Abstract
In mammals, male sex determination starts when the Y chromosome Sry gene is expressed within the undetermined male gonad. One of the earliest effect of Sry expression is to induce up-regulation of Sox9 gene expression in the developing gonad. SOX9, like SRY, contains a high mobility group domain and is sufficient to induce testis differentiation in transgenic XX mice. Before sexual differentiation, SOX9 protein is initially found in the cytoplasm of undifferentiated gonads from both sexes. At the time of testis differentiation and anti-Müllerian hormone expression, it becomes localized to the nuclear compartment in males whereas it is down-regulated in females. In this report, we used NIH 3T3 cells as a model to examine the regulation of SOX9 nucleo-cytoplasmic shuttling. SOX9-transfected cells expressed nuclear and cytoplasmic SOX9 whereas transfected cells treated with the nuclear export inhibitor leptomycin B, displayed an exclusive nuclear localization of SOX9. By using SOX9 deletion constructs in green fluorescent protein fusion proteins, we identified a functional nuclear export signal sequence between amino acids 134 and 147 of SOX9 high mobility group box. More strikingly, we show that inhibiting nuclear export with leptomycin B in mouse XX gonads cultured in vitro induced a sex reversal phenotype characterized by nuclear SOX9 and anti-Müllerian hormone expression. These results indicate that SOX9 nuclear export signal is essential for SOX9 sex-specific subcellular localization and could be part of a regulatory switch repressing (in females) or triggering (in males) male-specific sexual differentiation.
Expression of Sry (1, 2) in the undetermined male gonad induces a variety of morphogenetic events including cell proliferation, cell migration, Sertoli cell fate determination, and subsequent sex cord formation (3–6). The earliest downstream effect of Sry may be up-regulation of Sox9 expression in the developing gonad (7), although it has yet to be shown at the molecular level. SOX9 is related to SRY by its high mobility group (HMG) domain. Interestingly, SOX9 is better conserved during evolution and, like SRY, is sufficient to induce testis differentiation in female transgenic mice (8). Furthermore, heterozygous mutations in SOX9 lead to campomelic dysplasia, a skeletal malformation syndrome associated with sex reversal in 75% of XY patients (9, 10).
Immunofluorescence studies on mouse and human XX and XY embryo gonad sections revealed that cytoplasmic SOX9 protein is present in undifferentiated gonads of both sexes, but that in the male gonad it becomes nuclear at the onset of testis differentiation (7, 11). These results, together with the previous identification of two nuclear localization signal (NLS) sequences in the HMG box (12), let us to hypothesize that SOX9 is able to shuttle between the nucleus and the cytoplasm and thus may contain a nuclear export signal (NES). Prototypic NESs are short hydrophobic sequences rich in leucine residues (13–16) regulating the subcellular localization of several proteins through interaction with the CRM1 nuclear export receptor (17, 18). CRM1-dependent nuclear export can be inhibited by leptomycin B (LMB), a Streptomyces metabolite (19), which blocks the interaction between CRM1 and NES (20, 21). Here, we report the identification and characterization of a functional LMB-sensitive NES located between the two NLSs in the SOX9 HMG domain. Furthermore, we show in mouse gonad culture experiments, that blocking NES function with LMB interferes with the sex-specific gene expression pattern of female gonads, and thus mimicking a sex reversal phenotype. We hypothesize that SOX9 subcellular localization plays a key role in the regulation of the onset of male vs. female sexual differentiation pathways.
Materials and Methods
Green Fluorescent Protein (GFP)-Reporter Constructs.
The SOX9 expression vector (pcDNA-SOX9) was described (22). SOX9 HMG box fragments were amplified by PCR by using the following oligo-nucleotides: HMG1: 5′-CCGCACGTCAAG-CGGCCCATGAAC (spanning SOX9 amino acids 103–110), HMG2: 5′-CGCCTGCCCGTTCTTCACCGACTT (amino acids 180–187), NES1: 5′-GCGGACCAGTACCCGCACTTGCAC (amino acids 124–131), and NES2: 5′-GTACTTGTAAT-CCGGGTGGTCCTT (amino acids 167–174). PCR fragments HMG (HMG1/HMG2), ΔNLS1–2 (NES1/NES2), ΔNLS1 (NES1/HMG2), ΔNLS2 (HMG1/NES2), and double-stranded oligo-nucleotides corresponding to SOX9 NES (amino acids 134–147: ELSKTLGKLWRLLN) and to mutant SOX9 NESs (5L-5A: EASKTAGKAWRAAN and L142A: ELSKTLGKAWRLLN) were cloned in-frame, downstream of GFP, in the pcDNA3.1/NT-GFP-TOPO vector (Invitrogen). The L142A mutation was introduced in the pcDNA-SOX9 HMG-GFP construct by site-directed mutagenesis (Stratagene QuickChange kit).
Cell Transfection and GFP Detection.
For GFP detection, 5 × 104 cells were seeded onto coverslips 24 h before transfection. Plasmid DNA (200–500 ng) was transfected by using Dc Chol liposome (23) in OptiMEM-glutamax medium (Invitrogen). After incubation for 36 h, cells were fixed in 3.7% (vol/vol) paraformaldehyde/PBS for 10 min at room temperature and washed in PBS. Cell nuclei were stained by Hoechst 33286, and GFP was visualized by fluorescence microscopy. On average, 100 cells were scored.
Collection and Sexing of Gonad Explants.
Swiss mouse embryos were collected between 10.5 and 12.5 days postcoitum (dpc) and staged according to the tail somite (TS) number. Pairs of gonad/mesonephros were excised from embryos in dissecting medium [10% (vol/vol) FCS/25 mM Hepes, pH 7.4, in DMEM, GIBCO/Invitrogen] and kept in individual tissue culture wells at room temperature. Concurrently, the chromosomal sex of all embryos was determined by scoring for the presence or absence of Barr bodies in Toluidine blue-stained amniotic cells (24).
Cell Culture and Embryonic Gonad Culture.
COS-7 and NIH 3T3 cells were maintained in DMEM supplemented with 10% (vol/vol) FCS, glutamine, and penicillin. LMB and actinomycin D (AcD) were purchased from Sigma. LMB (2.5 ng/ml) and AcD (5 μg/ml) were added 3 h before fixation of the cells. Cycloheximide (Sigma) was added at a concentration of 15 μg/ml. The tissues were cultured individually in 24-well tissue culture plates on top of 1.5% agar blocks such that the biopsies were soaking in 300 μl of gonad culture medium [10% (vol/vol) FCS/2 mM glutamine/0.5 mM pyruvate/0.1 mM β-mercaptoethanol/100 units of penicillin/0.05 mg/ml streptomycin in GIBCO DMEM] (4) supplemented with fungizone (1,5 μg/ml, GIBCO) and, when indicated, with LMB (5 ng/ml, Sigma). Culture medium was exchanged every 24 h for up to 4 days.
Cryosection and Immunofluorescence.
Tissues were washed twice in PBS, fixed in 3.7% (vol/vol) paraformaldehyde/PBS for 18 h at 4°C, washed twice in PBS, and incubated overnight in 30% (wt/vol) sucrose/PBS. Samples were embedded in OCT medium (Tissue-Tek) and frozen at −70°C, and serial 10-μm sections were cut with a cryostat (Leica). After drying, the sections were rehydrated in PBS for 5 min and processed to reveal alkaline phosphatase activity of the primordial germ cells. Tissue sections processing for immunofluorescence and polyclonal human SOX9 rabbit serum (used at a 1/100 dilution) were described (22). Double SOX9 and anti-Müllerian hormone (AMH) detection was performed according to ref. 25. Rabbit anti-AMH Ab (1/400) was a gift from R. Rey (École Normale Supérieur, Montrouge, France; ref. 26).
Results
Nuclear SOX9 Is Exported to the Cytoplasm by a CRM1-Dependent Mechanism.
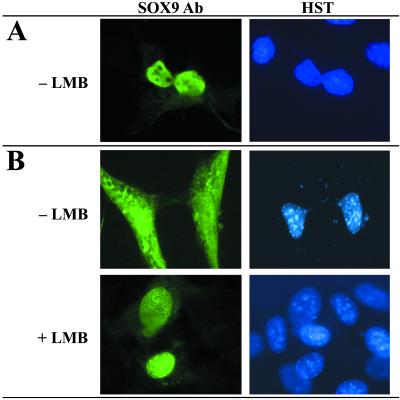
We found that human SOX9 protein contains a putative NES sequence located between the two NLSs in the HMG DNA-binding domain (Fig. 1A). The leucine-rich sequence is found in all SOX proteins and is conserved among mammalian species. Four of the five hydrophobic residues are present among SOX proteins of the seven different SOX groups (Fig. 1B) whereas leucine-142 (L142, * in Fig. 1A) is specific to the SOX9 group (see ref. 27). To study the nuclear-cytoplasmic shuttling of SOX9, we analyzed its intracellular distribution in transfected COS-7 and NIH 3T3 cells. In COS-7 cells, SOX9 protein shows an exclusive nuclear localization (Fig. 2A and ref. 12) whereas both cytoplasmic and nuclear SOX9 were detected in NIH 3T3 cells (Fig. 2B, −LMB). The presence of different cellular factors in COS-7 and NIH 3T3 cells (respectively derived from monkey kidney and from mouse fibroblast cell lines) could explain this differential localization. Transfected NIH 3T3 treated with LMB to specifically block the nuclear export receptor CRM1, showed an exclusive nuclear localization of SOX9 (Fig. 2B, +LMB). Hence, SOX9 is exported from the nucleus by a CRM1-dependent mechanism involving either the SOX9-NES or interaction of SOX9 with a NES-containing protein. The effect of LMB treatment was not affected by cotreatment with cycloheximide (not shown), indicating that relocalization was not due to de novo protein synthesis but solely to protein translocation.
Fig 1.
SOX proteins contain conserved NES sequences. (A) Alignment of SOX9 NES sequence (amino acids 134–147) with conserved leucine-rich functional NES sequences (16). (B) Alignment of the HMG box NES sequences of SOX9 with human SOX proteins representative of each SOX family group (for the classes, see ref. 27). Group A: SRY (SwissProt accession no. Q 05066); group B: SOX2 (P 48431); group C: SOX4 (O 15370); group D: SOX5 (P 35711); group E: SOX9 (P 48436); group F: SOX7 (AAH 04299 and ref. 46), and group G: SOX20 (O 60248). Conserved hydrophobic residues are boxed. SOX9 leucine-142 (L142) is specific to group E SOX proteins and is indicated by * in A.
Fig 2.
SOX9 contains a CRM1-dependent NES. COS-7 (A) and NIH 3T3 (B) cells were transiently transfected with a SOX9 expression vector and treated with (2.5 ng/ml) LMB when indicated (+LMB). Cells were costained with SOX9 Ab (Left) and Hoechst dye (HST, Right). (A) In COS-7 cells, SOX9 was strictly nuclear and was not affected by LMB (not shown). (B) Conversely in NIH 3T3 cells, SOX9 is also cytoplasmic (−LMB). This localization is the result of nuclear export because after inhibition by LMB all SOX9 remains in the nucleus (+LMB).
SOX9 Contains a Functional NES Sequence.
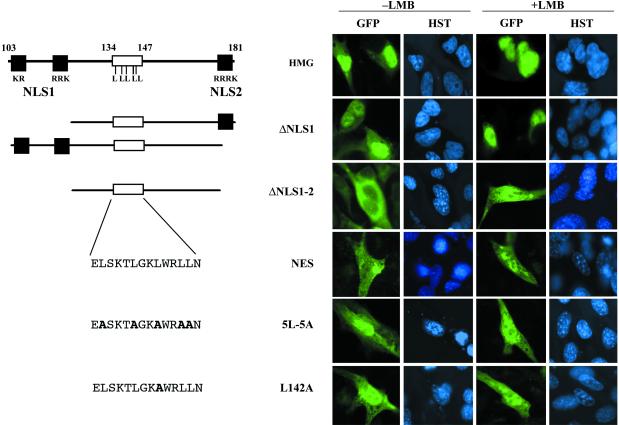
To prove the functionality of the SOX9-NES, the subcellular localization of different SOX9 HMG-GFP fusion proteins was tested in NIH 3T3 cells (Fig. 3). SOX9 HMG-GFP control fusion protein was found to accumulate in the nucleus and the cytoplasm as predicted (Fig. 3, HMG). SOX9 HMG-GFP deleted for either one of the two NLS (Fig. 3, ΔNLS1, not shown for ΔNLS2) resulted in a phenotype close to that of the HMG clone whereas deletion of both NLSs lead to an exclusive cytoplasmic localization (Fig. 3, ΔNLS1–2). Because the GFP reporter alone diffuses to the nucleus (not shown), this result suggests that the ΔNLS1–2-HMG box fragment (amino acids 124–174) is sufficient to export GFP from the nucleus. When nuclear export was blocked by LMB, a decrease in HMG-, ΔNLS1-, and ΔNLS2-GFP cytoplasmic staining was observed whereas ΔNLS1–2-GFP became mainly nuclear (Fig. 3, ΔNLS1–2, +LMB) thus confirming that nuclear export is CRM1 dependent. To narrow down this activity to the putative SOX9 NES itself (amino acids 134–147: ELSKTLGKLWRLLN), we fused GFP to synthetic double-stranded oligo-nucleotides coding for this sequence and for two mutant NESs containing either substitutions by alanine of the five leucines or of the single SoxE group-specific leucine L142 (respectively NES-, 5L-5A-, and L142A clones in Fig. 3). The NES-GFP fusion protein was cytoplasmic but localized mainly to the nucleus in the presence of LMB (Fig. 3, NES +LMB). The 5L-5A- and the L142A-GFP fusion proteins localized mainly to the nucleus with or without LMB (Fig. 3, 5L-5A and L142A). Hence, the SOX9 NES oligo-peptide alone (amino acids 134–147) is sufficient to drive protein nuclear export and is inactivated by the L142A substitution.
Fig 3.
Mapping of the SOX9 NES. Different HMG box constructs and NES oligos were fused to GFP reporter and transfected into NIH 3T3 cells. The GFP and Hoechst dye (HST) were analyzed in control cells (−LMB) or in cells treated with 2.5 ng/ml LMB (+LMB). Schematic drawings of the different reporter constructs and their names are indicated below the complete SOX9-HMG box and to the left of the corresponding transfection experiments. HMG-GFP, ΔNLS1-GFP (lines HMG and ΔNLS1) and ΔNLS2-GFP (not shown) are nuclear and cytoplasmic but are mainly nuclear in the presence of LMB. ΔNLS1–2-GFP is cytoplasmic because of a lack of NLS peptide; when LMB is applied, the fusion protein is able to remain in the nucleus (line ΔNLS1–2). Synthetic double-stranded oligos NES-GFP is predominantly cytoplasmic but accumulates in the nucleus when nuclear export is inhibited (line NES, with LMB). Conversely, the two mutant NES oligos 5L-5A-GFP and L142A-GFP are essentially nuclear regardless of LMB inhibition (lines 5L–5A and L142A). Black boxes are NLS sequences; white boxes are the NES oligo sequence within the HMG box.
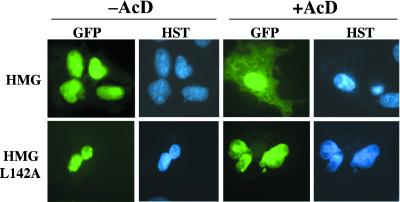
The activities of the wild-type and the L142A mutant NESs within the complete SOX9 HMG box were distinguished by their ability to shift GFP fusion proteins to the cytoplasm despite the presence of NLS sequences. A common method to test for nuclear export of proteins is to block nuclear import with the transcriptional inhibitor AcD (see ref. 28). In transfected COS-7 cells, both fusion proteins were maintained in the nucleus by the NLS sequences (Fig. 4, HMG and HMG L142A, −AcD). Upon AcD treatment, the wild-type SOX9 HMG-GFP protein was partially shifted to the cytoplasm (Fig. 4, HMG, +AcD) whereas the mutant protein remained exclusively nuclear (Fig. 4, HMG L142A, +AcD). These results indicate that SOX9 subcellular localization depends on the presence of active NES and NLS sequences and thus on regulation of nuclear import/export activities.
Fig 4.
Substitution of leucine-142 is sufficient to block nuclear export. COS-7 cells were transiently transfected with wild-type (HMG) and L142 mutant SOX9 HMG (HMG-L142A) expression vectors. After 36 h of expression, cells were treated with the nuclear import inhibitor AcD 3 h before fixing (+AcD) or used as controls (−AcD). Cells were stained with Hoechst dye (HST), and GFP subcellular localization (GFP) was analyzed. When nuclear import is impaired (+AcD), some HMG-GFP fusion protein is able to accumulate in the cytoplasm and becomes visible. In the absence of AcD, the balance is in the favor of nuclear HMG-GFP, whereas inhibition by AcD reveals the nuclear export activity driven by the NES. This export activity is completely abolished by the L142A mutation, and only nuclear L142A-GFP could be detected.
LMB Induces Gonadal Sex Reversal in Cultured XX Mouse Gonads.
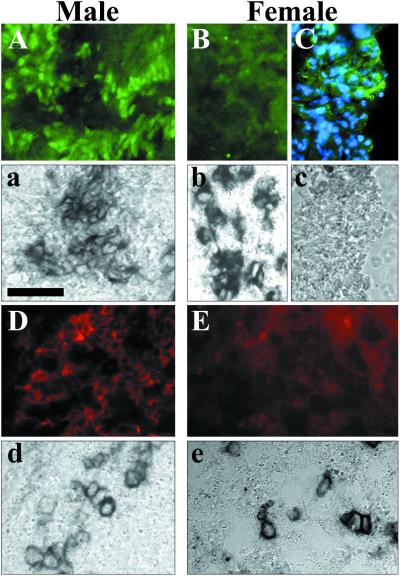
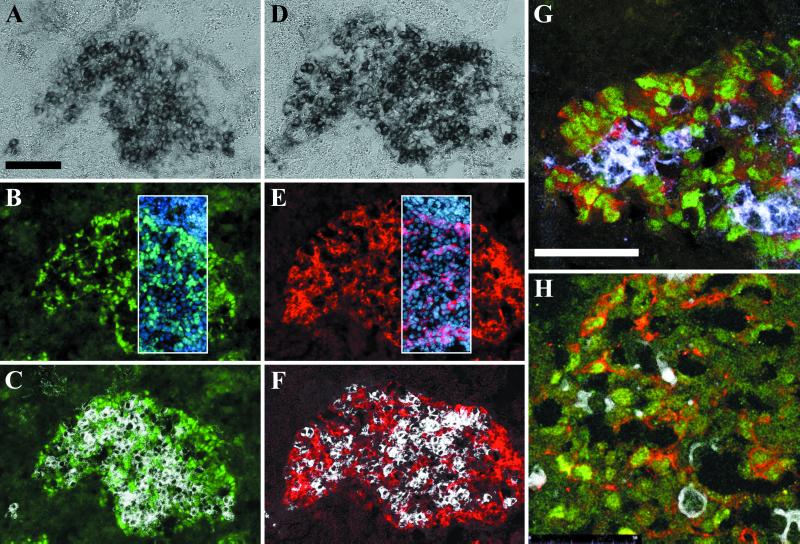
To evaluate the importance of SOX9 nuclear export for sexual differentiation, we analyzed the effect of LMB treatment on mouse gonad explant cultures. Dissected sexually indifferent gonadal ridges and associated mesonephros (herein after referred to as mesogonads) from 10.5 dpc mouse embryos (5–10 TS stage) are able to differentiate into sexually dimorphic organs in vitro. Although mesogonads develop at a lower rate than in vivo, sexual differentiation normally proceeds according to the sex genotype (29, 30). We assessed the differentiation potential of mesogonads cultured for 0–4 days (J0–J4) by comparison to gonads of different developmental stages dissected from 10.5–13.5 dpc embryos. Development was slower and incomplete but still resulted in detectable sexually dimorphic protein expression and tissue organization. At J0, gonads from both sexes only contained low levels of cytoplasmic SOX9 whereas at J1 and J2 SOX9 became nuclear in male gonads (not shown). At J4, male mesogonads had Sertoli cells partially organized into sex cord around germ cells and expressing SOX9 and AMH (Fig. 5 A, a, D, and d). Control female mesogonad cultures never expressed AMH (Fig. 5 E and e). Some female explants expressed SOX9 but only in the cytoplasm (Fig. 5 C and c) and not in the areas where female germ cells were detected (Fig. 5 B and b). When cultures were performed in the presence of LMB, the differentiation of male mesogonads was indistinguishable from control cultures (Fig. 6G). Conversely, in treated female mesogonads, the protein distribution and gene expression mimicked the pattern found in male gonads. This effect was first observed in J1 cultures where SOX9 was first detected in the nucleus (not shown). At J4, female mesogonads contained Sertoli-like cells in the vicinity of alkaline phosphatase-positive germ cells (Fig. 6 A, C, D, and F) expressing nuclear SOX9 (Fig. 6 B and C) and AMH (Fig. 6 E and F). These cells were partially organized in sex-cord-like structures surrounding germ cells (circular structures in Fig. 6 B, C, E, and F). The colocalization of SOX9 and AMH in males and treated females was confirmed by double labeling and confocal microscopy analysis (Fig. 6 G and H). In four separate experiments, 16 of 21 (80%) female mesogonad pairs (from 5–10 TS embryos) treated with LMB displayed the same reversal phenotype. Mesogonads dissected from 10–15 TS embryos yielded fewer reversal (3/13) whereas 15–20 TS embryos did not yield any sex reversal phenotype (0/19). Sex reversal was never observed in untreated female mesogonads (0/17) and male mesogonads cultured in the presence of LMB (n = 31; Fig. 6G) were indistinguishable from male control cultures (n = 29; Fig. 5 A, a, D, and d). The differentiation of female somatic cells into Sertoli-like cells and the reorganization of gonads suggest a sex reversal phenomenon, induced by LMB treatment.
Fig 5.
SOX9 and AMH expression in gonad cultures. Dissected pairs of genital ridge and mesonephros (mesogonads) from 10.5 dpc male and female mouse embryos were cultured in vitro for 4 days and sectioned. The subcellular localization of SOX9 (green in A–C) and AMH (red in D and E) were revealed with specific Abs. Germ cells were concomitantly detected by endogenous alkaline-phosphatase activity on the same sections (dark staining in a, b, d, and e and not detected in c). In male gonads, (A) nuclear SOX9- and (D) cytoplasmic AMH-expressing cells were found in areas positive for germ cell (a and d, respectively). In female gonads, germ cell-positive regions were devoid of any SOX9 (B and b) or AMH (E and e) expression. When cytoplasmic SOX9 could be observed (not colocalized with blue nuclear Hoechst staining in C), it was not in the direct vicinity of germ cells (c). (Bar = 50 μm.)
Fig 6.
LMB induces sex reversal in female gonad cultures. Dissected pairs of mesogonads from 10.5 dpc embryos were cultured in vitro with LMB for 4 days and sectioned. SOX9 (green in B, C, G, and H) and AMH (red in E, F, G, H) were revealed by specific Abs. Germ cells were concomitantly detected by endogenous alkaline-phosphatase activity on the same sections (dark staining in A and D and white in C, F, G, and H). Female gonads treated with LMB displayed a sex-reversal phenotype resembling male-expression pattern: (i) cells expressing nuclear SOX9 (B) surrounded germ cells (black in A and white in C); (ii) in an adjacent section, cytoplasmic AMH was expressed in the same area (E) and surrounded germ cells (D and F). Subcellular localization was confirmed by blue nuclear Hoechst staining (frame in B and E); (iii) nuclear SOX9-positive cells are organized in sex-cord-like circles surrounding germ cells (B and C). Expression of AMH in SOX9-positive cells was recorded on a confocal microscope after double immuno-staining in a male (G) and in a treated female mesogonad culture (H, germ cells marked in white). [Bars = 100 μm (A–F) or 50 μm (G–H).]
Discussion
The dynamic subcellular redistribution of SOX9 protein at the time of sexual differentiation was reported in mouse and human gonads (7, 11). However, the regulation of this process and its relevance to sex determination had not been reported. The two NLSs of the SOX9 HMG box (12), drive the exclusively nuclear localization of SOX9 in COS-7 cells. However, SOX9 is also cytoplasmic in NIH 3T3-transfected cells and together with previous in vivo observations (11), this let us hypothesize that SOX9 could bear a NES allowing its nucleo-cytoplasmic shuttling. The presence of a NES sequence was further suggested by the nuclear accumulation of SOX9 in transfected NIH 3T3 cells incubated in the presence of the export inhibitor, LMB. Indeed, by comparing the human SOX9 primary amino acid sequence to the leucine-rich sequence of conserved NES sequences (16), we found that the central part of the HMG domain contained a potential NES sequence. We narrowed down this sequence to the amino acids 134–147 peptide, containing five hydrophobic residues (only leucine residues in SOX9), a key feature of all NES-containing proteins. The spacing of the two central leucines appears characteristic for NES sequences found in other proteins like MAPKK1 (31), IκBα (32, 33) whereas surrounding sequence is less conserved. The NES motif is well conserved among the SOX9 protein from different species. Four of five hydrophobic amino acids are conserved in proteins from all Sox groups, whereas SOX9 leucine L142 is specific to the SOX9 group E. By using SOX9-GFP fusion proteins, we confirmed that this NES sequence is functional and sensitive to LMB inhibition and that SOX9 NES belongs to the class of leucine-rich CRM1-dependent NESs.
We found that like for STAT1 (34) or cABL (35), a single leucine substitution (SOX9 leucine-142 to alanine) was sufficient to block SOX9 export. The nuclear accumulation of L142A-NES-GFP was only partial because of the lack of both SOX9 NLS sequences. However, the L142A SOX9 HMG-GFP mutant was completely nuclear even in the presence of nuclear import inhibitor AcD. This result indicates that SOX9 is only exported via its own NES and that no other CRM1-independent export mechanism is involved to exclude SOX9 from the nucleus.
When sexually indifferent female gonads were cultured in the presence of LMB, we observed a sex reversal phenotype. Control gonads from both sex and male gonads treated with LMB differentiated according to their sexual genotype. The sex reversal phenotype in female gonads was primarily characterized by the appearance of nuclear SOX9 protein. Second, the same cells expressed AMH (which is a male-specific trait at this stage). Third, these cells usually encircled alkaline-phosphatase-positive germ cells and were often organized in cord-like structures. The association with germ cells indicates that Sertoli cell differentiation is not due to the loss of XX germ cells, another cause for sex reversal phenotype in females (36). Furthermore it points out a population of uncommitted cells that are likely the Sertoli and granulosa common precursor-cell (25). These results show a potential role for NES-driven nuclear export in the regulation of the sex-specific gene expression. Furthermore, they suggest a role played by SOX9-NES in the protein sex-specific subcellular localization and in the subsequent dual choice between sexual differentiation pathways. We cannot conclude whether or not nuclear localization of SOX9 is sufficient to induce a sex reversal phenotype in XX gonads because other factors, trapped in the nucleus by the action of LMB, could also be necessary to achieve this transformation. However, the former hypothesis agrees with the direct regulation of AMH expression by SOX9 in mammals (22, 37) and with evidences pointing to SOX9 as an essential and sufficient switch of Sertoli cells and testis differentiation with or without SRY (8, 38). The lack of sex reversal in older tissues indicates that the responsiveness of genetically female mesogonads to LMB treatment is lost after sexual differentiation onset, when SOX9 is no longer present in female cells. Indeed, in male embryos, SOX9 enters the nucleus of pre-Sertoli cells between the 10 and 15 TS stages (unpublished observation). Thus, the restricted localization of SOX9 and AMH-positive cells and time-window for efficient export inhibition show that LMB treatment itself is not enough to cause SOX9 and AMH expression but rather brings about and mimics what normally occurs in male cells: the shift of SOX9 to the nucleus.
Therefore, we suggest that beside the up-regulation of SOX9 expression in males (7), the subcellular localization of SOX9 protein resulting from a nuclear import/export equilibrium could be the regulatory switch which represses (in females) or triggers (in males) male-specific sexual differentiation. The subcellular localization of SOX9 alone could be responsible for its own up-regulation in males and loss of expression in females previously suggested (39). Thus, sex reversal in XX transgenic mice overexpressing Sox9 (8, 38) could be explained by the saturation of the nuclear export mechanism causing increased levels of SOX9 in the nucleus and resulting in testis differentiation. Our results suggest an active “anti-maleness” role for nuclear export during female gonad differentiation to keep SOX9 out of the nucleus to retain the capacity to enter the feminization pathway. This example adds to the list of transcriptional activities modulated by subcellular localization of NES-containing regulatory factors such as REV (40, 41), Stat1 (34), APC (42, 43), P53 (44), STAT1 (34), and SMAD4 (45). Future studies should focus on determining the mechanisms underlying SOX9 shift to the nucleus, such as up-regulation of SOX9 to override the CRM1-dependent export, association with a specific factor or protein modification to mask the NES peptide. Other important studies should reveal the role played by SRY in this process and whether or not, in vertebrate species lacking SRY, the nuclear localization of SOX9 is the prime activator of testis differentiation. Last but not least, the potent effect of LMB on sex determination illustrates the risk carried by epigenetic environmental factors able to affect protein subcellular localization and hence to trigger abnormal differentiation programs.
Acknowledgments
We thank Prof. J. Demaille for continual support, Dr. R. Rey and Dr. N. Josso for the gift of their AMH Ab, Dr. S. MacKay for her advice on Barr body detection, Prof. A. Schedl and Dr. J. Teixeira for critical reading of the manuscript, B. Moniot for helpful discussions and advice on histology and immunofluorescence techniques, P. Atger for graphical artwork, N. Lautredou (Centre Régional d'Imagerie Cellulaire, Montpellier) for confocal microscopy, and D. Haddou for mice caretaking. This work was funded by the European Economic Community through the Fifth Framework Program and by ARC Grant 5210. J.C. was supported by Marie Curie Fellowship GLG2-CT-1999-00741.
Abbreviations
AMH, anti-Müllerian hormone
GFP, green fluorescent protein
HMG, high mobility group
LMB, leptomycin B
NES, nuclear export signal
NLS, nuclear localization signal
TS, tail somite
dpc, days postcoitum
References
- 1.Sinclair A. H., Berta, P., Palmer, M. S., Hawkins, J. R., Griffiths, B. L., Smith, M. J., Foster, J. W., Frischauf, A. M., Lovell-Badge, R. & Goodfellow, P. N. (1990) Nature (London) 346, 240-244. [DOI] [PubMed] [Google Scholar]
- 2.Gubbay J., Collignon, J., Koopman, P., Capel, B., Economou, A., Munsterberg, A., Vivian, N., Goodfellow, P. & Lovell-Badge, R. (1990) Nature (London) 346, 245-250. [DOI] [PubMed] [Google Scholar]
- 3.Schmahl J., Eicher, E. M., Washburn, L. L. & Capel, B. (2000) Development (Cambridge, U.K.) 127, 65-73. [DOI] [PubMed] [Google Scholar]
- 4.Buehr M., Gu, S. & McLaren, A. (1993) Development (Cambridge, U.K.) 117, 273-281. [DOI] [PubMed] [Google Scholar]
- 5.Martineau J., Nordqvist, K., Tilmann, C., Lovell-Badge, R. & Capel, B. (1997) Curr. Biol. 7, 958-968. [DOI] [PubMed] [Google Scholar]
- 6.Tilmann C. & Capel, B. (1999) Development (Cambridge, U.K.) 126, 2883-2890. [DOI] [PubMed] [Google Scholar]
- 7.Morais da Silva S., Hacker, A., Harley, V., Goodfellow, P., Swain, A. & Lovell-Badge, R. (1996) Nat. Genet. 14, 62-68. [DOI] [PubMed] [Google Scholar]
- 8.Vidal V. P., Chaboissier, M. C., de Rooij, D. G. & Schedl, A. (2001) Nat. Genet. 28, 216-217. [DOI] [PubMed] [Google Scholar]
- 9.Wagner T., Wirth, J., Meyer, J., Zabel, B., Held, M., Zimmer, J., Pasantes, J., Bricarelli, F. D., Keutel, J., Hustert, E., et al. (1994) Cell 79, 1111-1120. [DOI] [PubMed] [Google Scholar]
- 10.Foster J. W., Dominguez-Steglich, M. A., Guioli, S., Kowk, G., Weller, P. A., Stevanovic, M., Weissenbach, J., Mansour, S., Young, I. D., Goodfellow, P. N., et al. (1994) Nature (London) 372, 525-530. [DOI] [PubMed] [Google Scholar]
- 11.de Santa Barbara P., Moniot, B., Poulat, F. & Berta, P. (2000) Dev. Dyn. 217, 293-298. [DOI] [PubMed] [Google Scholar]
- 12.Sudbeck P. & Scherer, G. (1997) J. Biol. Chem. 272, 27848-27852. [DOI] [PubMed] [Google Scholar]
- 13.Wen W., Meinkoth, J. L., Tsien, R. Y. & Taylor, S. S. (1995) Cell 82, 463-473. [DOI] [PubMed] [Google Scholar]
- 14.Bogerd H. P., Fridell, R. A., Benson, R. E., Hua, J. & Cullen, B. R. (1996) Mol. Cell. Biol. 16, 4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda M., Gotoh, I., Gotoh, Y. & Nishida, E. (1996) J. Biol. Chem. 271, 20024-20028. [DOI] [PubMed] [Google Scholar]
- 16.Henderson B. R. & Eleftheriou, A. (2000) Exp. Cell Res. 256, 213-224. [DOI] [PubMed] [Google Scholar]
- 17.Fornerod M., Ohno, M., Yoshida, M. & Mattaj, I. W. (1997) Cell 90, 1051-1060. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda M., Asano, S., Nakamura, T., Adachi, M., Yoshida, M., Yanagida, M. & Nishida, E. (1997) Nature (London) 390, 308-311. [DOI] [PubMed] [Google Scholar]
- 19.Nishi K., Yoshida, M., Fujiwara, D., Nishikawa, M., Horinouchi, S. & Beppu, T. (1994) J. Biol. Chem. 269, 6320-6324. [PubMed] [Google Scholar]
- 20.Kudo N., Wolff, B., Sekimoto, T., Schreiner, E. P., Yoneda, Y., Yanagida, M., Horinouchi, S. & Yoshida, M. (1998) Exp. Cell Res. 242, 540-547. [DOI] [PubMed] [Google Scholar]
- 21.Kudo N., Matsumori, N., Taoka, H., Fujiwara, D., Schreiner, E. P., Wolff, B., Yoshida, M. & Horinouchi, S. (1999) Proc. Natl. Acad. Sci. USA 96, 9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Santa Barbara P., Bonneaud, N., Boizet, B., Desclozeaux, M., Moniot, B., Sudbeck, P., Scherer, G., Poulat, F. & Berta, P. (1998) Mol. Cell. Biol. 18, 6653-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X. & Huang, L. (1991) Biochem. Biophys. Res. Commun. 179, 280-285. [DOI] [PubMed] [Google Scholar]
- 24.Palmer S. J. & Burgoyne, P. S. (1991) Development (Cambridge, U.K.) 113, 709-714. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht K. H. & Eicher, E. M. (2001) Dev. Biol. 240, 92-107. [DOI] [PubMed] [Google Scholar]
- 26.Rey R., Lordereau-Richard, I., Carel, J. C., Barbet, P., Cate, R. L., Roger, M., Chaussain, J. L. & Josso, N. (1993) J. Clin. Endocrinol. Metab. 77, 1220-1226. [DOI] [PubMed] [Google Scholar]
- 27.Bowles J., Schepers, G. & Koopman, P. (2000) Dev. Biol. 227, 239-255. [DOI] [PubMed] [Google Scholar]
- 28.Pinol-Roma S. & Dreyfuss, G. (1992) Nature (London) 355, 730-732. [DOI] [PubMed] [Google Scholar]
- 29.Mackay S. & Smith, R. A. (1989) Cell Differ. Dev. 27, 19-28. [DOI] [PubMed] [Google Scholar]
- 30.McLaren A. & Buehr, M. (1990) Cell Differ. Dev. 31, 185-195. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda M., Gotoh, I., Adachi, M., Gotoh, Y. & Nishida, E. (1997) J. Biol. Chem. 272, 32642-32648. [DOI] [PubMed] [Google Scholar]
- 32.Johnson C., Van Antwerp, D. & Hope, T. J. (1999) EMBO J. 18, 6682-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang T. T., Kudo, N., Yoshida, M. & Miyamoto, S. (2000) Proc. Natl. Acad. Sci. USA 97, 1014-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mowen K. & David, M. (2000) Mol. Cell. Biol. 20, 7273-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taagepera S., McDonald, D., Loeb, J. E., Whitaker, L. L., McElroy, A. K., Wang, J. Y. & Hope, T. J. (1998) Proc. Natl. Acad. Sci. USA 95, 7457-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taketo T., Saeed, J., Manganaro, T., Takahashi, M. & Donahoe, P. K. (1993) Biol. Reprod. 49, 13-23. [DOI] [PubMed] [Google Scholar]
- 37.Arango N. A., Lovell-Badge, R. & Behringer, R. R. (1999) Cell 99, 409-419. [DOI] [PubMed] [Google Scholar]
- 38.Bishop C. E., Whitworth, D. J., Qin, Y., Agoulnik, A. I., Agoulnik, I. U., Harrison, W. R., Behringer, R. R. & Overbeek, P. A. (2000) Nat. Genet. 26, 490-494. [DOI] [PubMed] [Google Scholar]
- 39.Swain A. & Lovell-Badge, R. (1999) Genes Dev. 13, 755-767. [DOI] [PubMed] [Google Scholar]
- 40.Fischer U., Huber, J., Boelens, W. C., Mattaj, I. W. & Luhrmann, R. (1995) Cell 82, 475-483. [DOI] [PubMed] [Google Scholar]
- 41.Fritz C. C. & Green, M. R. (1996) Curr. Biol. 6, 848-854. [DOI] [PubMed] [Google Scholar]
- 42.Neufeld K. L., Nix, D. A., Bogerd, H., Kang, Y., Beckerle, M. C., Cullen, B. R. & White, R. L. (2000) Proc. Natl. Acad. Sci. USA 97, 12085-12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosin-Arbesfeld R., Townsley, F. & Bienz, M. (2000) Nature (London) 406, 1009-1012. [DOI] [PubMed] [Google Scholar]
- 44.Stommel J. M., Marchenko, N. D., Jimenez, G. S., Moll, U. M., Hope, T. J. & Wahl, G. M. (1999) EMBO J. 18, 1660-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe M., Masuyama, N., Fukuda, M. & Nishida, E. (2000) EMBO Rep. 1, 176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takash W., Canizares, J., Bonneaud, N., Poulat, F., Mattei, M. G., Jay, P. & Berta, P. (2001) Nucleic Acids Res. 29, 4274-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]