Abstract
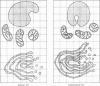
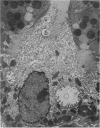
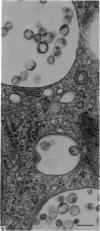
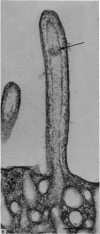
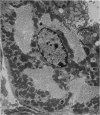
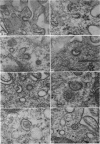
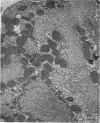
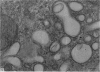
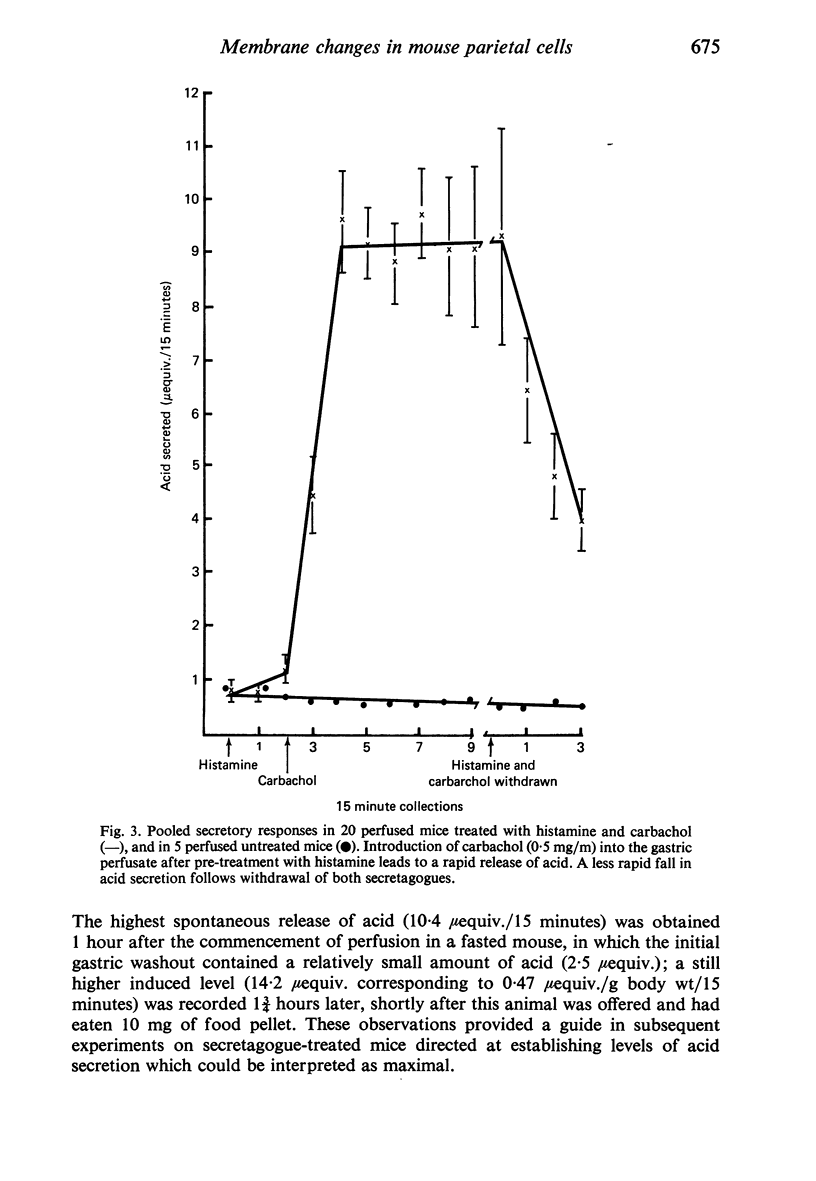
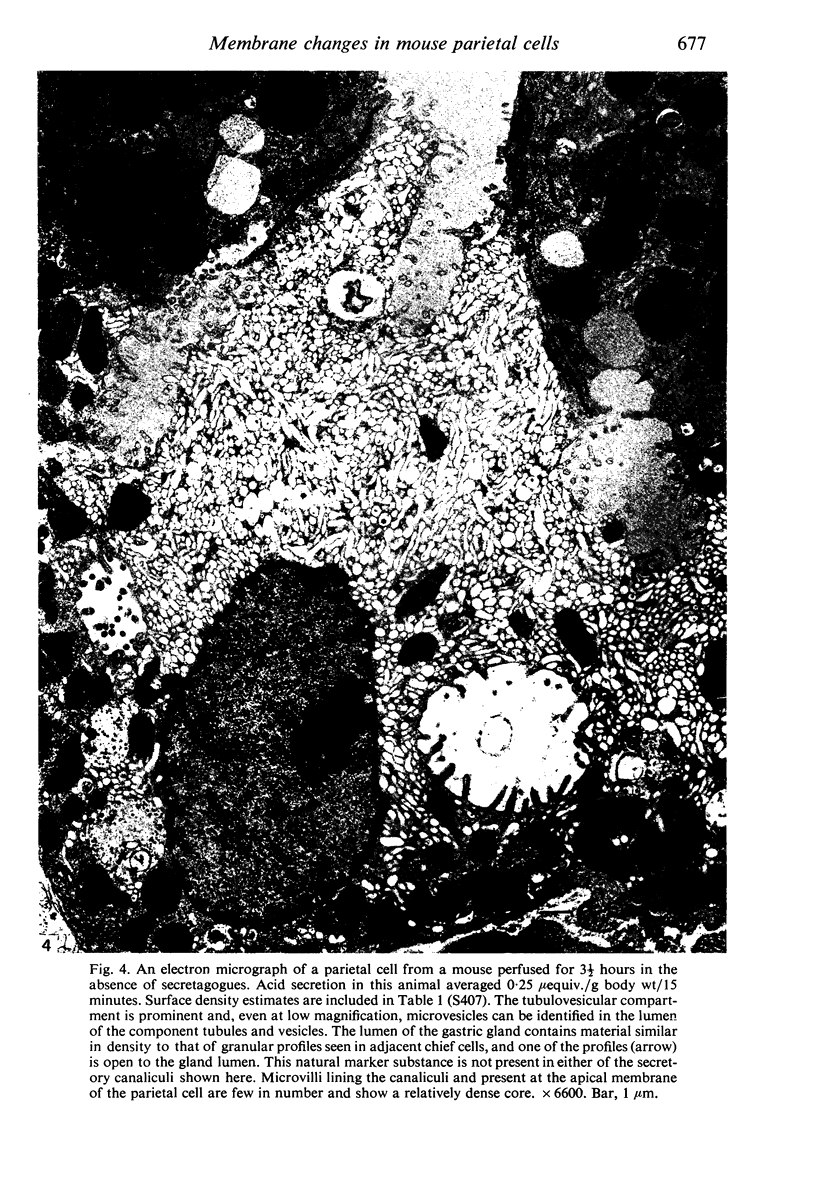
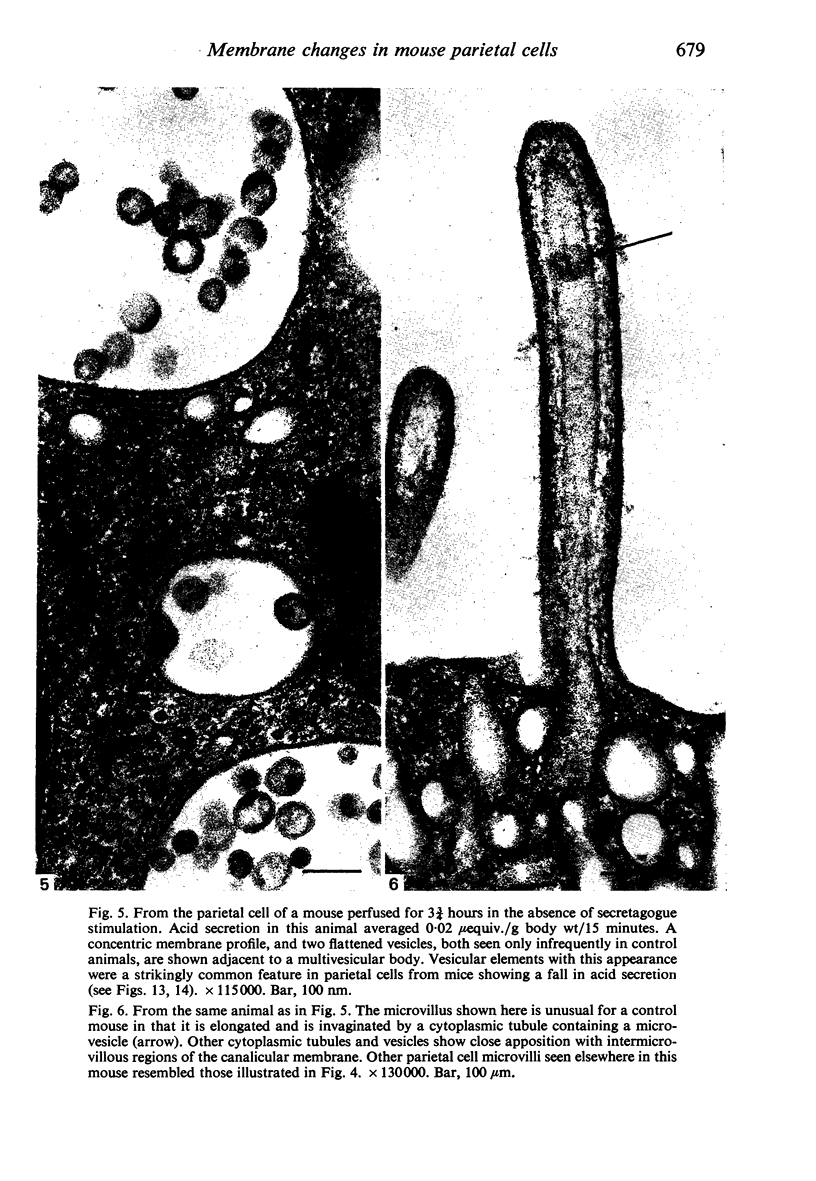
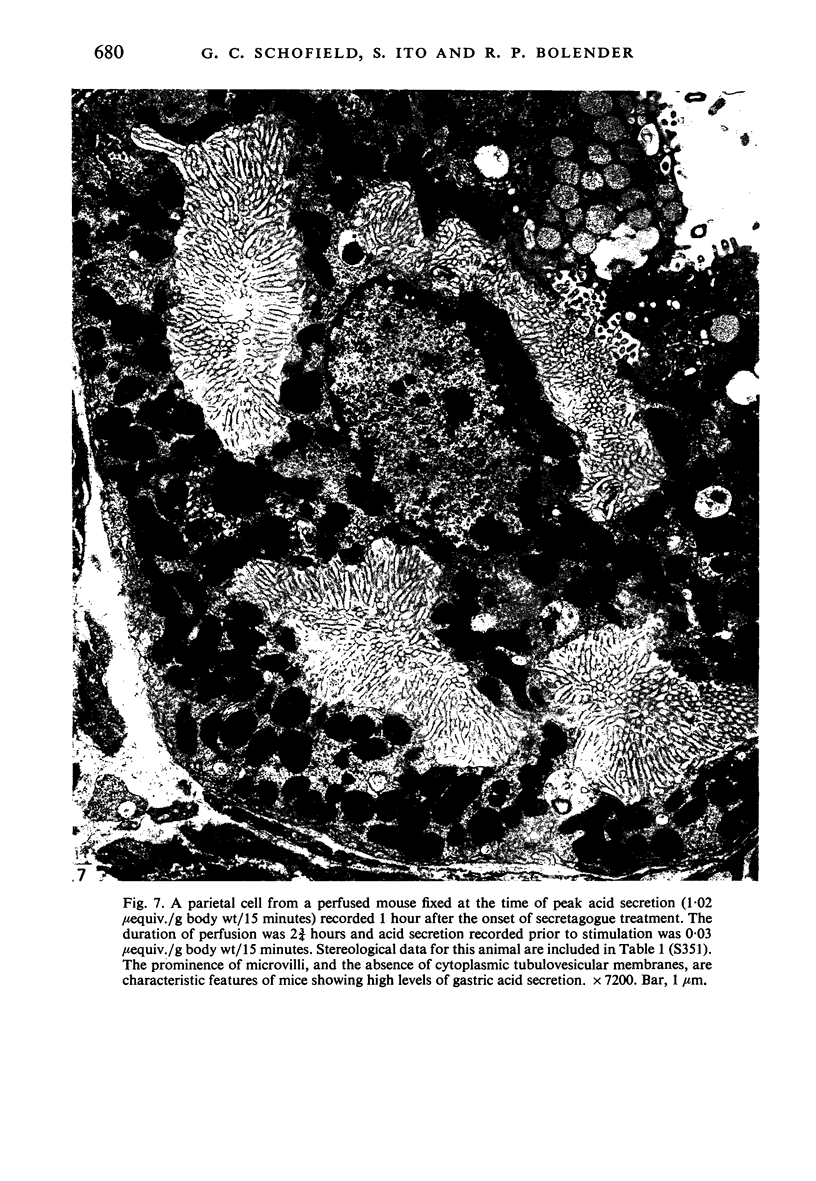
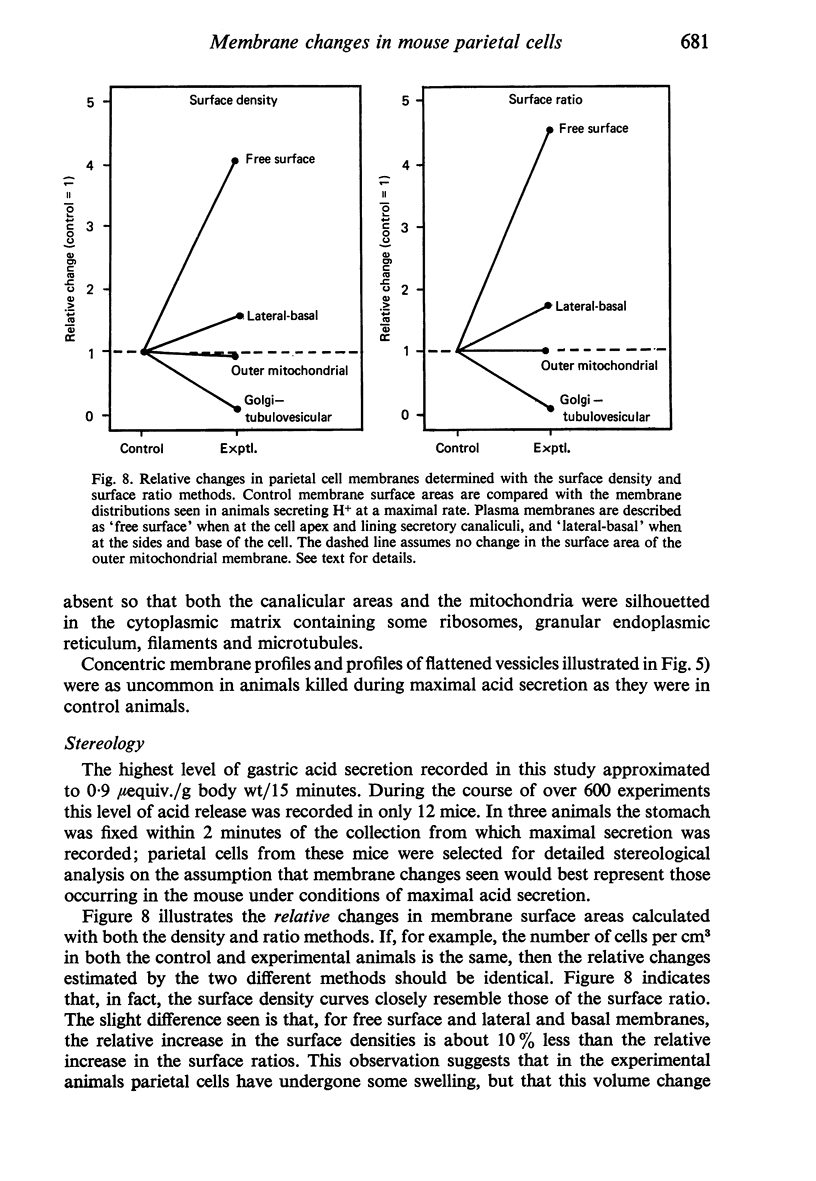
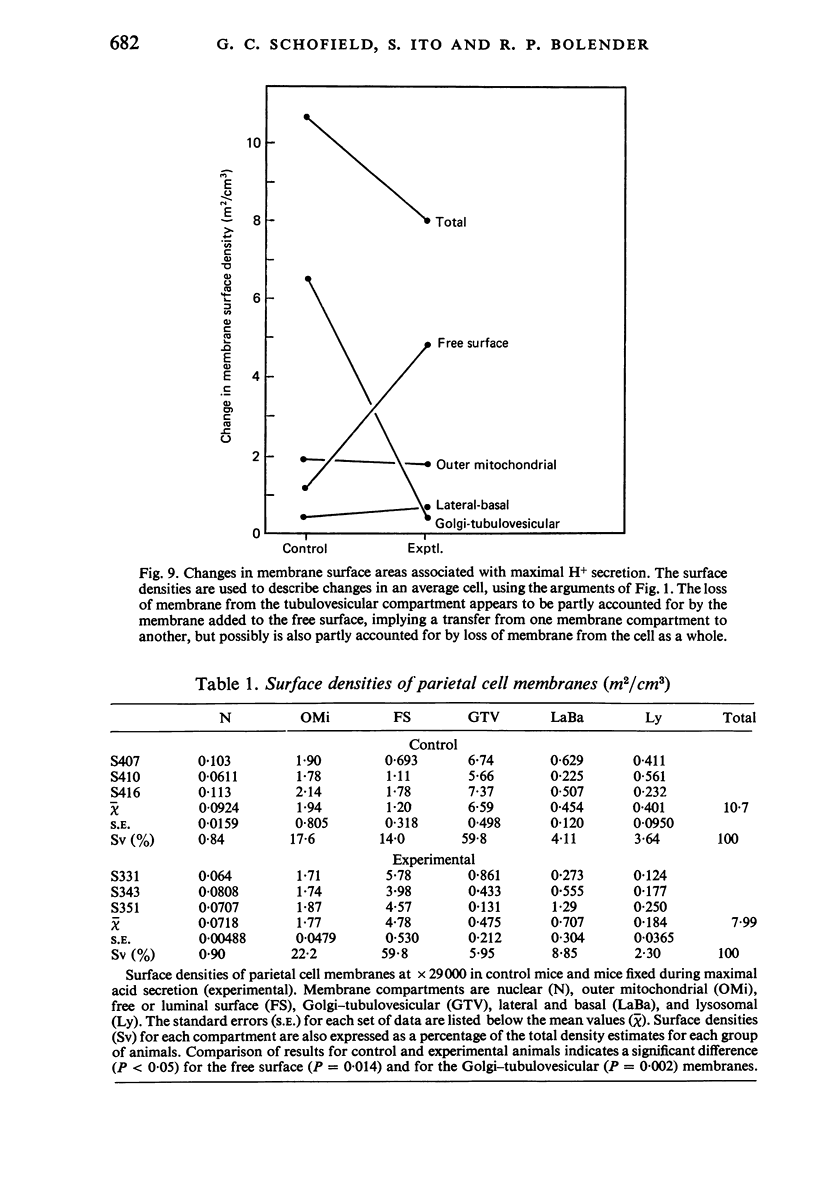
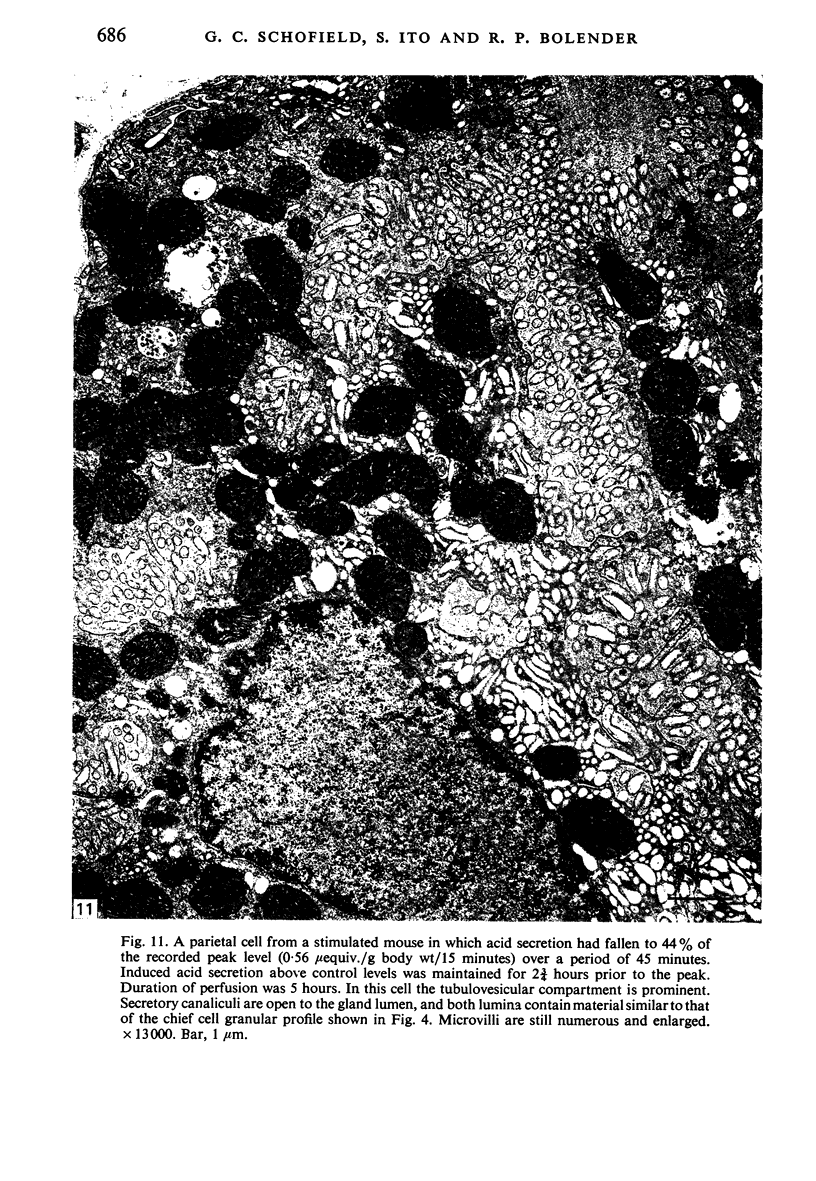
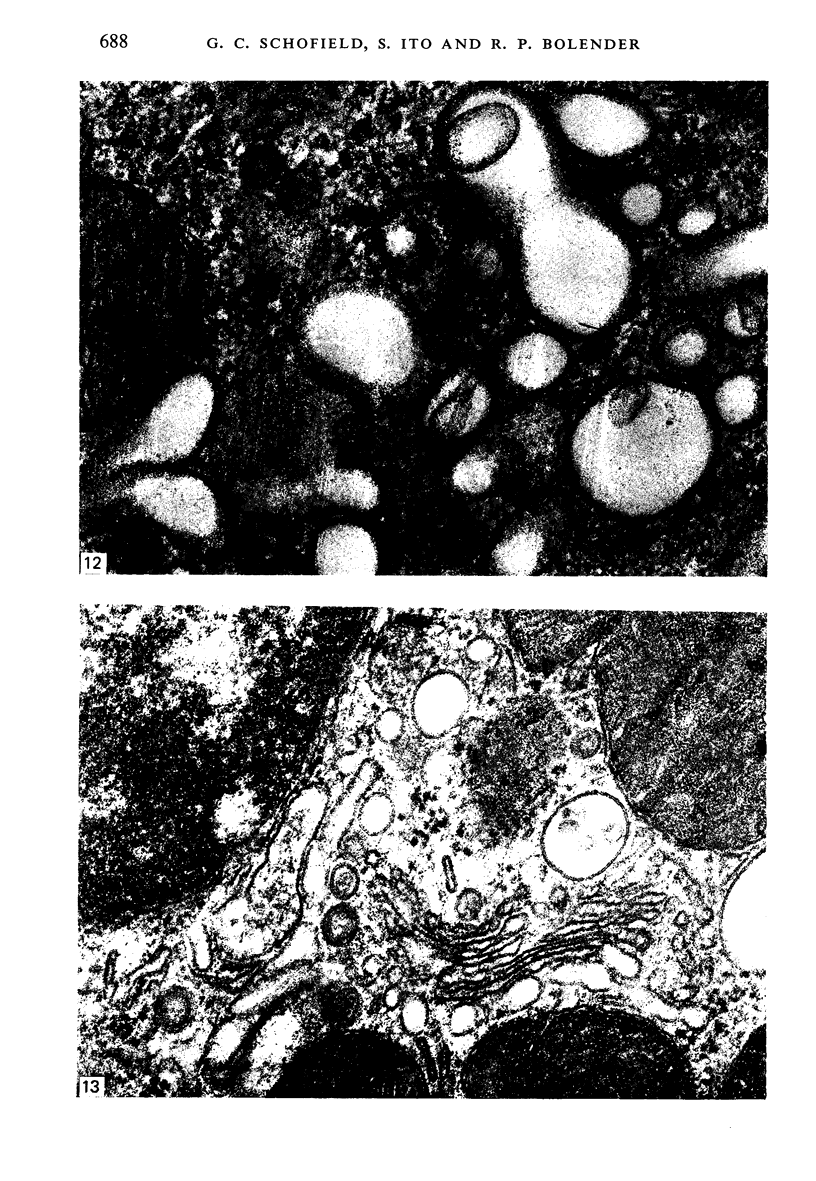
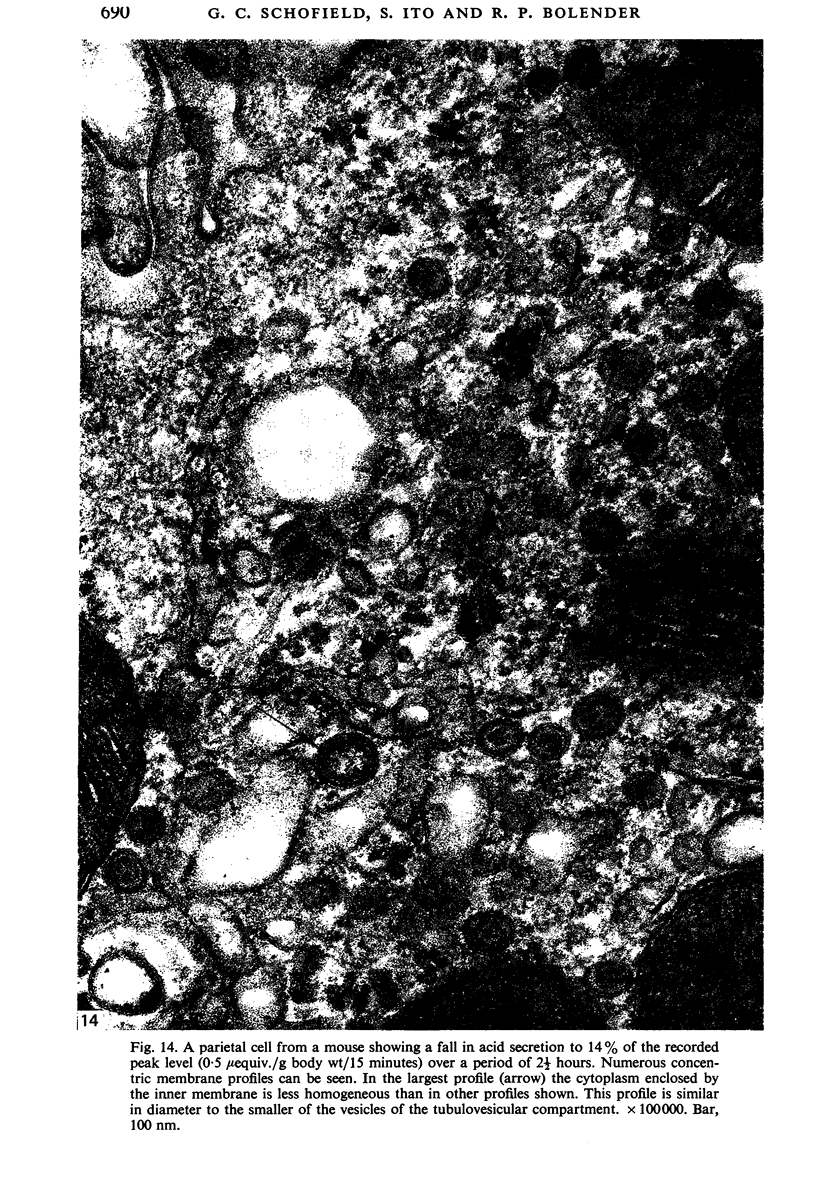
Levels of gastric acid secretion which may be maximal for the mouse were recorded following treatment with histamine and carbachol. A 30-fold increase over control levels was obtained in perfused animals, corresponding to a fourfold increase over highest levels recorded previously for stimulated mice. Stereological methods were used to estimate surface areas of membrane compartments of parietal cells in control and stimulated animals. Estimates of relative changes in membrane surface areas using a surface ratio method in this case substantiated changes detected by calculating surface densities. Main changes in membrane compartments of parietal cells from animals showing maximal acid secretion were a fourfold increase in free (luminal) surface, a 50% increase approximately in lateral and basal membrane, and a 90% reduction approximately in the tubulovesicular membrane compartment. Following withdrawal of secretagogues, acid secretion usually returned to control levels within 3 hours, but complete reconstitution of the tubulovesicular compartment was not seen within any survival period up to 5 hours. Reappearance of tubulovesicular elements first occurred shortly after the peak of a secretory response in focal cytoplasmic areas containing spherical and indented coated vesicles, and also numerous concentric membrane profiles not previously described in parietal cells. The way in which movement of membrane from the tubulovesicular compartment to the free surface occurs is not yet clear. However, reconstitution of the tubulovesicular compartment during a fall in acid secretion appears to involve movement of membrane from the free surface through coated vesicles, and their progression through indented forms and concentric membrane profiles to vesicles of the tubulovesicular compartment.
Full text
PDF



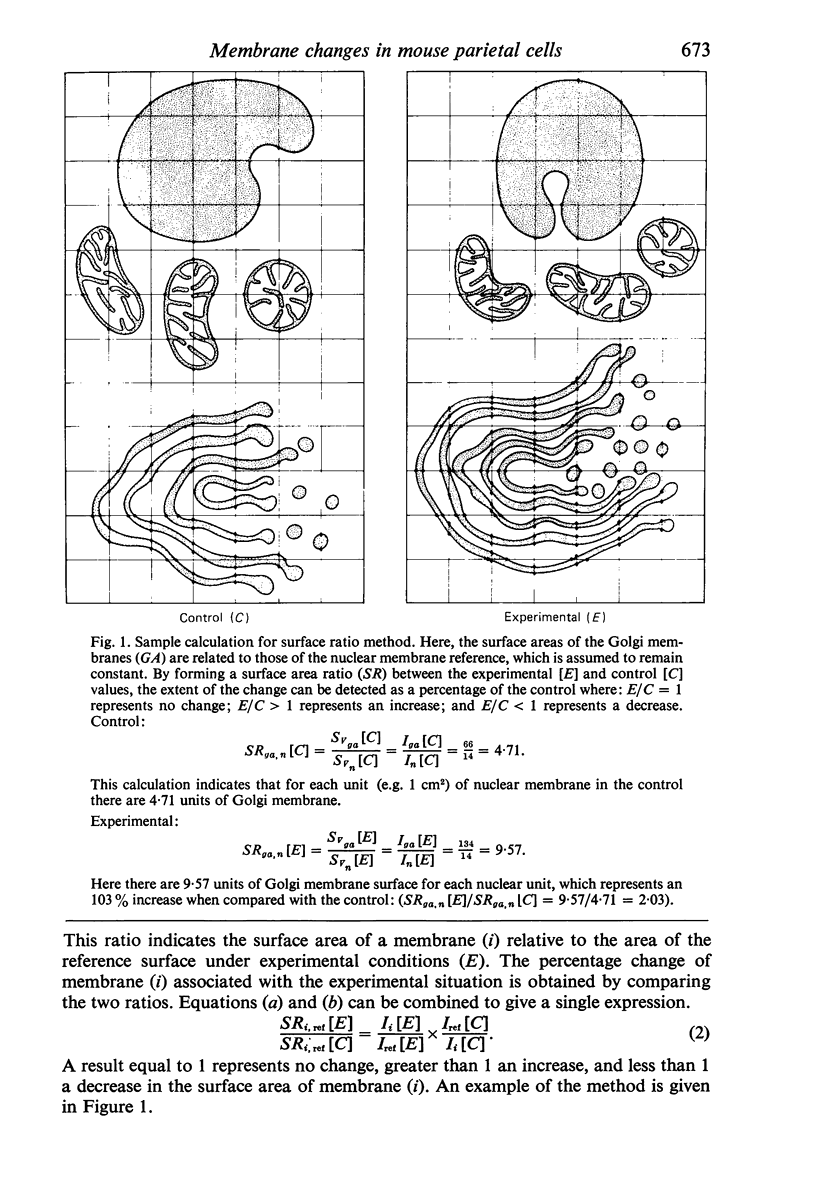
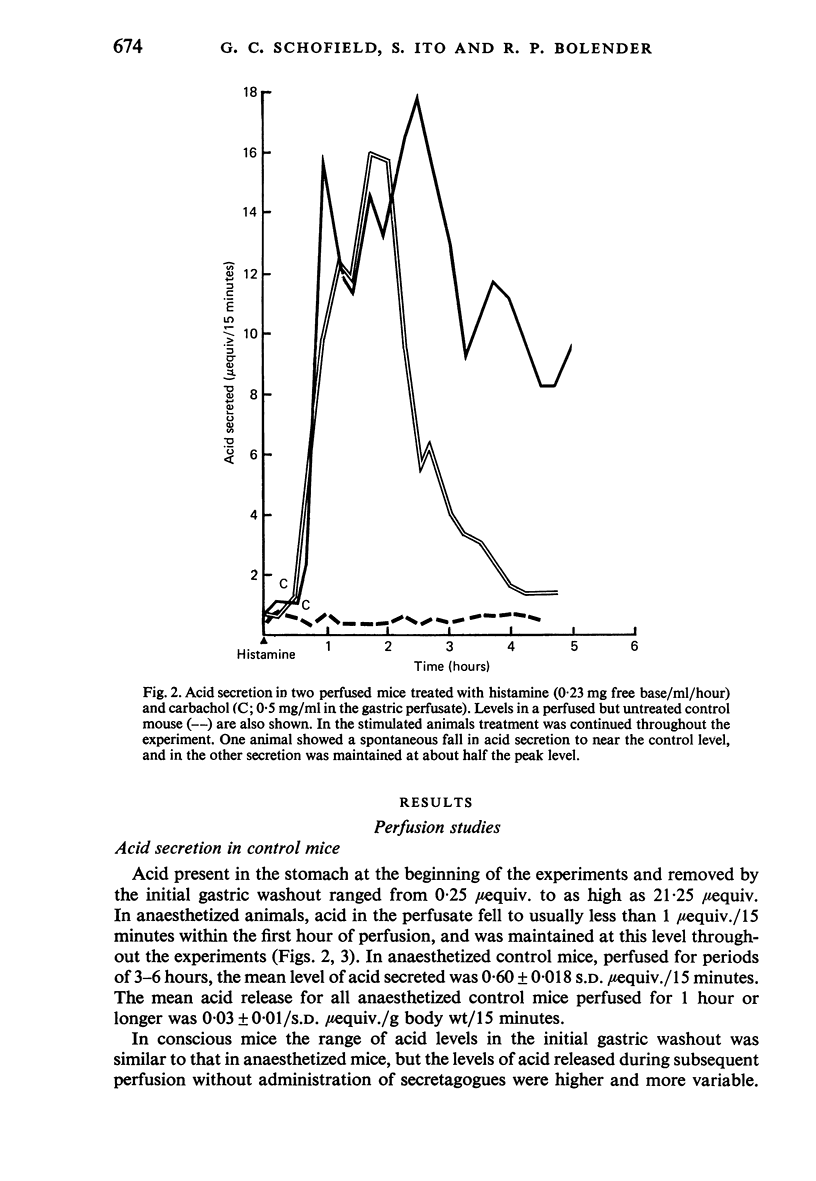








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins R. B., Jr, Ende N., Gobbel W. G., Jr A correlation of parietal cell activity with ultrastructural alterations. Surgery. 1967 Dec;62(6):1059–1069. [PubMed] [Google Scholar]
- BERENBLUM I., FOGEL-KAUFMAN H. Chemical analysis of the gastric contents of the mouse. II. Response in acid secretion to histamine, atropine, acetylcholine, and pilocarpine. Gastroenterology. 1957 Feb;32(2):286–294. [PubMed] [Google Scholar]
- Bolender R. P., Paumgartner D., Losa G., Muellener D., Weibel E. R. Intergrated stereological and biochemical studies of hepatocytic membranes. I. Membrane recoveries in subcellular fractions. J Cell Biol. 1978 May;77(2):565–583. doi: 10.1083/jcb.77.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol. 1974 May;61(2):269–287. doi: 10.1083/jcb.61.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT H. W., CHAVRE V. J. Conditions affecting acid secretion by mouse stomachs in vitro. Gastroenterology. 1950 Jul;15(3):467–480. [PubMed] [Google Scholar]
- Flexinos J., Carballido M., Louis A., Ribet A. Effects of pentagastrin stimulation on human parietal cells. An electron microscopic study with quantitative evaluation of cytoplasmic structures. Am J Dig Dis. 1971 Dec;16(12):1065–1074. doi: 10.1007/BF02235162. [DOI] [PubMed] [Google Scholar]
- GHOSH M. N., SCHILD H. O. Continuous recording of acid gastric secretion in the rat. Br J Pharmacol Chemother. 1958 Mar;13(1):54–61. doi: 10.1111/j.1476-5381.1958.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad A., Bennett G., Leblond C. P. Formation and turnover of plasma membrane glycoproteins in kidney tubules of young rats and adult mice, as shown by radioautography after an injection of 3H-fucose. Am J Anat. 1977 Feb;148(2):241–273. doi: 10.1002/aja.1001480205. [DOI] [PubMed] [Google Scholar]
- Helander H. F., Hirschowitz B. I. Quantitative ultrastructural studies on gastric parietal cells. Gastroenterology. 1972 Dec;63(6):951–961. [PubMed] [Google Scholar]
- Hübner G., Klein H. J., Eder M. Feinstrukturelle Untersuchungen an der Fundusschleimhaut des Rattenmagens nach Stimulierung mit Pentagastrin und Betazol. Verh Dtsch Ges Pathol. 1969;53:247–253. [PubMed] [Google Scholar]
- Ito S., Schofield G. C. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974 Nov;63(2 Pt 1):364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSA F. ULTRASTRUCTURE OF THE PARIETAL CELL OF THE HUMAN GASTRIC MUCOSA IN THE RESTING STATE AND AFTER STIMULATION WITH HISTALOG. Gastroenterology. 1963 Sep;45:354–363. [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]