Abstract
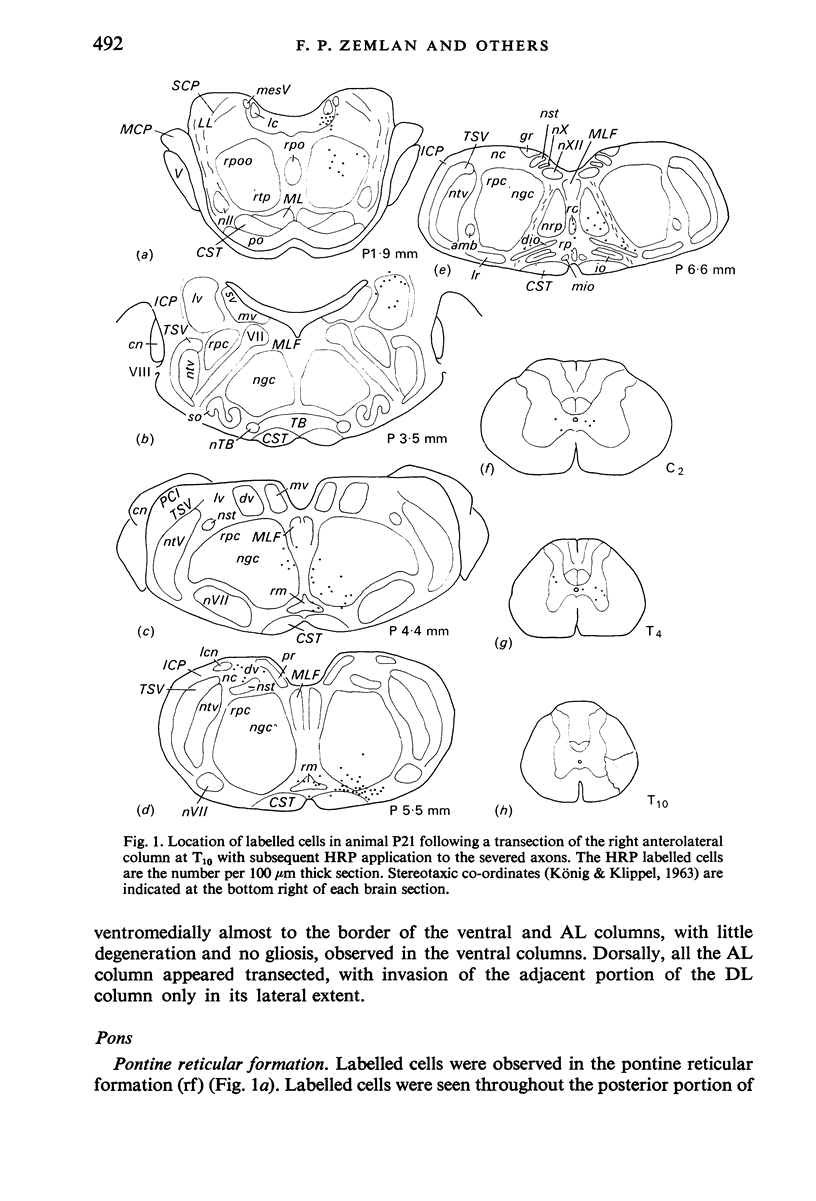
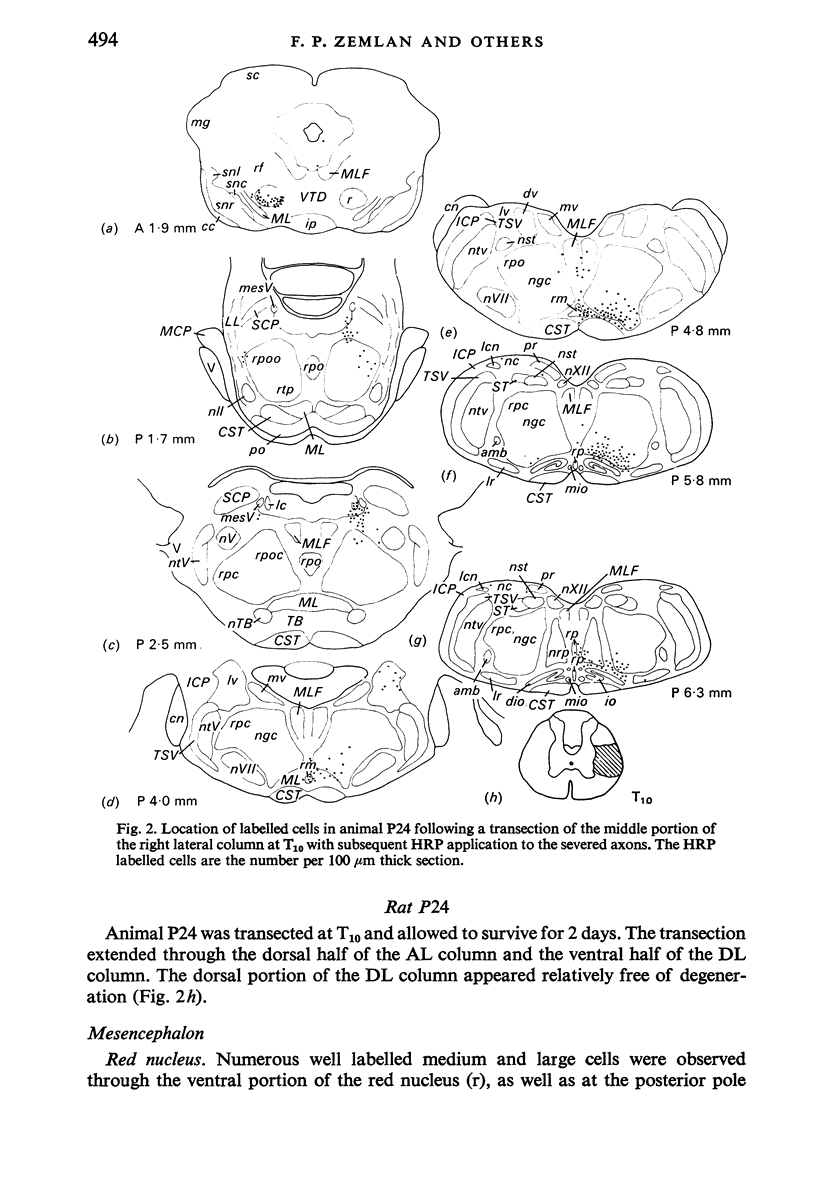
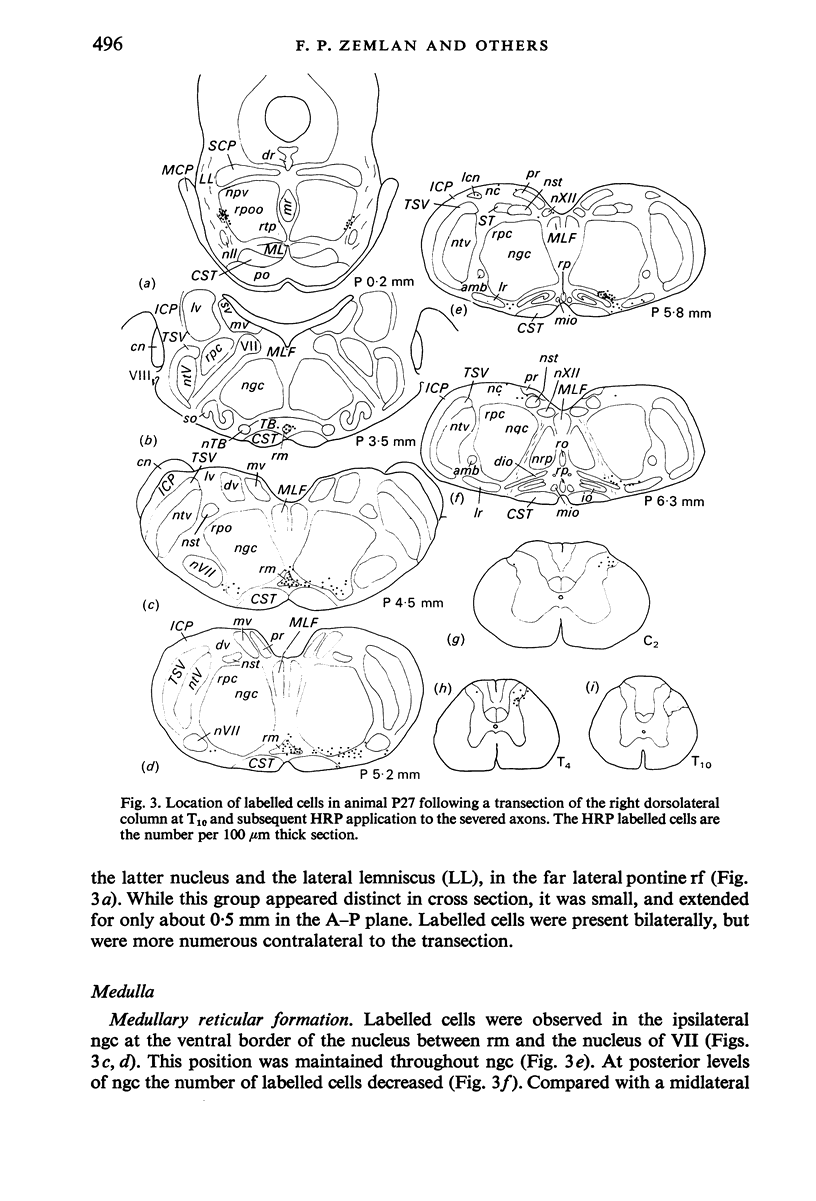
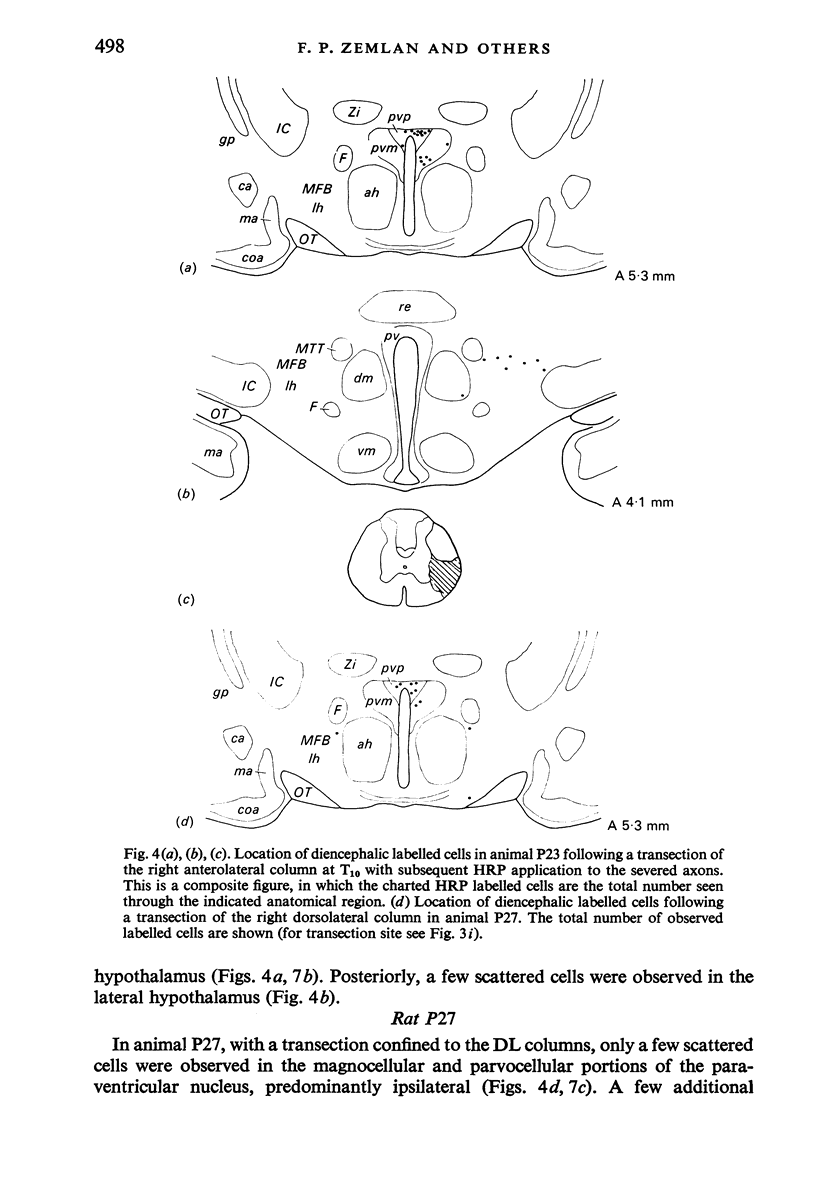
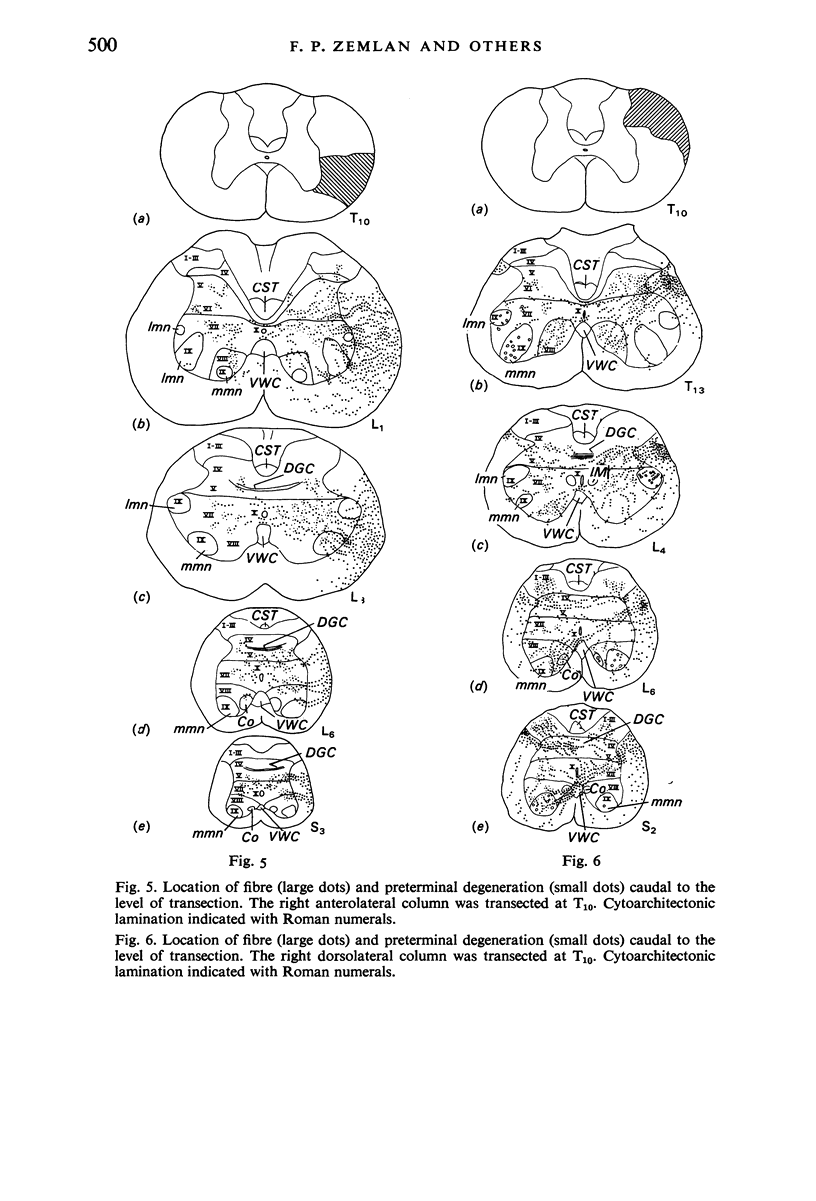
The location of the cells of origin and the projection areas of descending fibre tracts of the spinal cord lateral columns were examined in rats. Unilateral micro-transections of subpopulations of lateral column fibres, at C2 or T10, with subsequent application of horseradish peroxidase to the severed axons, allowed identification, by retrograde labelling, of those cell groups projecting to the spinal cord through the lateral columns. Additionally, the pattern of fibre and preterminal degeneration below the level of transection was examined using the Fink-Heimer silver impregnation technique. The largest number of labelled cells was observed in the ventral portion of nucleus gigantocellularis, projecting ipsilaterally through both the anterolateral (AL) and dorsolateral (DL) columns. Labelled cells were observed in the dorsal portion of the lateral vestibular nucleus (lv) following a T10 transection, and throughout the necleus following a C2 transection. Protein marker was observed in the large Deiters' cells of the lv, ipsilaterally. Also following an AL, but not a DL, column transection, retrograde labelled cells occurred throughout necleus reticularis pontis oralis (rpoo), bilaterally. At the border of rpoo and the lateral lemniscus, a discrete group of labelled cells was observed bilaterally following a DL column transection. This group of reticulospinal cells was located in a position similar to that of the A7 cell group reported in histofluorescence studies. The most extensive group of labelled cells following a DL column transection occurred in the magnocellular portion of the contralateral red nucleus. Although lavelled cells were observed in the red nucleus following either a C2 or T10 DL column transection, labelled cells were more numbeous and extended further rostrally and dorsally, following a high cervical transection. Labelled cells in nucleus raphe magnus were also more numerous following a DL column transection. Additional groups of labelled cells were seen following both an AL or DL column transection. These groups included necleus subcoeruleus ipsilaterally, and nucleus reticularis ventralis and the nucleus of the tract of spinal V, bilaterally. Labelled cells were observed as far forwards as the hypothalamus, occurring predominantly in the paraventricular nucleus, ipsilaterally. A few labelled cells were observed in the lateral hypothalamus. Some cell groups were labelled only after a C2 transection. These included the interstitial nucleus of Cajal, ipsilaterally, the descending vestibular necleus and the deep layers of the superior colliculus, contralaterally, and the central grey matter and nucleus raphe pallidus. Fibre and preterminal degeneration resulting from unilateral AL or DL column transection was examined. Following an AL column transection degeneration was most intense in the ipsilateral laminae V, VI and VII...
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTMAN J., CARPENTER M. B. Fiber projections of the superior colliculus in the cat. J Comp Neurol. 1961 Apr;116:157–177. doi: 10.1002/cne.901160206. [DOI] [PubMed] [Google Scholar]
- BRODAL A., GOGSTAD A. C. Rubro-cerebellar connections; an experimental study in the cat. Anat Rec. 1954 Mar;118(3):455–485. doi: 10.1002/ar.1091180302. [DOI] [PubMed] [Google Scholar]
- Bobillier P., Seguin S., Petitjean F., Salvert D., Touret M., Jouvet M. The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976 Sep 3;113(3):449–486. doi: 10.1016/0006-8993(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Brown L. T. Rubrospinal projections in the rat. J Comp Neurol. 1974 Mar 15;154(2):169–187. doi: 10.1002/cne.901540205. [DOI] [PubMed] [Google Scholar]
- Bunt A. H., Hendrickson A. E., Lund J. S., Lund R. D., Fuchs A. F. Monkey retinal ganglion cells: morphometric analysis and tracing of axonal projections, with a consideration of the peroxidase technique. J Comp Neurol. 1975 Dec 1;164(3):265–285. doi: 10.1002/cne.901640302. [DOI] [PubMed] [Google Scholar]
- Burton H., Loewy A. D. Projections to the spinal cord from medullary somatosensory relay nuclei. J Comp Neurol. 1977 Jun 15;173(4):773–792. doi: 10.1002/cne.901730408. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B., Harbison J. W., Peter P. Accessory oculomotor nuclei in the monkey: projections and effects of discrete lesions. J Comp Neurol. 1970 Oct;140(2):131–154. doi: 10.1002/cne.901400202. [DOI] [PubMed] [Google Scholar]
- Courville J. Rubrobulbar fibres to the facial nucleus and the lateral reticular nucleus (nucleus of the lateral funiculus). An experimental study in the cat with silver impregnation methods. Brain Res. 1966 May-Jun;1(4):317–337. doi: 10.1016/0006-8993(66)90125-9. [DOI] [PubMed] [Google Scholar]
- De Vito J. L., Clausing K. W., Smith O. A. Uptake and transport of horseradish peroxidase by cut end of the vagus nerve. Brain Res. 1974 Dec 27;82(2):269–271. doi: 10.1016/0006-8993(74)90604-0. [DOI] [PubMed] [Google Scholar]
- Edwards S. B. The ascending and descending projections of the red nucleus in the cat: an experimental study using an autoradiographic tracing method. Brain Res. 1972 Dec 24;48:45–63. doi: 10.1016/0006-8993(72)90170-9. [DOI] [PubMed] [Google Scholar]
- Fink R. P., Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967 Apr;4(4):369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- Fraser E. H. An experimental research into the relations of the posterior longitudinal bundle and Deiters' nucleus. J Physiol. 1901 Dec 23;27(4-5):372–397. doi: 10.1113/jphysiol.1901.sp000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furstman L., Saporta S., Kruger L. Retrograde axonal transport of horseradish peroxidase in sensory nerves and ganglion cells of the rat. Brain Res. 1975 Feb 7;84(2):320–324. doi: 10.1016/0006-8993(75)90987-7. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- HINMAN A., CARPENTER M. B. Efferent fiber projections of the red nucleus in the cat. J Comp Neurol. 1959 Aug;113:61–82. doi: 10.1002/cne.901130104. [DOI] [PubMed] [Google Scholar]
- Halperin J. J., LaVail J. H. A study of the dynamics of retrograde transport and accumulation of horseradish peroxidase in injured neurons. Brain Res. 1975 Dec 19;100(2):253–269. doi: 10.1016/0006-8993(75)90482-5. [DOI] [PubMed] [Google Scholar]
- Hancock M. B., Fougerousse C. L. Spinal projections from the nucleus locus coeruleus and nucleus subcoeruleus in the cat and monkey as demonstrated by the retrograde transport of horseradish peroxidase. Brain Res Bull. 1976 Mar-Apr;1(2):229–234. doi: 10.1016/0361-9230(76)90072-1. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Nauta W. J. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977 May 1;173(1):123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., FLEMING W. R., FARINHOLT J. W. Subcorticospinal projections in the rhesus monkey. J Comp Neurol. 1962 Feb;118:107–137. doi: 10.1002/cne.901180109. [DOI] [PubMed] [Google Scholar]
- Keefer D. A., Christ J. F. Distribution of endogenous diaminobenzidine-staining cells in the normal rat brain. Brain Res. 1976 Nov 5;116(2):312–316. doi: 10.1016/0006-8993(76)90909-4. [DOI] [PubMed] [Google Scholar]
- Kow L. M., Montgomery M. O., Pfaff D. W. Effects of spinal cord transections on lordosis reflex in female rats. Brain Res. 1977 Mar 4;123(1):75–88. doi: 10.1016/0006-8993(77)90644-8. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Retrograde transport of horseradish peroxidase in transected axons. 1. Time relationships between transport and induction of chromatolysis. Brain Res. 1974 Oct 11;79(1):101–109. doi: 10.1016/0006-8993(74)90569-1. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y., Sjöstrand J. Axonal uptake and retrograde transport of exogenous proteins in the hypoglossal nerve. Brain Res. 1971 Sep 24;32(2):399–406. doi: 10.1016/0006-8993(71)90332-5. [DOI] [PubMed] [Google Scholar]
- Kuypers H. G., Kievit J., Groen-Klevant A. C. Retrograde axonal transport of horseradish peroxidase in rats forebrain. Brain Res. 1974 Feb 22;67(2):211–218. doi: 10.1016/0006-8993(74)90273-x. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., LaVail M. M. Retrograde axonal transport in the central nervous system. Science. 1972 Jun 30;176(4042):1416–1417. doi: 10.1126/science.176.4042.1416. [DOI] [PubMed] [Google Scholar]
- Liebeskind J. C., Paul L. A. Psychological and physiological mechanisms of pain. Annu Rev Psychol. 1977;28:41–60. doi: 10.1146/annurev.ps.28.020177.000353. [DOI] [PubMed] [Google Scholar]
- Litchy W. J. Uptake and retrograde transport of horseradish peroxidase in frog sartorius nerve in vitro. Brain Res. 1973 Jun 29;56:377–381. doi: 10.1016/0006-8993(73)90356-9. [DOI] [PubMed] [Google Scholar]
- Lloyd R. E. On chromatolysis in Deiters' nucleus after hemisection of the cord. J Physiol. 1900 Feb 2;25(3):191–195. doi: 10.1113/jphysiol.1900.sp000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modianos D. T., Pfaff D. W. Brain stem and cerebellar lesions in female rats. II. Lordosis reflex. Brain Res. 1976 Apr 16;106(1):47–56. doi: 10.1016/0006-8993(76)90072-x. [DOI] [PubMed] [Google Scholar]
- Modianos D. T., Pfaff D. W. Facilitation of the lordosis reflex in female rats by electrical stimulation of the lateral vestibular nucleus. Brain Res. 1977 Oct 7;134(2):333–345. doi: 10.1016/0006-8993(77)91077-0. [DOI] [PubMed] [Google Scholar]
- NYBERG-HANSEN R., BRODAL A. SITES AND MODE OF TERMINATION OF RUBROSPINAL FIBRES IN THE CAT. AN EXPERIMENTAL STUDY WITH SILVER IMPREGNATION METHODS. J Anat. 1964 Apr;98:235–253. [PMC free article] [PubMed] [Google Scholar]
- NYBERG-HANSEN R., MASCITTI T. A. SITES AND MODE OF TERMINATION OF FIBERS OF THE VESTIBULOSPINAL TRACT IN THE CAT. AN EXPERIMENTAL STUDY WITH SILVER IMPREGNATION METHODS. J Comp Neurol. 1964 Jun;122:369–383. doi: 10.1002/cne.901220307. [DOI] [PubMed] [Google Scholar]
- NYBERG-HANSEN R. SITES AND MODE OF TERMINATION OF RETICULO-SPINAL FIBERS IN THE CAT. AN EXPERIMENTAL STUDY WITH SILVER IMPREGNATION METHODS. J Comp Neurol. 1965 Feb;124:71–99. doi: 10.1002/cne.901240107. [DOI] [PubMed] [Google Scholar]
- Nauta H. J., Pritz M. B., Lasek R. J. Afferents to the rat caudoputamen studied with horseradish peroxidase. An evaluation of a retrograde neuroanatomical research method. Brain Res. 1974 Feb 22;67(2):219–238. doi: 10.1016/0006-8993(74)90274-1. [DOI] [PubMed] [Google Scholar]
- ORIOLI F. L., METTLER F. A. The rubrospinal tract in Macaca mulatta. J Comp Neurol. 1956 Dec;106(2):299–318. doi: 10.1002/cne.901060204. [DOI] [PubMed] [Google Scholar]
- Oliveras J. L., Besson J. M., Guilbaud G., Liebeskind J. C. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974 Apr 30;20(1):32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- POMPEIANO O., BRODAL A. Experimental demonstration of a somatotopical origin of rubrospinal fibers in the cat. J Comp Neurol. 1957 Oct;108(2):225–251. doi: 10.1002/cne.901080204. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Jacobowitz D. M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (mesencephalon, rhombencephalon). J Comp Neurol. 1974 Sep 1;157(1):29–42. doi: 10.1002/cne.901570104. [DOI] [PubMed] [Google Scholar]
- Petras J. M. Cortical, tectal and tegmental fiber connections in the spinal cord of the cat. Brain Res. 1967 Oct;6(2):275–324. doi: 10.1016/0006-8993(67)90196-5. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Segal M., Bloom F. E. A radioautographic study of the efferent pathways of the nucleus locus coeruleus. J Comp Neurol. 1974 May 1;155(1):15–42. doi: 10.1002/cne.901550103. [DOI] [PubMed] [Google Scholar]
- Poirier L. J., Bouvier G. The red nucleus and its efferent nervous pathways in the monkey. J Comp Neurol. 1966 Oct;128(2):223–244. doi: 10.1002/cne.901280208. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Reis D. J. Effects of lesions of locus coeruleus on regional distribution of dopamine-beta-hydroxylase activity in rat brain. Brain Res. 1974 Jun 14;73(1):161–166. doi: 10.1016/0006-8993(74)91016-6. [DOI] [PubMed] [Google Scholar]
- Réthelyi M., Szentágothai J. The large synaptic complexes of the substantia gelatinosa. Exp Brain Res. 1969;7(3):258–274. doi: 10.1007/BF00239033. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. NEURONAL AND SYNAPTIC ARRANGEMENT IN THE SUBSTANTIA GELATINOSA ROLANDI. J Comp Neurol. 1964 Apr;122:219–239. doi: 10.1002/cne.901220207. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. Direct hypothalamo-autonomic connections. Brain Res. 1976 Nov 26;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Steiner T. J., Turner L. M. Cytoarchitecture of the rat spinal cord. J Physiol. 1972 Apr;222(2):123P–125P. [PubMed] [Google Scholar]
- TORVIK A., BRODAL A. The origin of reticulospinal fibers in the cat; an experimental study. Anat Rec. 1957 May;128(1):113–137. doi: 10.1002/ar.1091280110. [DOI] [PubMed] [Google Scholar]
- VALVERDE F. Reticular formation of the albino rat's brain stem cytoarchitecture and corticofugal connections. J Comp Neurol. 1962 Aug;119:25–53. doi: 10.1002/cne.901190105. [DOI] [PubMed] [Google Scholar]
- VERHAART W. J., VAN BEUSEKOM G. T. Fibre tracts in the cord in cat. Acta Psychiatr Neurol Scand. 1958;33(3):359–376. doi: 10.1111/j.1600-0447.1958.tb03525.x. [DOI] [PubMed] [Google Scholar]
- WALBERG F. On the termination of the rubrobulbar fibers; experimental observations in the cat. J Comp Neurol. 1958 Aug;110(1):65–73. doi: 10.1002/cne.901100103. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. T. Demonstration of geniculocortical and callosal projection neurons in the squirrel monkey by means of retrograde axonal transport of horseradish peroxidase. Brain Res. 1974 Oct 18;79(2):267–272. doi: 10.1016/0006-8993(74)90415-6. [DOI] [PubMed] [Google Scholar]