Abstract
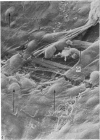
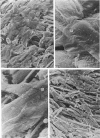
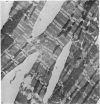
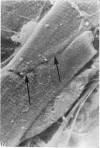
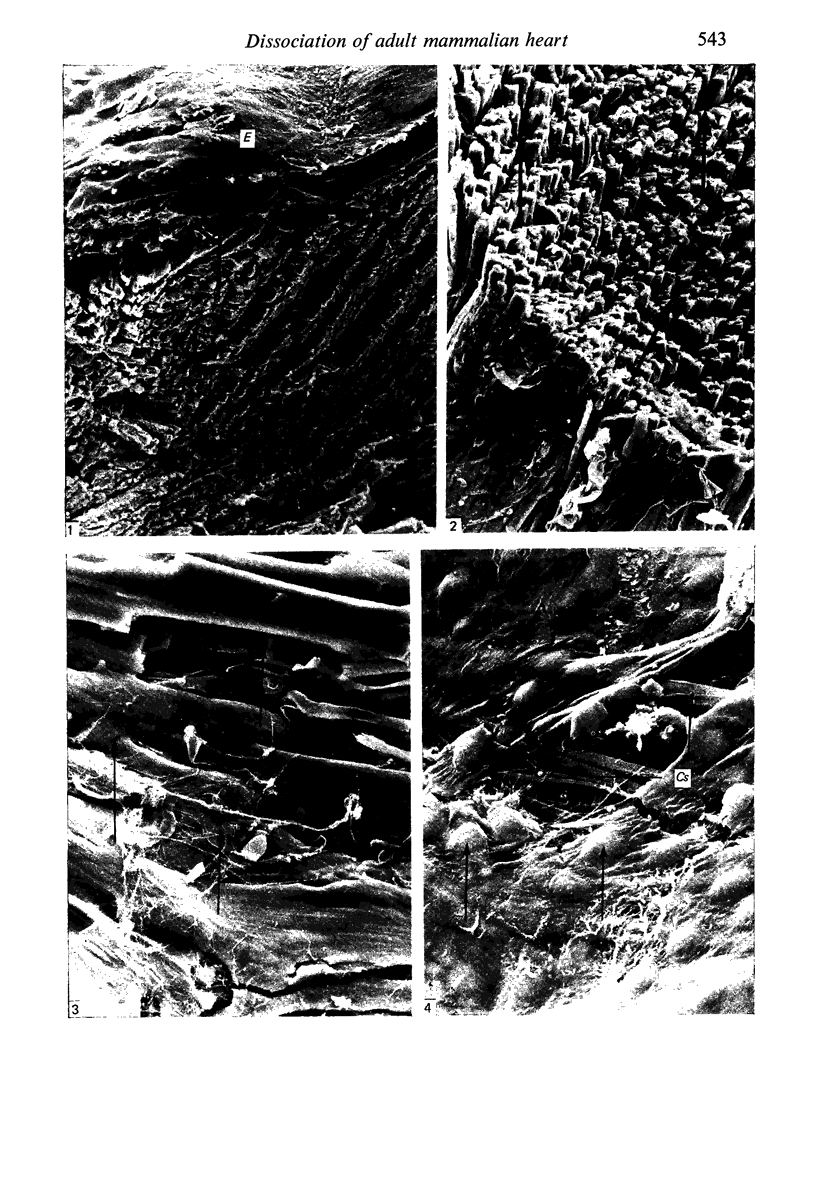
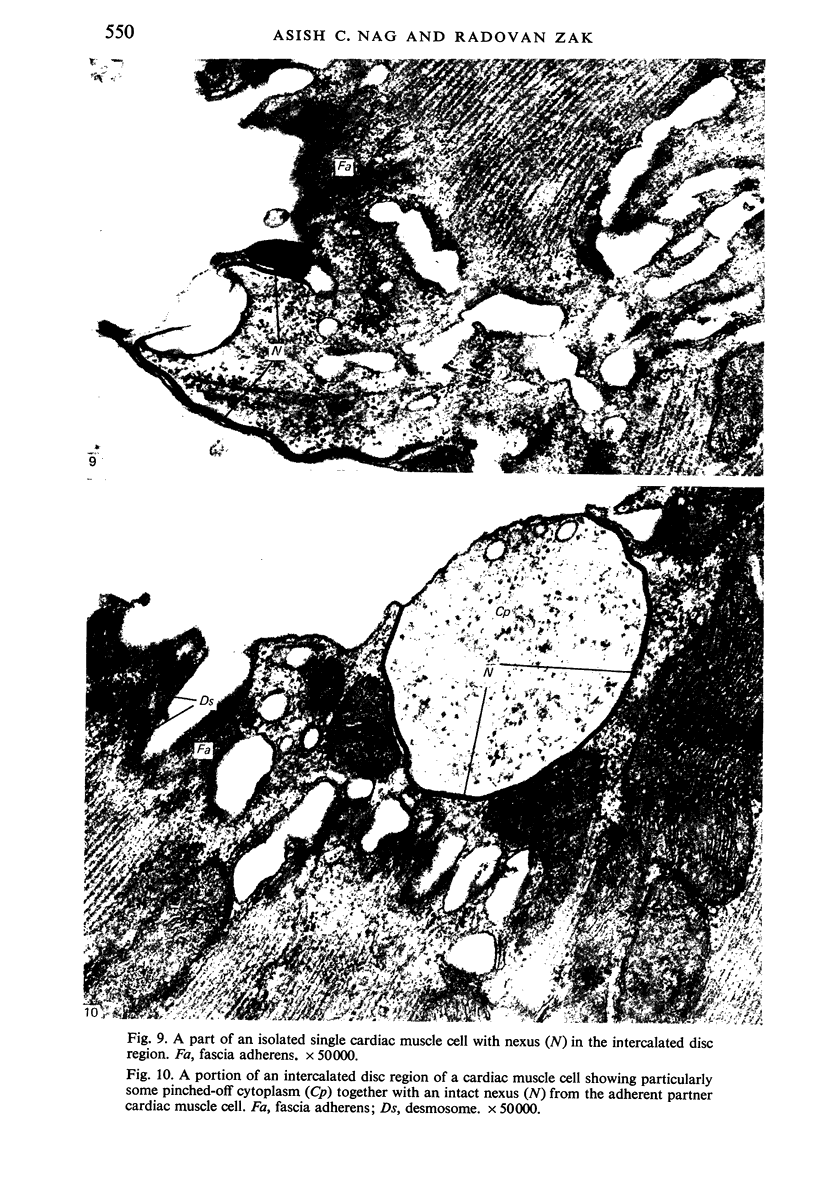
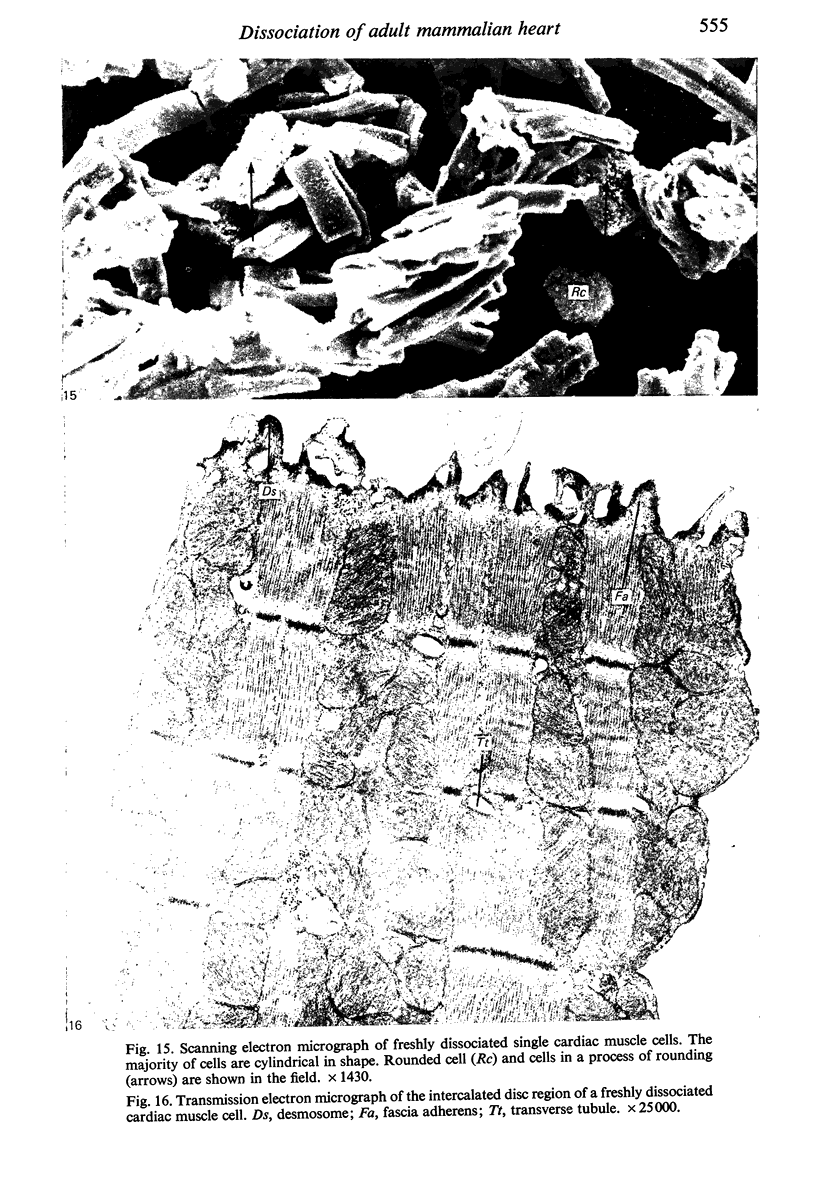
Adult rat heart was dissociated into a single cell suspension by a perfusion technique which used 0.05% collagenase and 0.1% hyaluronidase in Krebs-Ringer phosphate buffer (KRP). The non-muscle cells of the suspension were separated from the myocytes by centrifugation through 3% Ficoll solution in KRP with 0.01 mM Ca2+. An approximately 90% pure suspension of isolated single muscle cells was obtained with this method. The effects of the successive steps in the dissociation procedure on the ultrastructure of the heart were studied by scanning and transmission electron microscopy. After 30 minutes of enzyme digestion, dissociation of the inner endothelial lining of the ventricle into single cells or small groups of cells became apparent. In addition, the underlying cardiac skeleton began to disintegrate and linear arrays of cardiac muscle cells were observed. After 45 minutes of enzyme digestion the number of released single cells was higher because of the separation of intercalated discs. The majority of non-muscle cells were by now dissociated from the surfaces of muscle cells. Widening of the lateral intercellular spaces between the myocardial cells was associated with separation of desmosomes. In some regions of the heart, intact desmosomes, fasciae adherentes and gap junctions were observed even though lateral intercellular spaces had widened greatly. The majority of myocardial cells had become separated from one another after 60 minutes of enzyme digestion. Separation of gap junctional sites took place in two ways: (1) by 'unzipping' them through enzyme action; (2) by tearing them mechanically. Gap junction remnants were sometimes observed in a vesiculated state within the cell. The dissociation of the heart was ineffective when perfused with media containing 1.0 or 2 mM Ca2+. Alcian blue treatment after 60 minutes of enzyme digestion revealed that the basement membrane, and its accompanying collagen fibrils, was still present on the plasma membrane of dissociated single cells. The isolated myocardial cells retained their normal morphological characteristics. This study has enabled us to understand in detail how dismantlement of highly ordered adult cardiac tissue into a single cell suspension takes place. Cell suspensions of this type should be invaluable in the study of metabolic and synthetic activities in adult myocardial cells.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S., Scheuer J. Morphology and metabolism of intact muscle cells isolated from adult rat heart. Circ Res. 1970 Jun;26(6):679–687. doi: 10.1161/01.res.26.6.679. [DOI] [PubMed] [Google Scholar]
- Borysenko J. Z., Revel J. P. Experimental manipulation of desmosome structure. Am J Anat. 1973 Aug;137(4):403–421. doi: 10.1002/aja.1001370404. [DOI] [PubMed] [Google Scholar]
- Dani A. M., Cittadini A., Flamini G., Festuccia G., Terranova T. Preparation and some properties of isolated beating myocytes from adult rabbit heart. J Mol Cell Cardiol. 1977 Sep;9(9):777–784. doi: 10.1016/s0022-2828(77)80022-9. [DOI] [PubMed] [Google Scholar]
- Farmer B. B., Harris R. A., Jolly W. W., Hathaway D. R., Katzberg A., Watanabe A. M., Whitlow A. L., Besch H. R., Jr Isolation and characterization of adult rat hearts cells. Arch Biochem Biophys. 1977 Mar;179(2):545–558. doi: 10.1016/0003-9861(77)90143-6. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972 Jun;53(3):758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick M. R., Burns A. H., Reddy W. J. Dispersion and isolation of beating cells from adult rat heart. Anal Biochem. 1974 Sep;61(1):32–42. doi: 10.1016/0003-2697(74)90329-7. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies of single isolated beating heart cells. Science. 1960 Jun 3;131(3414):1674–1675. doi: 10.1126/science.131.3414.1674. [DOI] [PubMed] [Google Scholar]
- Kono T. Roles of collagenases and other proteolytic enzymes in the dispersal of animal tissues. Biochim Biophys Acta. 1969 Apr 22;178(2):397–400. doi: 10.1016/0005-2744(69)90410-0. [DOI] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Murphy G. E., Becker C. G. Occurrence of caterpillar nuclei within normal immature and normal appearing and altered mature heart muscle cells and the evolution of Anitschkow cells from the latter. Am J Pathol. 1966 Jun;48(6):931–957. [PMC free article] [PubMed] [Google Scholar]
- Nag A. C., Fischman D. A., Aumont M. C., Zak R. Studies of isolated adult rat heart cells: the surface morphology and the influence of extracellular calcium ion concentration on cellular viability. Tissue Cell. 1977;9(3):419–436. doi: 10.1016/0040-8166(77)90003-9. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D. ELECTRON MICROSCOPIC OBSERVATIONS ON THE ACCUMULATION OF DIVALENT CATIONS IN INTRAMITOCHONDRIAL GRANULES. J Cell Biol. 1964 Jan;20:95–111. doi: 10.1083/jcb.20.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., McCallister L. P. Studies on the intercalated disk of rat left ventricular myocardial cells. J Ultrastruct Res. 1973 Jun;43(5):388–411. doi: 10.1016/s0022-5320(73)90017-8. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- SEDAR A. W., FORTE J. G. EFFECTS OF CALCIUM DEPLETION ON THE JUNCTIONAL COMPLEX BETWEEN OXYNTIC CELLS OF GASTRIC GLANDS. J Cell Biol. 1964 Jul;22:173–188. doi: 10.1083/jcb.22.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahouny G. V., Wei R., Starkweather R., Davis C. Preparation of beating heart cells from adult rats. Science. 1970 Mar 20;167(3925):1616–1618. doi: 10.1126/science.167.3925.1616. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]