Abstract
G-protein coupled receptors (GPCRs) are ancient, ubiquitous sensors vital to environmental and physiological signaling throughout organismal life. With the publication of the Drosophila genome, numerous “orphan” GPCRs have become available for functional analysis. Here we characterize two groups of GPCRs predicted as receptors for peptides with a C-terminal amino acid sequence motif consisting of −PRXamide (PRXa). Assuming ligand-receptor coevolution, two alternative hypotheses were constructed and tested. The insect PRXa peptides are evolutionarily related to the vertebrate peptide neuromedin U (NMU), or are related to arginine vasopressin (AVP), both of which have PRXa motifs. Seven Drosophila GPCRs related to receptors for NMU and AVP were cloned and expressed in Xenopus oocytes for functional analysis. Four Drosophila GPCRs in the NMU group (CG11475, CG8795, CG9918, CG8784) are activated by insect PRXa pyrokinins, (−FXPRXamide), Cap2b-like peptides (−FPRXamide), or ecdysis triggering hormones (−PRXamide). Three Drosophila GPCRs in the vasopressin receptor group respond to crustacean cardioactive peptide (CCAP), corazonin, or adipokinetic hormone (AKH), none of which are PRXa peptides. These findings support a theory of coevolution for NMU and Drosophila PRXa peptides and their respective receptors.
Signaling via G protein-coupled receptors (GPCR) is a major route of cellular communication via the plasma membrane. Among the most common ligands associated with GPCR activation are neuropeptides and peptide hormones. A plethora of putative peptide ligands and GPCRs have been revealed with the publication of the Drosophila genome (1–3). Recent analysis predicts at least 41 of these GPCRs to be receptors for neuropeptides and peptide hormones (2, 4). Although numerous signaling peptides in insects are characterized by ability to activate visceral muscle, heartbeat, or gland secretion (reviewed in ref. 5), it is expected that authentic physiological functions for many of these remain to be determined. It now becomes possible to gain greater insight into their physiological functions by matching them to corresponding GPCRs, together with accurate tissue mapping using immunohistochemistry and in situ hybridization. Within the context of Drosophila, this provides an excellent opportunity to investigate functions of the peptide GPCRs and their ligand signaling peptides in a model genetic organism.
We examined GPCRs in phylogenetic groups likely to respond to peptides with the C-terminal −PRXamide motif (PRXa). PRXa peptides in Drosophila and other insects fall into three classes, including the pyrokinins (−FXPRXa; refs. 6 and 7), cardioactive CAP2b-like peptides (−FPRXa; refs. 8 and 9), and ecdysis triggering hormones (−PRXa; refs. 10–14). In the vertebrates, PRXa motifs are found in neuromedin U (NMU), vasopressin (AVP), and pancreatic polypeptide (PP), for which receptors already have been identified (15–17).
Assuming the PRXa C-terminal motif is a conserved evolutionary signature driving ligand-receptor coevolution, we predicted that receptors for peptides with this structural signature might be closely related. The functional cross reactivity of the vertebrate NMU receptor (also referred to as FM3) to SCPb, an invertebrate FPRXa peptide from snails, supports this assumption (15). Two alternative hypotheses are proposed; the insect PRXa peptides are evolutionarily related to NMU, or are related to AVP. As an initial step to test these alternative hypotheses, we conducted functional analysis of the two groups of Drosophila peptide GPCRs, four in the NMU receptor (NMUR) homologous group and three in the AVP receptor homologous group.
Our results demonstrate that Drosophila GPCRs occurring in a monophyletic group with vertebrate NMUR are activated by all three classes of Drosophila PRXa peptides. On the other hand, analysis of AVP-receptor homologous Drosophila GPCRs revealed putative receptors for crustacean cardioactive peptide (CCAP), corazonin, and adipokinetic hormone (AKH), none of which fall into the PRXa peptide group.
Materials and Methods
Prediction of Genes, Drosophila −PRXamide Peptides, and Peptide GPCRs.
Genes encoding Drosophila signaling peptides having PRXa C-terminal motifs were located by using blastp and tblastn searches with parameters for finding short matching sequences (PAM30, e = 5,000). Various insect PRXa peptides previously described (5) were used for query sequences. Mature peptides were predicted by the C-terminal sequence motif PRXG(K/R): G for amidation followed by a mono- or di-basic cleavage site. N termini were predicted after the dibasic cleavage sites (K/R)(K/R) in upstream positions proximal to the PRXG(K/R) motif. A total of three genes encoding seven mature peptides were predicted (Table 1), similar to previous reports (1, 2). We were unable to identify sequences similar to AVP or to the locust AVP-like insect diuretic hormone (18) in database searches with similar search parameters as above.
Table 1.
Sequences for −PRXamide signaling peptides in various organisms
| Peptide | Sequence |
|---|---|
| Mammalian −PRXamide | |
| Neuromedin-U25 | FRVDEEFQSPFASQSRGYFLFRPRNa |
| AVP | CYFQNCPRGa |
| Other invertebrate −PRXamide | |
| HezDH | NDVKDGAASGAHSDRLGLWFGPRLa |
| HezPBAN | LSDDMPATPADQEMYRQDPEQIDSRTKYFSPRLa |
| MasCap2b | pQLYAFPRVa |
| Aplysia SCPb | MNYLAFPRMa |
| MasETH | SNEAISPFDQGMMGYVIKTNKNIPRMa |
| MasPETH | SFIKPNNVPRVa |
| Drosophila −PRXamide | |
| hugγ | pQLQSNGEPAYRVRTPRLa |
| Drm-PK-2 | SVPFKPRLa |
| CAP2b-1 | GANMGLYAFPRVa |
| CAP2b-2 | ASGLVAFPRVa |
| CAP2b-3 | TGPSASSGLWFGPRLa |
| ETH1 | DDSSPGFFLKITKNVPRLa |
| ETH2 | GENFAIKNLKTIPRIa |
| Other peptides used in this study | |
| CCAP | PFCNAFTGCa |
| Corazonin | pQTFQYSRGWTNa |
| DrmAKH | pQLTFSPDWa |
| TaaAKH | pQLTFTPGWa |
| FMRFamide | FMRFa |
| Myomodulin | PMSMLRLa |
| Proctolin | RYLPT |
Lowercase “a” indicates C-terminal amidation, “pQ” at the N terminus indicates pyroglutamate. PRX motif sequences are underlined for FXPRX, FPRX, and PRX. Drosophila hugγ and Drm-PK-2 are encoded by gene hugin.
Two groups of Drosophila GPCRs were examined, one group related to the NMUR and the other to the AVP receptor. For prediction of Drosophila peptide GPCRs grouped with NMURs (15, 16), a distance tree was generated by pileup in GCG (Genetics Computer Group, Madison, WI). Two groups of genes were used to determine Drosophila peptide GPCR genes in the monophyletic group with NMUR; one of these consisted of closely related group of GPCRs, including NMUR 1 (FM3, NMUR1, AB038649), NMUR 2 (NMUR2, NP_071611), neurotensin receptor (NTR, JH0164), GH secretagogue receptor (GHSR, NP_114464), and TSH-releasing hormone receptor (TRHR, NP_037179). The second group consisted of highly diverged vertebrate peptide GPCRs, including bombesin receptor (NP_001718), somatostatin receptor (XP_009594), and tachykinin receptor (XP_005747). This analysis yielded four predicted Drosophila peptide GPCRs as CG8784, CG8795, CG9918, and CG14575.
A similar analysis was done for AVP receptor homologous GPCRs. The sequences used for this group of GPCRs were conopressin receptor 1 (CNPR1, AAA91998), conopressin receptor 2 (CNPR2, AAC46987), vasopressin V1a receptor (AVPR1a, P37288), vasopressin V1b receptor (AVPR1b, P47901), vasopressin V2 receptor (AVPR2, P30518), and gonadotropin-releasing hormone receptor (GNRHR, P30968). This analysis yielded three predicted Drosophila peptide GPCRs: CG6111, CG10698, and CG11325 (also named as gonadotropin releasing hormone receptor, refs. 19 and 20). For phylogenetic analysis, neighbor-joining distance trees were generated in paup (V4.0b8a) with bootstrapping analysis (Fig. 3).
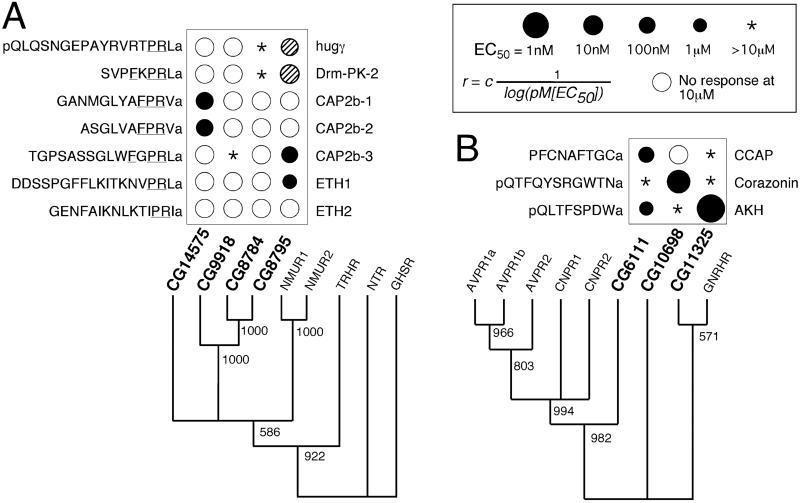
Fig 3.
Summary of the ligand-receptor specificity for Drosophila −PRXamide peptides and trees of the GPCRs for NMUR group (A) and Drosophila CCAP, corazonin, and AKH for AVPR group (B). Phylogenetic tree is generated in paup (V4.0b8a) for neighbor-joining distance method with numbers at nodes for bootstrapping value in 1,000 replications. Empty circles indicate no response at 10 μM concentration of peptide ligand. Asterisks represent small responses at 10 μM of ligand. Filled circles indicate EC50 by their size where the radius is inverse of log (pM EC50) multiplied by an arbitrary unit c. Hatched circles indicate approximated potency of the hugγ and Drm-PK-2 obtained by comparative measurements to that of ETH1 (Fig. 1 and text).
GPCR Cloning.
For each Drosophila GPCR, prediction of gene structure was made in FGENESH (www.softberry.com; ref. 21) by using about 20 kb of genomic sequence surrounding highly conserved regions, particularly for 5 prime and 3 prime ends of ORFs. Reverse transcriptase (RT)-PCR were performed by using primers designed for amplification of the largest ORF or primers designed on the conserved region encoding transmembrane domains. Complementary DNA was synthesized by using Superscript (Invitrogen) with mRNA isolated by Dynabeads (Dynal, Great Neck, NY) from whole flies (≈50 individuals of Canton S) in both larval and adult stages by priming at poly(A) sites. PCR conducted in 20-μl volumes contained 50 mM KCl, 2 mM MgCl2, 0.2 mM dNTP, 0.5 μM of each primer, 0.5 units Taq polymerase (Invitrogen), 0.5 units pfu polymerase (Stratagen), and 20 mM Tris-HCl, pH 8.4. The reaction mixture was denatured initially for 5 min at 94°C, then subjected to 35 cycles of 94°C for 1 min, 53°C for 1 min, and 72°C for 1 min 30 sec. Sequences reported in GenBank represent a consensus of two or more independent RT-PCR products, particularly for polymorphic sites compared with the Celera Drosophila genome sequences (22).
GPCR Expression in Xenopus Oocyte and Assay.
Composite clones in the Xenopus oocyte expression vector pGH19 were cut with NotI or XhoI restriction enzymes for runoff in vitro transcription using MEGAscript (Ambion, Austin, TX). Stage V and VI Xenopus laevis oocytes were prepared and injected by using standard protocols (23). Oocytes were injected with 30 ng of GPCR cRNA in 30 nl volumes of diethyl pyrocarbonate-treated water and incubated in ND-96 (96 mM NaCl/2 mM KCl/1 mM MgCl2/1 mM CaCl2/5 mM Hepes) for 1–5 days at 18°C before recordings.
Ligand-receptor interactions were detected electrophysiologically by using two-electrode voltage clamp (TEV-200, Dagan Instruments, Minneapolis). Inward currents recorded in response to application of ligand were acquired by using PCLAMP 6.0 (Axon Instruments, Foster City, CA) and analyzed with the ORIGIN 6.0 program (MicroCal Software, Northampton, MA). For perfusion application of ligands, a Perkin–Elmer Model 410 liquid chromatography pump and associated Rheodyne injection valve were used, supplying a continuous flow of ND-96 buffer at a flow rate of 0.9 or 1.2 ml/min. Various concentrations of ligands were applied in standard 100-μl volumes of ND-96 buffer through the injection valve, providing a 6.7- or 5-sec exposure of ligand solution to the oocyte. All negative responses to candidate ligands applied at 10 μM were confirmed by subsequent application of appropriate positive ligands. As positive controls, we used Drm-PK-2 for CG8784, ecdysis triggering hormone 1 (ETH1) for CG8795, CAP2b-3 for CG9918, CAP2b-1 for CG14575, CCAP for CG6111, corazonin for CG10698, and AKH for CG11325.
Concentration–response relationships were obtained from a single oocyte by perfusion of increasing concentrations of peptide ligands. Progressively higher concentrations of ligand were applied until the peak current response reached a plateau or desensitization occurred. Peak current measured after each treatment was normalized to the maximum peak current of the oocyte for the “% peak current.” All recordings were made at room temperature (22–25°C).
Receptor desensitization was defined as a reduction in current resulting from the second application of a given ligand applied at the same concentration. To minimize the effect of desensitization in data collections, oocytes were washed with a continuous flow (0.9 or 1.2 ml/min) of ND-96 buffer for longer than 2 min, which was the time for restoring the sensitivity determined in preliminary experiments. Desensitization of CG8784 and CG8795 was particularly severe after application of hugγ or Drm-PK-2, with little or no recovery evident after 1 hr of washing. For this reason, each data point obtained for these two peptides was obtained from a single egg treated with one concentration of ligand (Fig. 1C).
Fig 1.
Concentration–response relationships of Drosophila −PRXa signaling peptides on the Drosophila GPCRs in the NMUR group expressed in Xenopus oocytes. Error bars represent SEM. (A) CG14575 responds to CAP2b-1 and CAP2b-2. CG8795 responds to DrmETH1 and CAP2b-3 (B), and to Drm-PK-2 and hugγ (C) with rapid desensitization. Error bars represent SD. (D) Typical currents generated by progressive higher concentrations of ligand (CAP2b-1 in this case). Solid bar on the top indicates time for the ligand application.
Synthetic Peptides.
The peptides ETH1, ETH2, hugγ, and AKH were synthesized by Research Genetics (Huntsville, AL). Drm-PK-2, CAP2b-1, CAP2b-2, and CAP2b-3 were synthesized on a Milligen/Biosearch Model 9050 peptide synthesizer in the Department of Chemistry, Univ. of California, Riverside, and purified by HPLC. Manduca ETH (13, 14), Manduca PETH (14), and Bombyx ETH acid (10) were described previously. Other peptides used in this study, including proctolin, myomodulin, CCAP, HezPBAN, AVP, Tabanus atratus AKH, and corazonin, were purchased from Peninsula Laboratories.
Results
Bioinformatics: Identification of Drosophila −PRXamide Peptides and Putative GPCRs.
The PRXa C-terminal motif is found in a number of invertebrate and vertebrate peptides (Table 1). In the invertebrates, these include the PBAN-like FXPRXa motif characteristic of the pyrokinin group, FPRXa exemplified by small cardioactive peptide and CAP2b, and PRXa of ETH. Vertebrate PRXa peptides consist of pancreatic polypeptide (36 aa with C-terminal −NMLTRPRYa), AVP (−NXPRXa), and NMU-25 or -8 (25 or 8 aa with C-terminal −FXPRXa).
We searched the Drosophila genome database (www.fruitfly.org/blast/) for all genes encoding peptides with C-terminal amino acid −PRXa motifs and for G protein-coupled receptors likely to be activated by these ligands. The search for peptides yielded three genes: hugin (CG6371, GenBank accession no. AJ133105), cap2b-like (CG15520, capability, GenBank accession no. AF203878), and eth (CG18105; GenBank accession no. AF170922). The gene hugin encodes two peptides, referred to here as hugγ and Drm-PK-2 (ref. 24, Table 1), whose C-terminal motifs are related to the insect pyrokinins (5). The cap2b-like gene encodes three putative peptides related to cardioacceleratory peptides (CAPs; ref. 25), referred to here as CAP2b-1, -2, and -3. CAP2b-1 and CAP2b-2 contain a common C-terminal motif (FPRXa), whereas the C terminus of CAP2b-3 (−GLWFGPRLa) is identical to that of the diapause hormone of Lepidoptera (26–28). The peptides ETH1 and ETH2 encoded by the gene eth were shown to possess a C-terminal PRXa motif (11, 12).
Analysis of the three vertebrate PRXa peptides, NMU, AVP, and PP showed that the PRXa motifs are strictly conserved in NMU and AVP, whereas that of PP is likely a consequence of converging evolution from NPY/PYY/PP family, which includes Drosophila neuropeptide F [C-terminal motif (PH)R(YF)amide] (29, 30). In this fashion, our search for PRXa-activated GPCRs in Drosophila was narrowed to those related to the AVP and NMURs (15–17).
Phylogenetic analysis revealed that NMURs occur in a monophyletic clade with four Drosophila GPCRs: CG8784, CG8795, CG9918, and CG14575 (Fig. 3). Three Drosophila GPCRs homologous to AVP receptors are CG6111, CG11325 (also known as gonadotropin releasing hormone receptor; refs. 19 and 20), and CG10698. CG6111 is orthologous to the vasopressin/oxytocin receptor gene family (Fig. 3).
Cloning and Functional Expression of Putative PRXa Peptide GPCRs in Xenopus Oocytes.
Putative Drosophila GPCRs in the database were amplified by RT-PCR using primers based on gene predictions in the FGENESH gene finder (www.softberry.com; ref. 21). Conceptual translations of these genes aligned with other GPCRs present complete seven transmembrane domains (Figs. 4 and 5, which are published as supporting information on the PNAS web site, www.pnas.org). Sequences confirmed by at least two independent RT-PCR experiments revealed several polymorphic sites compared with the Celera Drosophila genomic sequences (Table 2 and Figs. 4 and 5).
Table 2.
Drosophila genes encoding GPCRs studied
| CG number (GenBank accession no.) | Predicted MW (no. of aa) | START..END for expression clone | Conflicts in sequence (substitutions in amino acid sequence) |
|---|---|---|---|
| Neuromedin U receptor group | |||
| CG8784 (AF522189) | 72.2 kDa (658 aa) | −47..17 | C328T (L110F), G357T, G861C, G1233A, CGGTGG1508del (GG503del), G1544T (G515V), A1593G, C1614A, T1728C, A1786C, A1807G (T603A) |
| CG8795 (AF522190) | 65.2 kDa (595 aa) | −18..17 | T66C, T192C, C312G, G417A, A456G, G711A, G804A, T1119C, A1134G, T1515C, C1577A (T526K), G1583T (G528V) |
| CG9918 (AF522191) | 47.3 kDa (430 aa) | −4..84 | A39T, G75A, C87G, A105G, G108T, T1089C, T1119C, G1140A |
| CG14575 (AF522193) | 54 kDa (477 aa) | −15..12 | G912A |
| Vasopressin receptor group | |||
| CG11325 (AF522194) | 51.4 kDa (455 aa) | −24..20 | C753T, A789G, C849T, C930A, G1005C, G1014A, C1035G, G1119T, A1125G, T1134A, C1281T, delGTAATTTCG, C1338A (D446E) |
| CG10698 (AF522192) | 64 kDa (579 aa) | −58..32 | T30C, G54A, T205C, T798C, C975T, A1020G, G1344A (M448I), C1654G (L552V) |
| CG6111 (AF522188) | 54.5 kDa (494 aa) | −19..122 | C134T, A156C, C906A, A1134C, A1314G |
Oocytes injected with cRNAs for the GPCRs generated inward currents up to ≈2.5 μA upon activation with appropriate ligands (Fig. 2D), whereas saline-injected controls showed no response (data not shown). We presume that ligand-activated inward currents in these experiments result from Gq activation of phospholipase C, liberation of inositol trisphosphate, and activation of chloride current by mobilization of intracellular calcium stores (31).
Fig 2.
Concentration-response relationships of Drosophila signaling peptides on the Drosophila GPCRs in the AVP receptor group expressed in Xenopus oocytes. Error bars represent SEM. (A) CG6111 responds to CCAP and AKH. (B) CG10698 responds to corazonin. (C) CG11325 responds to AKH.
Ligand-Receptor Specificity.
Drosophila GPCRs in the NMUR clade were activated by PRXa peptides with various levels of sensitivity and specificity (Figs. 1 and 3A). CG14575 was the most selective within this group, responding only to CAP2b-1 (EC50 150 nM) and CAP2b-2 (EC50 230 nM), which have an identical C-terminal −VFPRVamide motif. All other peptides were inactive on application at 10 μM. In contrast, CG8795 responded to a relatively wide range of ligands, including Drm-PK-2, hugγ, CAP2b-3, and ETH1, listed in order of decreasing potency. Drm-PK-2 and hugγ appeared to have highest potency (Figs. 1C and 3A), but also induced the most severe receptor desensitization. The high level of desensitization complicated efforts to produce quantitative determinations of potency for these ligands. On the other hand, ETH1 and CAP2b-3 treatment produced little or no desensitization.
CG9918 and CG8784 were insensitive to most ligands applied (Fig. 3A). CG9918 responded only to the highest concentration of CAP2b-3 applied (10 μM), and was otherwise insensitive to all other ligands applied at this concentration. Similarly, CG8784 was activated only by Drm-PK-2 or hugγ applied at 10 μM.
Drosophila GPCRs in the AVP receptor group responded to a completely different group of peptide ligands (Figs. 2 and 3B). The highest selectivity was exhibited by CG10698, which responded to picomolar concentrations of corazonin (EC50 1 nM). CG11325 was highly selective for AKH (EC50 0.3 nM). CG6111 responded to CCAP (EC50 130 nM), and AKH (EC50 240 nM). At the highest concentrations of ligands applied (10 μM), some cross-selectivity within the group was observed (Fig. 3B).
Discussion
Our analysis of Drosophila GPCRs was guided by an assumption of ligand-receptor coevolution, which led to construction of two alternative hypotheses. Insect PRXa peptides are evolutionarily related to vertebrate peptide NMU, or instead are related to AVP, both of which have PRXa C-terminal motifs. Deduction of evolutionary relationships between signaling peptides is a nontrivial task, because only a limited number of amino acids may be functionally conserved amid relatively fast evolution of overall amino acid sequences. Driving forces for the evolution of signaling peptides likely are maintenance of receptor binding affinity for specific activation, efficient processing during synthesis, and degradation. For maintenance of optimal receptor binding, receptor–ligand coevolution may be a common phenomenon (32). Clearly, demonstration of functional ligand–receptor interactions as described here in addition to the peptide sequence motifs must be considered in defining such evolutionary relationships.
A total of seven Drosophila GPCRs related to the vertebrate receptors for the PRXa peptides NMU and AVP were targeted for analysis. All four Drosophila GPCRs occurring in a monophyletic clade with neuromedin receptors responded only to Drosophila PRXa peptides, whereas GPCRs related to the AVP receptor were insensitive to all PRXa peptides tested, responding instead to corazonin, AKH, and CCAP. These findings suggest that the PRXa C-terminal motif is likely an evolutionarily conserved signature in vertebrate NMU and insect PRXa peptides (pyrokinin, CAP2b, and ETH). On the other hand, the PRXa motifs in AVP and in PP appear to be generated by converging evolutions.
Drosophila GPCRs in the NMUR Group Are Activated by PRXa Peptides.
Drosophila GPCRs in the NMUR group responded to the PRXa peptides, hugγ, Drm-PK-2, CAP2b-1 to -3, and ETH. Non-PRXa peptides such as proctolin, FMRFamide, and diuretic hormone produced no response at 10 μM, the highest concentration tested (see Table 3, which is published as supporting information on the PNAS web site). The range of ligand concentrations sufficient to activate each GPCR ranged from low nanomolar to micromolar. CG14575 was the most ligand-selective receptor in this group, responding only to low nanomolar concentrations of CAP2b-like peptides CAP2b1 and CAP2b-2 having FPRXa motifs, whereas CAP2b-3, a mature peptide from the same gene having FXPRXa motif had no effect on CG14575 at 10 μM.
It seems likely that CG14575 is involved in ion transport functions associated with diuresis in Drosophila. It has been shown that Drosophila CAP2b-1 and -2 act on principal cells of malphighian tubules, stimulating fluid secretion through the calcium–nitric oxide–cGMP pathway (33–36). It will be interesting to determine whether CG14575, the putative CAP2b-1/CAP2b-2 receptor from this study, is expressed in Malpighian tubules.
CG8795 responded to a different set of nonoverlapping PRXa peptides, being most sensitive to hugγ and Drm-PK-2. These peptides produced activation at low nanomolar concentrations accompanied by marked receptor desensitization, making it difficult to ascertain a reliable EC50 value for these peptides. CG8795 also showed moderate sensitivity to ETH1 and CAP2b-3, responding to mid- to high nanomolar concentrations. Interestingly, ETH2 had no effect at 10 μM. The responses of CG8795 to a wide range of peptides were unexpected. Although Drm-PK-2 was most active, hugγ, ETH1, and CAP2b-3 also produced robust responses. The ligands active on this receptor also include Manduca sexta MasETH (13, 14) and Heliothis virescens HezPBAN (6) at 10 μM concentration (data not shown). However, some obvious selectivity was apparent, with no responses registered to CAP2b-1 and -2, ETH2, and Manduca PETH applied at 10 μM.
Activation of CG8795 by both hugγ and ETH1 raises the possibility of its involvement in ecdysis. Such a possibility is indicated not only by its sensitivity to ETH1 that is known to be obligatory for ecdysis signaling (12). We recently learned that ecdysis deficiency is induced by ectopic expression of the hugin gene (24). Furthermore, the hugin gene product hugγ mimics ETH1 by inducing ecdysis behavior in wild-type flies and by rescuing ecdysis deficiency in buttoned-up eth null mutants (ref. 12, and Y.P. and M.E.A., unpublished data). Given that hugγ and ETH1 activate both CG8795 and ecdysis behavior, several interpretations are possible. CG8795 may be involved in ecdysis signal transduction, and both hugγ and ETH1 are ecdysis signaling molecules. Alternatively, CG8795 is not involved in ecdysis, but can be activated by relatively high concentrations of ETH1 acting as a hugγ agonist. According to this alternative scenario, CG8795 could be involved in other physiological functions such as pheromone biosynthesis as a hugγ and/or Drm-PK-2 receptor. Further work is needed to clarify authentic role of CG8795 and function of hugγ in the ecdysis signaling pathway.
The remaining GPCRs in the NMU group, CG8784 and CG9918, responded only to high levels (≥10 μM) of hugγ and Drm-PK-2, and CAP2b-3, respectively. It is possible that the endogenous signal transduction machinery in the Xenopus oocyte is inappropriate for mediation of functional receptor activation for CG8784 and CG9918. This assay system generates a presumed calcium-activated chloride current known to be activated exclusively by Gq coupled pathways (31). GPCRs can be coupled to a variety of G proteins, including Gi/o, Gs, and Gq, with various degrees of efficiency and specificity (37–40). Poor coupling of heterologously expressed GPCRs to Gq in the Xenopus oocyte clearly could result in artifactually low affinity estimates (37, 38). In particular, CG9918 and CG8784 were found to be largely insensitive to all ligands tested. Incorporation of alternative G proteins into the functional expression system, as well as expansion of studies to other related GPCRs for PRXa peptides are in progress (e.g., CG5911).
The functions of PRXa peptides known thus far in the vertebrates include activation of ion transport and contractile activity in intestine and arterial musculature via the NMUR (40, 41, 42). In invertebrates, functions for many of the PRXamide peptides remain uncertain, biological activity having been inferred from standard assays for visceral muscle contraction. For example, early demonstrations of activity for the pyrokinins (FXPRXa) were based on stimulation of gut, oviduct, and heart (5, 24), whereas more recent data implicating them in pheromone biosynthesis (6) and cuticle melanization (7) are more suggestive of authentic physiological functions. The FPRXa peptides, including small cardioactive peptides and cardioacceleratory peptide (CAP2b), were isolated based on their activity in heartbeat modulation (8, 9) but may be involved in water and ion transport (33, 34, 36). Finally, although all other PRXa peptides are produced in the central nervous system (CNS) (44, 45), ETH (PRXa) is produced peripherally in epitracheal Inka cells (13) and acts on CNS to trigger central pattern generators leading to ecdysis behavior (14). Knowledge of the expression patterns of the receptor GPCRs will likely provide new insights into the true physiological functions for the PRXa peptides.
Drosophila GPCRs in AVP Receptor Homologous Group: CCAP, Corazonin, and AKH Receptors.
Examination of the three Drosophila GPCRs homologous to the AVP receptor yielded serendipitous findings. CG6111, orthologous to the vasopressin receptor (Fig. 3B), is activated by CCAP and AKH (Fig. 2A). CG10698 and CG11325 are activated by corazonin and AKH, respectively (Fig. 2 B and C). The EC50 values for receptors in the AVP group are consistently lower than those observed in the NMU PRXa group.
It is surprising that CG6111, an orthologous gene of AVP receptor, is activated by CCAP and AKH, but not by AVP (data not shown). The presence of an insect vasopressin-like peptide was reported in locust (18), but searches of the Drosophila genome sequence to locate a candidate AVP-like peptide sequence were unsuccessful (refs. 1 and 2, and this study). CCAP and AVP both are C-terminally amidated, disulfide bridged peptides, but share no significant sequence similarity (Table 2). Our current data set favors assignment of CG6111 as an authentic CCAP receptor because of ligand cross-reactivity within this group of GPCRs (Fig. 3B and Table 3). It seems reasonable to have residual functional cross-activity within recent evolutionary diverged GPCRs. Further work is needed to verify whether CG6111 is an authentic CCAP receptor or is a receptor for unidentified Drosophila AVP-like peptide cross-reacting to the CCAP.
CG10698 is activated by corazonin with an EC50 of 1 nM. Similarly, CG11325, previously cloned by its homology to GNRHR (19), is activated by AKH with an EC50 of 0.3 nM (ref. 20 and in this study, Fig. 2 B and C). These evolutionarily related GPCRs, activated by structurally similar signaling peptides (46), reveals a clear case of receptor–ligand coevolution.
CCAP, corazonin, and AKH have overlapping biological functions, and thus it is not unexpected that their receptors would fall into an evolutionarily related group. CCAP was initially identified by its cardioacceleratory action on the heart of the shore crab (47) and in the tobacco hawkmoth, Manduca (8). The primary structure of this peptide appears to be strictly conserved across the arthropods (47, 48). Additional functions of CCAP include myotropic actions (25, 49), induction of AKH release in corpora cardiaca of locust (50), and induction of ecdysis behaviors (51, 52). Corazonin is known for its cardioactive function in cockroach (46) and pigment modulation in locust (53, 54). AKH and related peptides, grouped with red pigment concentrating hormone of crustacea are cardioacceleratory and have metabolic functions such as lipid and carbohydrate mobilization (reviewed in ref. 5).
Conclusions
The present findings favor a hypothesis that the PRXa motif is an evolutionarily conserved signature in both vertebrate NMU and insect PRXa peptides. Examination of potential cross-activity of NMU and insect PRXa peptides for their receptors may provide further support for the theory of ligand–receptor coevolution in the PRXa peptide–receptor group. Another clear case of ligand-receptor coevolution was shown for recently diverging corazonin and AKH (46), and their receptors (this study).
The ligand-activated GPCR responses described in this report provide an important first step in defining authentic physiological roles for these signaling peptides. Our findings help to promote a subset of GPCRs from “orphan” to “putative” receptors, and provide a direction for further characterization of receptors for PRXa peptides, CCAP, corazonin, and AKH. Expanded studies in Drosophila and in other insects will help to validate these initial findings.
The multiple ligand sensitivity exhibited by certain GPCRs such as CG8795 and CG6111 raises the obvious question of physiological significance. One possible interpretation is that, in contrast to a one ligand-one receptor model, certain receptors may be involved in the transduction of a multiple peptide signals, thus providing a pleiotropic model of functional regulation. This possibility deserves careful examination in more physiologically relevant bioassays, as well as in vivo.
Supplementary Material
Acknowledgments
We thank Sarjeet Gill and Timothy Kingan (Univ. of California, Riverside) and Dusan Zitnan (Institute of Zoology, Slovak Academy of Sciences, Bratislava) for helpful discussions. We are indebted to Christophe Roos (Univ. of Helsinki, Finland) for providing valuable information about the hugin gene and ectopic hugin mutant flies before the publication. This work was supported by National Institutes of Health Grant AI 40555 and National Science Foundation Grant IBN-9514678.
Abbreviations
GPCR, G protein-coupled receptor
CCAP, crustacean cardioactive peptide
AKH, adipokinetic hormone
AVP, arginine vasopressin
NMU, neuromedin U
NMUR, NMU receptor
ETH, ecdysis-triggering hormone
PP, pancreatic polypeptide
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vanden Broeck J. (2001) Peptides (Tarrytown, NY) 22, 241-254. [DOI] [PubMed] [Google Scholar]
- 2.Hewes R. S. & Taghert, P. H. (2001) Genome Res. 11, 1126-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathoo A. N., Moeller, R. A., Westlund, B. A. & Hart, A. C. (2001) Proc. Natl. Acad. Sci. USA 98, 14000-14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brody T. & Cravchik, A. (2000) J. Cell Biol. 150, F83-F88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gäde G., Hoffmann, K. H. & Spring, J. H. (1997) Physiol. Rev. 77, 963-1032. [DOI] [PubMed] [Google Scholar]
- 6.Raina A. K., Jaffe, H., Kempe, T. G., Keim, P., Blacher, R. W., Fales, H. M., Riley, C. T., Klun, J. A., Ridgway, R. L. & Hayes, D. K. (1989) Science 244, 796-798. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto S., Kitamura, A., Nagasawa, H., Kataoka, H., Orikasa, C., Mitsui, T. & Suzuki, A. (1990) J. Insect Physiol. 36, 427-432. [Google Scholar]
- 8.Huesmann G. R., Cheung, C. C., Loi, P. K., Lee, T. D., Swiderek, K. M. & Tublitz, N. J. (1995) FEBS Lett. 371, 311-314. [DOI] [PubMed] [Google Scholar]
- 9.Morris H. R., Panico, M., Karplus, A., Lloyd, P. E. & Riniker, B. (1982) Nature (London) 300, 643-645. [DOI] [PubMed] [Google Scholar]
- 10.Adams M. E. & Zitnan, D. (1997) Biochem. Biophys. Res. 230, 188-191. [DOI] [PubMed] [Google Scholar]
- 11.Park Y., Zitnan, D., Gill, S. S. & Adams, M. E. (1999) FEBS Lett. 463, 133-138. [DOI] [PubMed] [Google Scholar]
- 12.Park Y., Filippov, V., Gill, S. S. & Adams, M. E. (2002) Development (Cambridge, U.K.) 129, 493-503. [DOI] [PubMed] [Google Scholar]
- 13.Zitnan D., Kingan, T. G., Hermesman, J. & Adams, M. E. (1996) Science 271, 88-91. [DOI] [PubMed] [Google Scholar]
- 14.Zitnan D., Ross, L. S., Zitnanova, I., Hermesman, J. L., Gill, S. S. & Adams, M. E. (1999) Neuron 23, 1-20. [DOI] [PubMed] [Google Scholar]
- 15.Fujii R., Hosoya, M., Fukusumi, S., Kawamata, Y., Habata, Y., Hinuma, S., Onda, H., Nishimura, O. & Fujino, M. (2000) J. Biol. Chem. 275, 21068-21074. [DOI] [PubMed] [Google Scholar]
- 16.Howard A. D., Wang, R., Pong, S.-S., Mellin, T. N., Strack, A., Guan, X.-M., Zeng, Z., Williams, D. L., Feighner, S. D., Nunes, C. N., et al. (2000) Nature (London) 406, 70-74. [DOI] [PubMed] [Google Scholar]
- 17.Thibonnier M., Auzan, C., Madhun, Z., Wilkins, P., Berti-Mattera, L. & Clauser, E. (1994) J. Biol. Chem. 269, 3304-3310. [PubMed] [Google Scholar]
- 18.Proux J. P., Miller, C. A., Li, J. P., Carney, R. L., Girardie, A., Delaage, M. & Schooley, D. A. (1987) Biochem. Biophys. Res. 149, 180-186. [DOI] [PubMed] [Google Scholar]
- 19.Hauser F., Sondergaard, L. & Grimmelikhuijzen, C. J. P. (1998) Biochem. Biophys. Res. 249, 822-828. [DOI] [PubMed] [Google Scholar]
- 20.Staubli F., Jorgensen, T. J. D., Cazzamali, G., Williamson, M., Lenz, C., Sondergaard, L., Roepstorff, P. & Grimmelikhuijzen, C. J. P. (2002) Proc. Natl. Acad. Sci. USA 99, 3446-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salamov A. A. & Solovyev, V. V. (2000) Genome Res. 10, 516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F., et al. (2000) Science 287, 2185-2195. [DOI] [PubMed] [Google Scholar]
- 23.Goldin A. L. & Sumikawa, K. (1992) Methods Enzymol. 207, 279-297. [DOI] [PubMed] [Google Scholar]
- 24.Meng, X., Wahlstrom, G., Immonen, T., Kolmer, M., Tirronen, M., Predel, R., Kalkkinen, N., Sariola, H., Heino, T. I. & Roos, C. (2002) Mech. Dev., in press. [DOI] [PubMed]
- 25.Tublitz N. J. & Truman, J. W. (1985) J. Exp. Biol. 114, 381-395. [DOI] [PubMed] [Google Scholar]
- 26.Ma P. W. K., Knipple, D. C. & Roelofs, W. L. (1994) Proc. Natl. Acad. Sci. USA 91, 6506-6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis M. T. B., Vakharia, V. N., Henry, J., Kempe, T. G. & Raina, A. K. (1992) Proc. Natl. Acad. Sci. USA 89, 142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawano T., Kataoka, H., Nagasawa, H., Isogai, A. & Suzuki, A. (1992) Biochem. Biophys. Res. 189, 221-226. [DOI] [PubMed] [Google Scholar]
- 29.Brown M. R., Crim, J. W., Arata, R. C., Cai, H. N., Chun, C. & Shen, P. (1999) Peptides (Tarrytown, NY) 20, 1035-1042. [DOI] [PubMed] [Google Scholar]
- 30.Larhammar D. (1996) Regul. Pept. 62, 1-11. [DOI] [PubMed] [Google Scholar]
- 31.Lee A. & Durieux, M. E. (1998) in Receptor Biochemistry and Methodology Series; Identification and Expression of G Protein-Coupled Receptors, ed. Lynch, K. R. (Wiley-Liss, New York), pp. 73–96.
- 32.Darlison M. G. & Richter, D. (1999) Trends Neurosci. 22, 81-88. [DOI] [PubMed] [Google Scholar]
- 33.Davies S. A., Huesmann, G. R., Maddrell, S. H. P., O'Donnell, M. J., Skaer, N. J. V., Dow, J. A. T. & Tublitz, N. J. (1995) Am. J. Physiol. 269, R1321-R1326. [DOI] [PubMed] [Google Scholar]
- 34.Davies S. A., Stewart, E. J., Huesmann, G. R., Skaer, N. J., Maddrell, S. H., Tublitz, N. J. & Dow, J. A. (1997) Am. J. Physiol. 273, R823-R827. [DOI] [PubMed] [Google Scholar]
- 35.Kean L., Cazenave, W., Costes, L., Broderick, K. E., Graham, S., Pollock, V. P., Davies, S. A., Veenstra, J. A. & Dow, J. A. T. (2002) Am. J. Physiol. 282, R1297-R1307. [DOI] [PubMed] [Google Scholar]
- 36.Rosay P., Davies, S. A., Yu, Y., Sozen, M. A., Kaiser, K. & Dow, J. A. T. (1997) J. Cell Sci. 110, 1683-1692. [DOI] [PubMed] [Google Scholar]
- 37.Kostenis E. (2001) Trends Pharmcol. Sci. 22, 560-564. [DOI] [PubMed] [Google Scholar]
- 38.Kenakin T. (1997) Trends Pharmcol. Sci. 18, 456-464. [DOI] [PubMed] [Google Scholar]
- 39.May L. G. & Gay, C. V. (1997) J. Cell. Biochem. 64, 161-170. [PubMed] [Google Scholar]
- 40.Allgeier A., Laugwitz, K.-L., Van Sande, J., Schultz, G. & Dumont, J. E. (1997) Mol. Cell. Endocrinol. 127, 81-90. [DOI] [PubMed] [Google Scholar]
- 41.Brown D. R. & Quito, F. L. (1988) Eur. J. Pharm. 155, 159-162. [DOI] [PubMed] [Google Scholar]
- 42.Minamino N., Kangawa, K. & Matsuo, H. (1985) Biochem. Biophys. Res. 130, 1078-1085. [DOI] [PubMed] [Google Scholar]
- 43.Gardiner S. M., Compton, A. M., Bennett, T., Domin, J. & Bloom, S. R. (1990) Am. J. Physiol. 258, R32-R38. [DOI] [PubMed] [Google Scholar]
- 44.Kingan T. G., Blackburn, M. B. & Raina, A. K. (1992) Cell Tissue Res. 270, 229-240. [Google Scholar]
- 45.Ma P. W. K., Garden, R. W., Niermann, J. T., O'Connor, M., Sweedler, J. V. & Roelofs, W. L. (2000) J. Insect Physiol. 46, 221-230. [DOI] [PubMed] [Google Scholar]
- 46.Veenstra J. A. (1989) FEBS Lett. 250, 231-234. [DOI] [PubMed] [Google Scholar]
- 47.Stangier J., Hilbich, C., Beyruether, K. & Keller, R. (1987) Proc. Natl. Acad. Sci. USA 84, 575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loi P. K., Emmal, S. A., Park, Y. & Tublitz, N. J. (2001) J. Exp. Biol. 204, 2803-2816. [DOI] [PubMed] [Google Scholar]
- 49.Nichols R., Kaminski, S., Walling, E. & Zornik, E. (1999) Peptides (Tarrytown, NY) 20, 1153-1158. [DOI] [PubMed] [Google Scholar]
- 50.Veelaert D., Passier, P., Devreese, B., Vanden Broeck, J., Van Beeumen, J., Vullings, H. G. B., Diederen, J. H. B., Schoofs, L. & De Loof, A. (1997) Endocrinology 138, 138-142. [DOI] [PubMed] [Google Scholar]
- 51.Ewer J. & Truman, J. W. (1996) J. Comp. Neurol. 370, 330-341. [DOI] [PubMed] [Google Scholar]
- 52.Phlippen M. K., Webster, S. G., Chung, J. S. & Dircksen, H. (2000) J. Exp. Biol. 203, 521-536. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka S. (2000) J. Insect Physiol. 46, 1535-1544. [DOI] [PubMed] [Google Scholar]
- 54.Tawfik A. I., Tanaka, S., De Loof, A., Schoofs, L., Baggerman, G., Waelkens, E., Derua, R., Milner, Y., Yerushalmi, Y. & Pener, M. P. (1999) Proc. Natl. Acad. Sci. USA 96, 7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.