Abstract
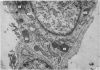
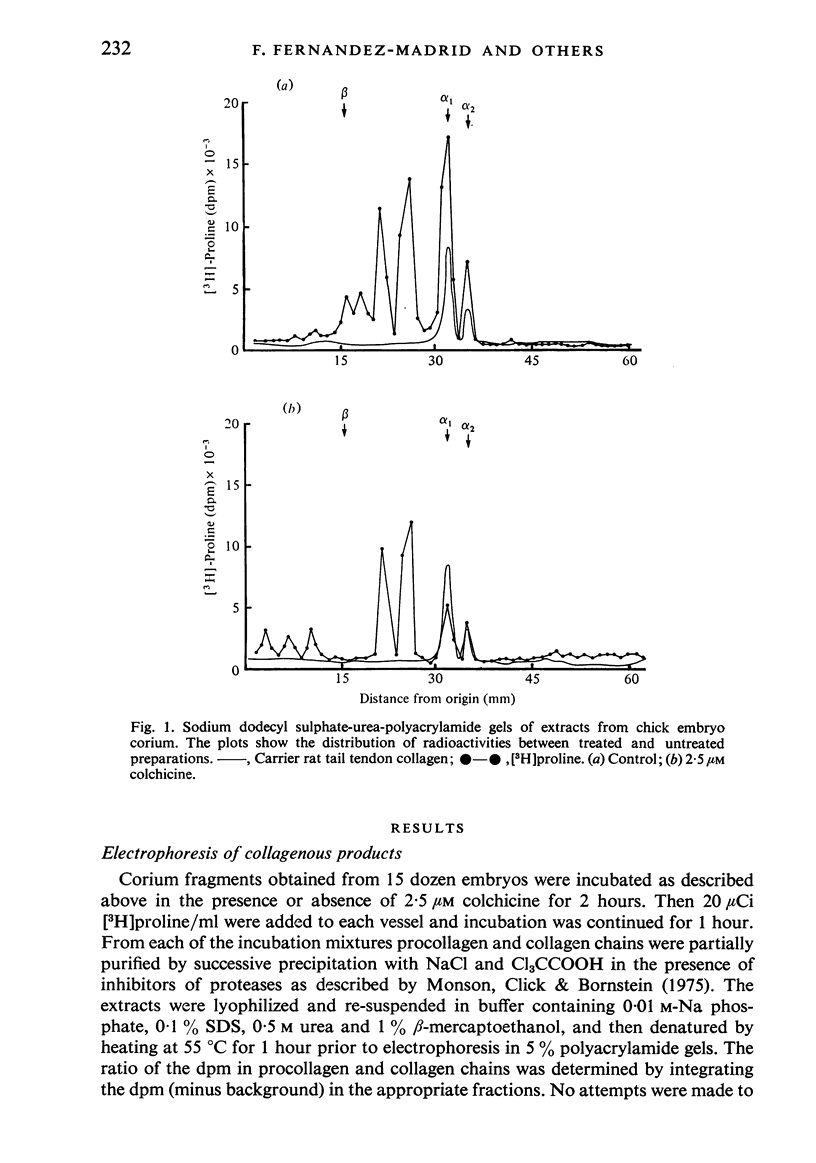
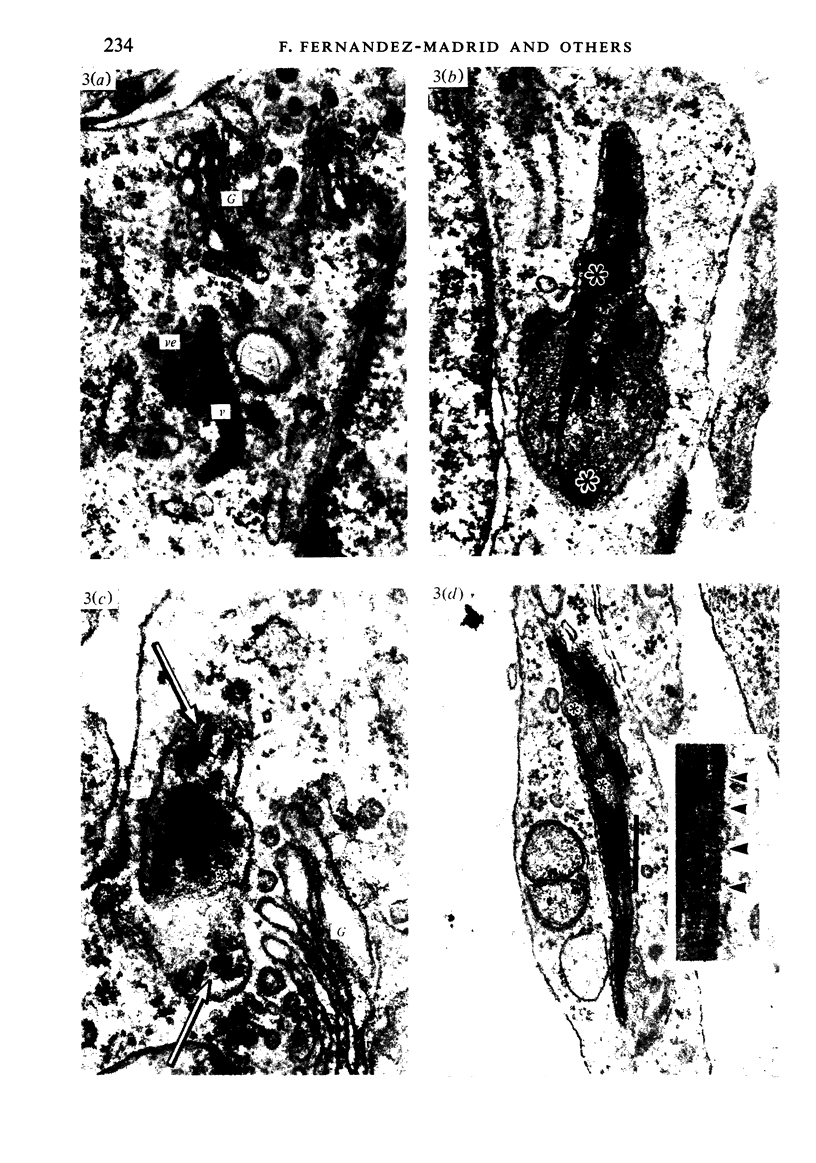
Corium fragments incubated in vitro with colchicine or obtained from chick embryos 11 days old after in vivo injection of this alkaloid were studied with the electron microscope. The well known effect of colchicine in producing an accumulation of procollagen was demonstrated by electrophoretic separation of the collagenous products in polyacrylamide gels after incubation of corium fragments in vitro with labelled amino acids. Electron microscopical studies showed various types of cytoplasmic vacuoles containing collagenous fibrillar structures. Some of these vacuoles contained non-striated filaments, fibres with the periodicity of native collagen, and segment long spacing (SLS) crystallites, embedded in an electrondense substance. The central aspect of this communication is the finding of intracellular SLS in cytoplasmic vacuoles. These crystallites were morphologically intact, and had no additional bands at the NH2 or COOH ends, suggesting that they were formed by the aggregation of tropocollagen molecules. Since the presence of intact SLS in cytoplasmic vacuoles suggests that these are secretory, we believe that inhibition of the transcellular transport system by colchicine permitted the visualization of vacuoles containing the products of tropocollagen interconversions. We interpret these findings as an example of autophagocytosis, with intracellular processing of procollagen, occurring in chick embryo fibroblasts when the secretion of procollagen is inhibited by colchicine.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Cox R. W., Grant R. A., Horne R. W. The structure and assembly of collagen fibrils. I. Native-collagen fibrils and their formation from tropocollagen. J R Microsc Soc. 1967;87(1):123–142. doi: 10.1111/j.1365-2818.1967.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Intermediates in the limited proteolytic conversion of procollagen to collagen. Biochemistry. 1975 Nov 18;14(23):5188–5194. doi: 10.1021/bi00694a026. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972 Aug 30;238(87):257–260. doi: 10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Ross R., Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974 Aug;62(2):390–405. doi: 10.1083/jcb.62.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit H., Barondes S. H. Colchicine-binding activity in particulate fractions of mouse brain. J Neurochem. 1970 Sep;17(9):1355–1364. doi: 10.1111/j.1471-4159.1970.tb06870.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Madrid F. Biochemical and physicochemical characterization of collagen-synthesizing polyribosomes. J Cell Biol. 1967 Apr;33(1):27–42. doi: 10.1083/jcb.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler L. I., Morris N. P., Fessler J. H. Procollagen: biological scission of amino and carboxyl extension peptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4905–4909. doi: 10.1073/pnas.72.12.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. M. Etude autoradiographique de la dentinogenese en microscopie electronique a l'aide de la proline tritiee chez le chat. Arch Oral Biol. 1970 Jul;15(7):583–596. doi: 10.1016/0003-9969(70)90128-7. [DOI] [PubMed] [Google Scholar]
- GROSS J. The behavior of collagen units as a model in morphogenesis. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):261–274. doi: 10.1083/jcb.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand A. R. The effects of acute starvation on parotid acinar cells. Ultrastructural and cytochemical observations on ad libitum-fed and starved rats. Am J Anat. 1972 Sep;135(1):71–92. doi: 10.1002/aja.1001350107. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The sub-cellular location of inter-chain disulfide bond formation during procollagen biosynthesis by embryonic chick tendon cells. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1188–1196. doi: 10.1016/s0006-291x(73)80020-8. [DOI] [PubMed] [Google Scholar]
- Hay E. D., Dodson J. W. Secretion of collagen by corneal epithelium. I. Morphology of the collagenous products produced by isolated epithelia grown on frozen-killed lens. J Cell Biol. 1973 Apr;57(1):190–213. doi: 10.1083/jcb.57.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindelang-Gertner C., Stoeckel M. E., Porte A., Stutinsky F. Colchicine effects on neurosecretory neurons and other hypothalamic and hypophysial cells, with special reference to changes in the cytoplasmic membranes. Cell Tissue Res. 1976 Jul 20;170(1):17–41. doi: 10.1007/BF00220108. [DOI] [PubMed] [Google Scholar]
- Hirsimäki Y., Arstila A. U., Trump B. F. Autophagocytosis: in vitro induction by microtuble poisons. Exp Cell Res. 1975 Apr;92(1):11–14. doi: 10.1016/0014-4827(75)90630-8. [DOI] [PubMed] [Google Scholar]
- Imura S., Tanaka S., Takase B. Intracytoplasmic segment long spacing fibrils in chondrosarcoma. J Electron Microsc (Tokyo) 1975;24(2):87–95. [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. Effects of colchicine and chloramphenicol on the oxidative metabolism and phagocytic activity of human neutrophils. J Infect Dis. 1973 Jan;127(1):40–48. doi: 10.1093/infdis/127.1.40. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. The fine structure of connective tissue. I. The fibroblast. Exp Mol Pathol. 1962 Dec;1:509–534. doi: 10.1016/0014-4800(62)90040-0. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Bensch K. G. Human polymorphonuclear leukocytes: demonstration of microtubules and effect of colchicine. Science. 1967 Apr 28;156(3774):521–522. doi: 10.1126/science.156.3774.521. [DOI] [PubMed] [Google Scholar]
- Nist C., Von Der Mark K., Hay E. D., Olsen B. R., Bornstein P., Ross R., Dehm P. Location of procollagen in chick corneal and tendon fibroblasts with ferritin-conjugated antibodies. J Cell Biol. 1975 Apr;65(1):75–87. doi: 10.1083/jcb.65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble N. L., Boucek R. J. Incorporation of sulphate by chick-embryo corium. Nature and products of the process in vitro. Biochem J. 1965 Nov;97(2):432–439. doi: 10.1042/bj0970432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Prockop D. J. Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc Natl Acad Sci U S A. 1974 May;71(5):2033–2037. doi: 10.1073/pnas.71.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pérez-Tamayo R. Collagen resorption in carrageenin granulomas. II. Ultrastructure of collagen resorption. Lab Invest. 1970 Feb;22(2):142–159. [PubMed] [Google Scholar]
- REVEL J. P., HAY E. D. AN AUTORADIOGRAPHIC AND ELECTRON MICROSCOPIC STUDY OF COLLAGEN SYNTHESIS IN DIFFERENTIATING CARTILAGE. Z Zellforsch Mikrosk Anat. 1963 Oct 8;61:110–144. doi: 10.1007/BF00341524. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., MILLER F., BARRNETT R. J. ALDEHYDE FIXATION FOR MORPHOLOGICAL AND ENZYME HISTOCHEMICAL STUDIES WITH THE ELECTRON MICROSCOPE. J Histochem Cytochem. 1964 Feb;12:57–71. doi: 10.1177/12.2.57. [DOI] [PubMed] [Google Scholar]
- SHELDON H., KIMBALL F. B. Studies on cartilage. III. The occurrence of collagen within vacuoles of the golgi apparatus. J Cell Biol. 1962 Mar;12:599–613. doi: 10.1083/jcb.12.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherft J. P., Heersche J. N. Accumulation of collagen-containing vacuoles in osteoblasts after administration of colchicine. Cell Tissue Res. 1975;157(3):353–365. doi: 10.1007/BF00225526. [DOI] [PubMed] [Google Scholar]
- Schmitt F. O., Gross J., Highberger J. H. A New Particle Type in Certain Connective Tissue Extracts. Proc Natl Acad Sci U S A. 1953 Jun;39(6):459–470. doi: 10.1073/pnas.39.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer M. L., Church R. L., Yaeger J. A., Wampler D. E., Park E. Procollagen: intermediate forms containing several types of peptide chains and non-collagen peptide extensions at NH2 and COOH ends. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3009–3013. doi: 10.1073/pnas.71.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. Vacuoles in the embryonic chick corneal epithelium, an epithelium which produces collagen. J Cell Biol. 1971 Mar;48(3):689–694. doi: 10.1083/jcb.48.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Domnina L. V., Ivanova O. Y., Komm S. G., Olshevskaja L. V. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970 Nov;24(3):625–640. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinstock M. Collagen formation--observations on its intracellular packaging and transport. Z Zellforsch Mikrosk Anat. 1972;129(4):455–470. doi: 10.1007/BF00316743. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after (3H)proline administration. J Cell Biol. 1974 Jan;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Possible role of microtubules in thyroid secretion. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1901–1908. doi: 10.1073/pnas.67.4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]