn2+ chelator, o-phenanthroline, the protein still exists primarily as dimer, indicating that the dimerization does not involve a Zn-bound state. Evidence that the dimerization more likely involves formation of intermolecular disulfide bridges comes from our observation that the oxidized dimer cannot react with the thiol-reactive fluorescent probe monobromobimane on SDS/PAGE (Fig. 3D, lane 1), whereas in the presence of 10 mM DTT, the reduced monomer shows a strong fluorescent signal.
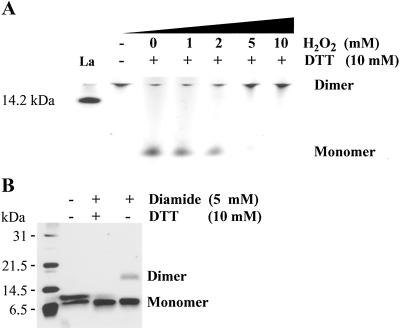
Elevation in H2O2 levels such as that occurring in vivo in cotton fibers (28) might be one means by which dimerization to promote cellulose synthase complex assembly could occur. Dimers of H-A1ZD (Fig. 4A, lane 2) were reduced in the presence of 10 mM DTT to the monomer form (Fig. 4A, lane 3) and further incubated with increasing amounts of H2O2. By progressively increasing the molar ratio of DTT/H2O2, a gradual shift from monomer to dimer occurred, and dimers were detected only at a 1:1 molar ratio (Fig. 4A, lane 7).
Fig 4.
Redox potential regulates dimerization of H-A1zd. (A) H-A1zd pretreated with 10 mM DTT at 4°C for 15 min was incubated for an additional 15 min at 4°C in the presence of increasing concentrations of H2O2 as indicated and analyzed on 15% nondenaturing PAGE developed by silver staining. Untreated protein (30 min at 4°C) served as control. The 14.2-kDa lactalbumin marker is indicated. (B) H-A1zd was incubated in the presence of DTT and diamide as indicated and subjected to 15% SDS/PAGE developed by silver staining.
Under nonreducing conditions on SDS/PAGE, two bands were visualized reflecting the reduced (Fig. 4B Upper, lane 2) monomer and an oxidized (Lower, lane 2) monomer, which presumably formed by intramolecular disulfide bonds. Addition of 10 mM DTT and the oxidizing agent diamide to H-A1ZD gradually reduced the upper band and increased the lower band, whereas incubation in the presence of 5 mM diamide alone resulted in a single oxidized monomer band and some dimer with the expected molecular mass of about 17 kDa. These results suggest that the CesA1 Zn domain can exist as a dimer and/or, under some conditions, as two distinct forms of monomer: an oxidized form in which intermolecular disulfide bonds may be formed between cysteine residues and the reduced form that on oxidation forms a dimer via intramolecular disulfide bonds.
Full-Length GhCesA1 Can Also Form Dimers and Tetramers.
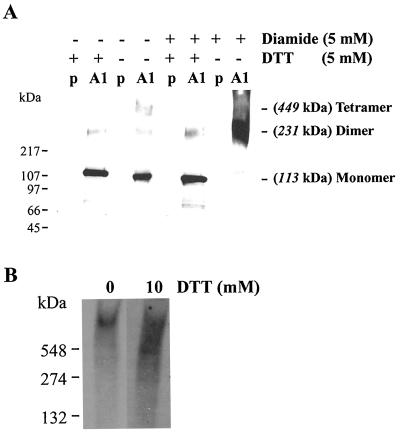
In yeast cells expressing full-length cDNA for GhCesA1 under control of the GAL-inducible promoter (PGAL1-CesA1), the protein is highly expressed and detectable in the membrane fraction using anti-GhCesA1 Zn domain antibodies. In the presence of 5 mM DTT in the SDS/PAGE gel sample buffer, GhCesA1 migrates mainly as monomer (113 kDa) with a slight amount of dimer (231 kDa) (Fig. 5A, lane 2). In the absence of DTT, an additional band with a calculated molecular mass of about 450 kDa was detected, suggesting that under nonreduced conditions CesA1 forms monomers, dimers, and tetramers (Fig. 5A, lane 4). Diamide added with DTT did not alter these patterns (Fig. 5A, lane 6), but in diamide without DTT, monomer disappeared and the protein was primarily a dimer with possibly other higher molecular weight forms as well (Fig. 5A, lane 8), suggesting again that dimerization is the main form under fully oxidative conditions. Although it is difficult to estimate the size of detergent-solubilized GhCesA1 from cotton fibers, on native gels we see an indication that a higher molecular weight form does begin to disassemble when oxidizing conditions are shifted in the presence of DTT (Fig. 5B).
Fig 5.
Redox state affects dimerization and complex formation of the full-length GhCesA1 and complex formation in vivo. (A) Western blot of total membrane proteins (20 μg) extracted from yeast expressing the plasmid PGAL1 (P) and the full-length GhCesA1 (PGAL1-CesA1: A1) subjected to gradient (4–12%) SDS/PAGE, blotted, and immunodetected with anti GhCesA1 Zn domain antibodies. Samples were pretreated with DTT and diamide as indicated. (B) Western blot of detergent-solubilized plasma membrane proteins extracted from 24 DPA cotton fibers and separated on nondenaturing gradient PAGE (4–12%). The blot was immunodecorated as described in A.
The Herbicide CGA 325′625 May Inhibit Crystalline Cellulose Formation by Altering Redox Conditions.
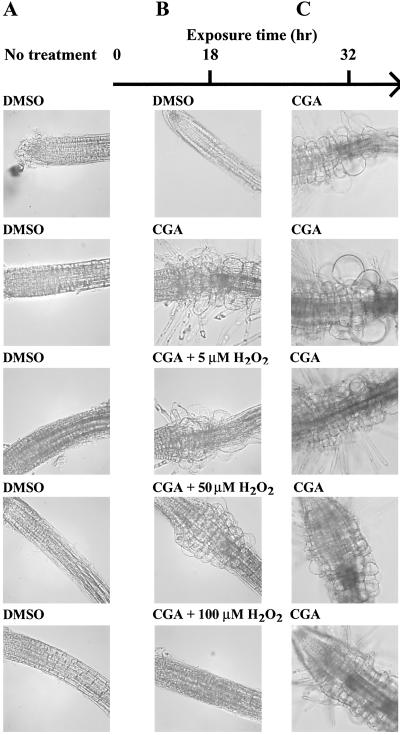
Inhibition of crystalline cellulose formation coupled with abrogation of rosette assembly and noncrystalline glucan accumulation by the herbicide CGA 325′615 (13) may indicate a situation in which active CesA subunits can function but cannot assemble crystalline microfibril arrays. This idea led us to test whether CGA 325′615 might prevent rosette formation by disturbing oxidative conditions that allow CesA dimers or higher oligomers to form. If so, restoring oxidative conditions by addition of H2O2 might reverse the effects of this herbicide. Exposure of Arabidopsis seedlings to 10 nM CGA 325′615 for 18 hr induced the radial swelling phenotype characteristic of phenotypes in which cellulose synthesis is inhibited in the root tips and also induced ectopic root hair formation in this region (compare Fig. 6 A with B). Addition of increasing concentration of H2O2 to the CGA 325′615-treated seedlings significantly reduced the radial swelling and completely abrogated root hair induction, and 100 μM H2O2 completely reversed all effects of the herbicide (Fig. 6B). To show this effect was reversible and not caused by other unknown effects of H2O2, treated seedlings were transferred for an additional 14 hr to CGA 325′615. Seedlings pretreated with herbicide and H2O2 completely recovered the CGA 325′615 phenotype on removal of the H2O2 and possessed similar phenotypes to those obtained in seedlings treated for 32 hr with CGA 325′615 alone (Fig. 6C). As a control, we determined that CGA 325′615 activity was not destroyed by pretreatment with H2O2 (not shown). To demonstrate that the H2O2 effect specifically relates to the mode of action of CGA 325′615, the herbicide DCB, which presumably possesses a different mode of action (13), was also incubated in the presence and absence of H2O2. The induced radial swelling phenotype obtained by DCB was not abrogated by exposure of Arabidopsis seedlings to DCB and H2O2 (not shown).
Fig 6.
Effect of H2O2 on the phenotype of Arabidopsis seedlings treated with CGA 325′615. Four-day seedlings were analyzed prior to (A) and after (B) treatment with CGA 325′615 in the presence of H2O2 concentrations as indicated. To recover the CGA 325′615 phenotype, treated seedlings were further incubated for additional 14 hr in the presence of herbicide (C).
Discussion
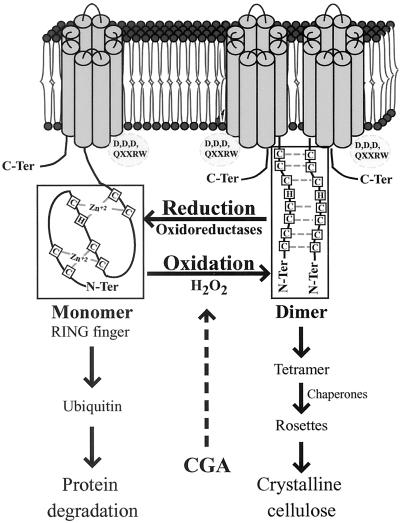
Taken collectively, the data presented have led us to consider a model in which dimerization of CesA subunits represents the first step in rosette assembly that occurs in the plasma membrane or alternatively in the outer face of the Golgi (Fig. 7). In general terms, this dimerization is proposed to occur via redox-regulated disulfide bond formation between at least some of the Cys resides in the Zn domains of two CesA subunits. Our evidence also suggests that CGA 325′625 may inhibit synthesis of crystalline cellulose by interfering with some aspect of the dimerization process. For cotton fibers at least, the H2O2 production that occurs during the onset of secondary wall formation (28) could create localized oxidative conditions that favor dimerization and capacity for synthesis of β-1,4-glucan chains, a process that could involve specific protein disulfide oxidoreductases. Final rosette assembly that would be required for synthesis of crystalline microfibrils may well involve further protein–protein interactions that have not been identified in this study. Similarly, the possibility that the reduced form on the Zn domain may serve as a target for ubiquitin-mediated degradation or recycling of CesA subunits remains an open question.
Fig 7.
Model for dimerization of two CesA subunits via the Zn domains under oxidative conditions. In this model, the hypothetical topology of a channel formed by single CesA1 is presented. Alternatively, this channel might be formed by higher interactions between two CesAs.
In support of this model, we have shown that the GhCesA1 Zn domain contains two active zinc-binding domains (Fig. 1). In the yeast two-hybrid system, GhCesA1 and GhCesA2 Zn domains can interact with themselves or with each other. Lack of activation of the two-hybrid system by the Zn domain fused to the GAL4 activating domain (Fig. 2A, 4 and 5) further supports the hypothesis that the Zn domain does not possess transcription factor properties. Using isolated recombinant proteins, these interactions were further verified in vitro. Clearly, in the reduced state promoted by addition of DTT, the GhCesA1 Zn domain exists preferentially in the monomeric state (Fig. 3). On oxidation by physiological levels of H2O2 (Fig. 4A) or by treatment with the strong oxidizing agent diamide that promotes disulfide formation (Fig. 4B), the preferred state of the Zn domain is a dimer. That full-length GhCesA1 expressed in yeast can also undergo redox-regulated dimerization indicates there is no other domain involved per se in this interaction. However, our finding that, in cotton fibers, whereas reducing conditions lead to partial disassembly but not to complete monomerization, forms higher than dimers may exist in vivo (Fig. 5B) and may suggest that other interactions may also be involved in total rosette assembly. This is also indicated by the fact that rosette assembly is impaired in the rsw1 mutant where the mutation lies in a region outside the Zn domain (5). By contrast, in membranes extracted from yeast cells expressing the full-length GhCesA1, the protein migrates as monomer, dimer, and tetramer with the dimer being the preferred form under completely oxidizing conditions (Fig. 5A). We note that other proteins that might participate in rosette assembly are not present in yeast, and we have not observed rosette formation or cellulose synthesis when GhCesA-1 alone is expressed (C. Haigler, Y.K., and D.D., unpublished work), although there is an ability to elongate supplied steryl glucoside to steryl cellotriose (10). Similarly, we note that intramolecular disulfide formation can also occur in the Zn domain (Fig. 4B); this interaction seems to abrogate the potential for dimerization and may be a factor that needs control in vivo. In this regard, our finding that a thioredoxin-like protein also interacts with the Zn domain in the yeast two-hybrid system (D.J.W. and D.D., unpublished work) may provide one means to minimize such a reaction, because thioredoxins are especially adept at reducing intramolecular disulfide bonds (31). Exactly which cysteine residues are preferred to interact for dimerization and how higher-order assembly of CesA subunits into rosettes occurs in vivo are important questions for the future.
Dimerization via RING-finger domains is well documented in plants and animals. In Arabidopsis, for example, the constitutive photomorphogenic 1 (COP1) interacts via its RING-finger domain type C3HC4 with the COP1 interacting protein 8 (CIP8) RING-H2 finger (32). As a result of the COP1–CIP8 interaction, the stability of the CIP8 in seedlings is increased. In addition, oxidative stress regulates the zinc finger of the eukaryotic replication protein A, which binds DNA in reduced conditions while under oxidized conditions and zinc release the protein dimerizes via formation of disulfide bonds (23). Graumann et al. (27) demonstrated that, on oxidative stress, the zinc-binding molecular chaperone Hsp33 dimerizes via two intramolecular disulfide bonds and becomes active as a molecular chaperone. The critical role of H2O2 in regulation of onset of secondary wall cellulose synthesis in cotton fibers (28) may therefore at least partially relate to its effects in vivo on CesA interactions via Zn domains.
The results obtained in vivo by using CGA 325′615 and H2O2 treatments (Fig. 6) can be explained if dimerization via the Zn domains occurs naturally in vivo under oxidative conditions, an event necessary for rosette assembly and function. These results support the previous finding that rosettes are disrupted on treatment with this herbicide (13), and our finding that addition of H2O2 completely abrogates the effects of the herbicide indeed supports the concept that CGA 325′615 somehow inhibits the oxidation and dimerization of CesA Zn domains. We also note that root hair growth is not affected at all by this herbicide; if the CslD proteins that lack Zn domains are the functional cellulose synthases in these tip-growing cells (19), lack of sensitivity to CGA 325′615 would be expected according to this model.
RING fingers also play important roles in transferring ubiquitin to heterologous proteins or to the RING-finger protein itself, namely by acting as E3 ubiquitin protein ligases (21, 22). Therefore, under reduced conditions, it may be suggested that the CesA Zn domain acts as an E3 ligase and promotes its own degradation. Our recent finding (I.K. and D.D., unpublished work) that GhCesA1 has a half-life in vivo in cotton fibers of less than 1 hr indeed suggests that regulation of CesA turnover may be another important aspect of regulation of cellulose synthesis. This could conceivably also relate to the question raised by other work (7, 17, 18) of whether a mixture of distinct forms of CesA are required for rosette assembly and/or function. According to the model presented by Scheible et al. (17), at least two nonidentical CesA1 subunits are required for active cellulose synthase complex. At present, our data do not indicate a preference for homo- vs. heterodimerization in the yeast two-hybrid system or in vitro. However, one cannot rule out the possibility that other domains are also involved in this interaction, and that chaperones and/or specific protein oxidoreductases might confer specificity in interactions that could not be detected in these studies. That mutant phenotypes are obtained when only one of several distinct CesAs expressed within the same cell type is impaired may imply that, in vivo, heterodimer formation is an essential step in rosette assembly and function.
In sum, the finding that dimerization of CesA subunits can occur via their Zn domains does point to new directions for future research on cellulose synthesis. Such future directions should include confirmation in vivo that these interactions are important for at least one step in rosette assembly, whether homo- or heterodimers are preferred, how redox control might regulate assembly, what other interactions between CesAs and/or other proteins are involved, and how turnover of the complexes in vivo is regulated.
Supplementary Material
Acknowledgments
We thank Pat Hogan for very helpful discussions and technical assistance and Klaus Kruez (Syngenta, Basel) for the gift of CGA 325′615. This work was supported by Grant DE-FG-03–963-ER-20238 to D.P.D. from the U.S. Department of Energy.
Abbreviations
CesA, cellulose synthase
Zn domain, zinc-finger domain
DPA, d postanthesis
GST, glutathione S-transferase
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Delmer D. P. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 245-276. [DOI] [PubMed] [Google Scholar]
- 2.Pear J., Kawagoe, Y., Schreckengost, W., Delmer, D. P. & Stalker, D. (1996) Proc. Natl. Acad. Sci. USA 93, 12637-12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richmond T. A. & Somerville, C. R. (2000) Plant Physiol. 124, 495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland N., Holland, D., Helentjaris, T., Dhugga, K., Xoconostle-Cazares, B. & Delmer, D. P. (2000) Plant Physiol 123, 1313-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arioli T., Peng, L., Betzner, A. S., Burn, J., Wittke, W., Herth, W., Camilleri, C., Hofte, H., Plazinski, J., Birch, R., et al. (1998) Science 279, 717-720. [DOI] [PubMed] [Google Scholar]
- 6.Taylor N. G., Scheible, W.-R., Cutler, S., Somerville, C. R. & Turner, S. R. (1999) Plant Cell 11, 769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor N. G., Laurie, S. & Turner, S. R. (2000) Plant Cell 12, 2529-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagard M., Desnos, T., Desprez, T., Goubet, F., Refregier, G., Mouille, G., McCann, M., Rayon, C., Vernhettes, S. & Hofte, H. (2000) Plant Cell 12, 2409-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner S. R., Taylor, N. & Jones, L. (2001) Plant Mol. Biol. 47, 209-219. [PubMed] [Google Scholar]
- 10.Peng L., Kawagoe, Y., Hogan, P. & Delmer, D. (2001) Science 295, 147-150. [DOI] [PubMed] [Google Scholar]
- 11.Kimura S., Laosinchai, W., Itoh, T., Cui, X., Linder, C. R. & Brown, R. M., Jr. (1999) Plant Cell 11, 2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura S., Sakurai, N. & Itoh, T. (1999) Plant Cell Physiol. 40, 335-338. [Google Scholar]
- 13.Peng L., Xiang, F., Roberts, E., Kawagoe, Y., Greve, L. C., Kreuz, K. & Delmer, D. P. (2001) Plant Physiol. 126, 981-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane D. R., Wiedemeier, A., Peng, L., Hofte, H., Vernhettes, S., Desprez, T., Hocart, C. H., Birch, R. J., Baskin, T. I., Burn, J. E., et al. (2001) Plant Physiol. 126, 278-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato S., Kato, T., Kakegawa, K., Ishii, T., Liu, Y. G., Awano, T., Takabe, K., Nishiyama, Y., Kuga, S., Sato, S., et al. (2001) Plant Cell Physiol. 42, 251-263. [DOI] [PubMed] [Google Scholar]
- 16.Haigler C. H., Ivanova-Datcheva, M., Hogan, P. S., Salnikov, V. V., Hwang, S., Martin, K. & Delmer, D. P. (2001) Plant Mol Biol. 47, 29-51. [PubMed] [Google Scholar]
- 17.Scheible W.-R., Eshed, R., Richmond, T., Delmer, D. & Somerville, C. (2001) Proc. Natl. Acad. Sci. USA 98, 10079-10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin R. M. (2001) Curr. Biol. 11, R213-R216. [DOI] [PubMed] [Google Scholar]
- 19.Doblin M. S., De Melis, L., Newbigin, E., Bacic, A. & Read, S. M. (2001) Plant Physiol. 125, 2040-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawagoe Y. & Delmer, D. P. (1997) Genet. Eng. 19, 63-87. [DOI] [PubMed] [Google Scholar]
- 21.Freemont P. S. (2000) Curr. Biol. 10, R84-R87. [DOI] [PubMed] [Google Scholar]
- 22.Joazeiro C. A. P. & Weissman, A. M. (2000) Cell 102, 549-552. [DOI] [PubMed] [Google Scholar]
- 23.Park J.-S., Wang, M., Park, S.-J. & Lee, S.-H. (1999) J. Biol. Chem. 274, 29075-29080. [DOI] [PubMed] [Google Scholar]
- 24.Delaunay A., Isnard, A. D. & Toledano, M. B. (2000) EMBO J. 19, 5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopalakrishna R. & Jaken, S. (2000) Free Radic. Biol. Med. 28, 1349-1361. [DOI] [PubMed] [Google Scholar]
- 26.Wang M., You, J. S. & Lee, S. H. (2001) Antioxid. Redox Signal. 3, 657-669. [DOI] [PubMed] [Google Scholar]
- 27.Graumann J., Lilie, H., Tang, X., Tucker, K. A., Hoffmann, J. H., Vijayalakshimi, J., Saper, M., Bardwell, C. A. & Jakob, U. (2001) Structure (London) 9, 377-387. [DOI] [PubMed] [Google Scholar]
- 28.Potikha T. S., Collins, C. C., Johnson, D. I., Delmer, D. P. & Levin, A. (1999) Plant Physiol. 119, 849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautier M.-F., Lullien-Pellerin, V., De Lamotte-Guery, F., Guirao, A. & Joudrier, P. (1998) Eur. J. Biochem. 252, 314-324. [DOI] [PubMed] [Google Scholar]
- 30.Baleja J. D., Marmorstein, R., Harrison, S. C. & Wagner, G. (1992) Nature (London) 356, 450-453. [DOI] [PubMed] [Google Scholar]
- 31.Holmgren A. (1989) J. Biol. Chem. 264, 13963-13966. [PubMed] [Google Scholar]
- 32.Torii K. U., Stoop-Mayer, C. D., Okamoto, H., Coleman, J. E., Matsui, M. & Deng, X. W. (1999) J. Biol. Chem. 274, 27674-27681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.