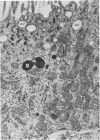
Abstract
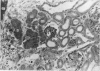
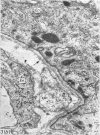
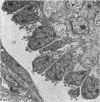
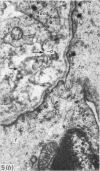
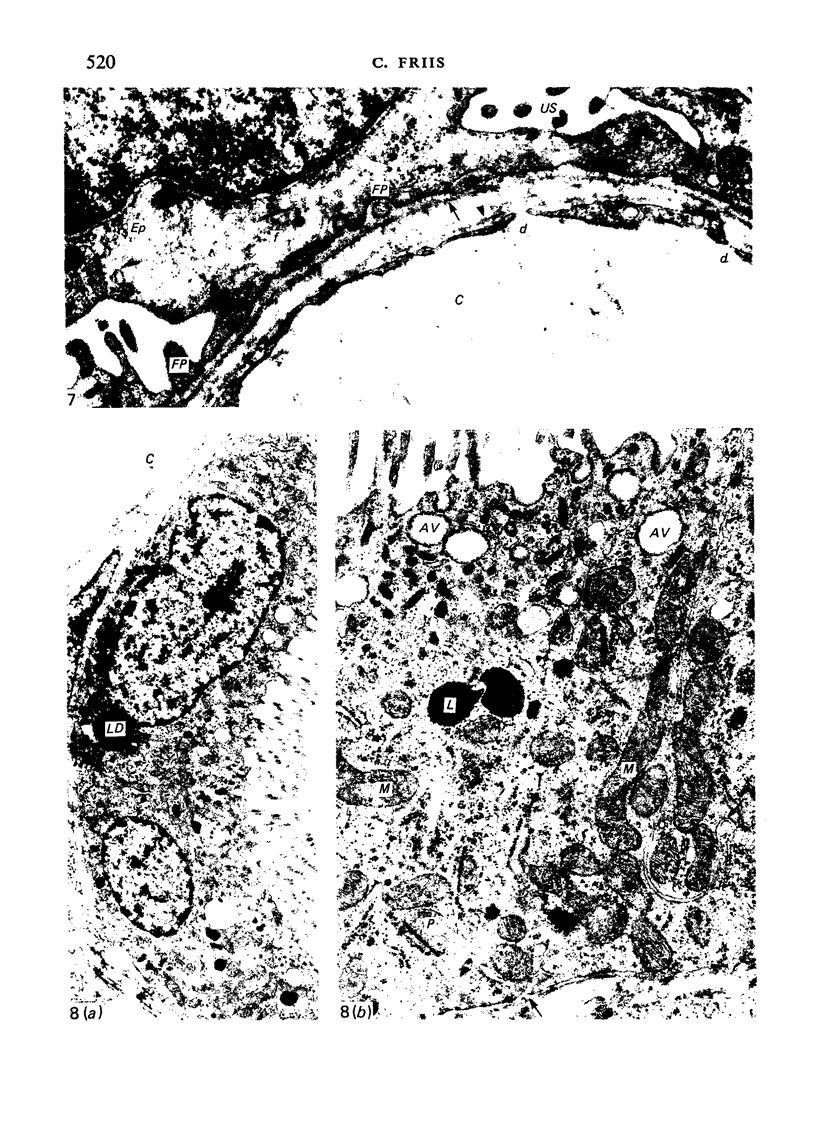
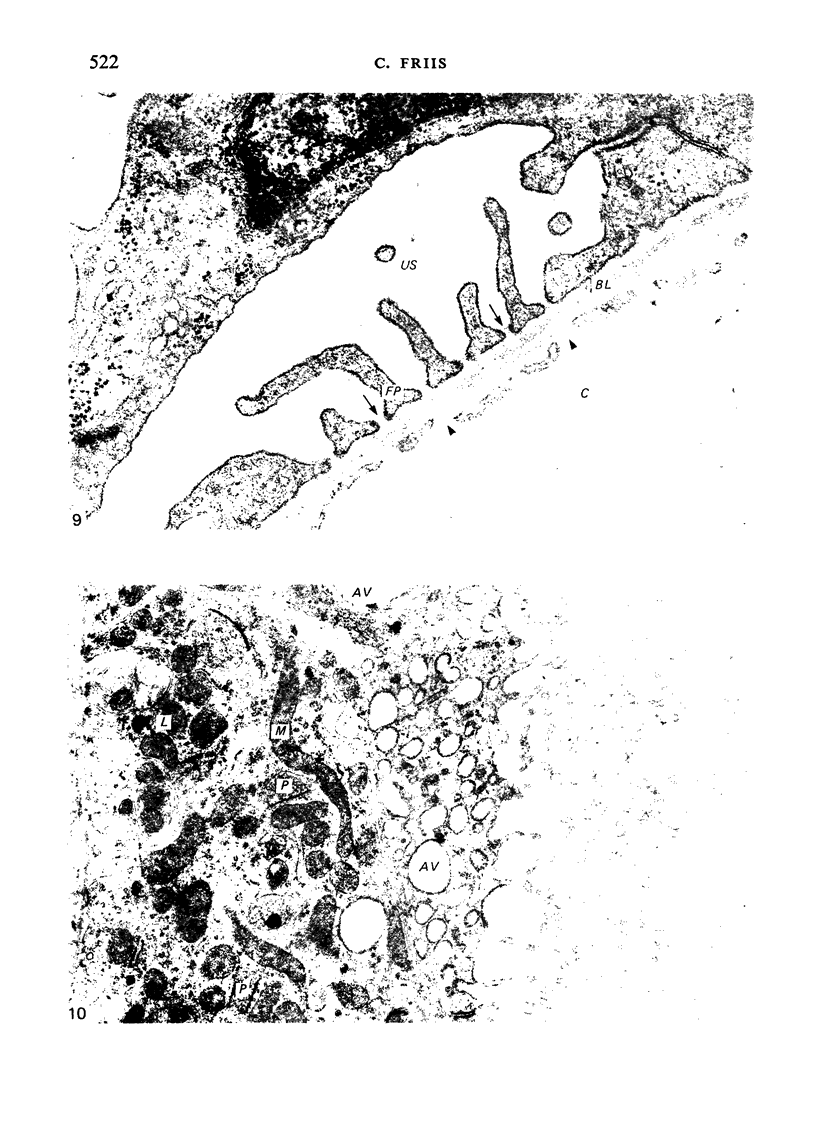
The detailed anatomy of the structures forming the glomerular filtration barrier and the proximal tubule was studied during postnatal development of the pig kidney. Development of the glomerular filtration barrier included three processes: Formation of visceral epithelial foot processes, flattening and fenestration of the endothelium and formation of a common epithelial and endothelial basal lamina. During formation of foot processes the distribution pattern of aggregated filaments in the epithelial cells was changed, suggesting that the filaments participate in the elongation of the foot processes. In the immature glomerulus the endothelial fenestrae were closed by thin diaphragms which disappeared during differentiation of the endothelium. Microvilli were observed in the proximal tubule cells in an early developmental stage, presumably before onset of glomerular filtration. Few cellular organelles were seen in the tubule anlage but the number of mitochondria, apical vacuoles and lysosome-like bodies increased markedly during maturation. Dense bodies identified as peroxisomes first appeared in a late development stage. The formation of nephrons continued up to about 3 weeks of age; after this time the morphological development was a differentiation of nephrons already present. The present observations, together with results of an accompanying functional study, demonstrate that changes in functional parameters reflect the structural development.
Full text
PDF
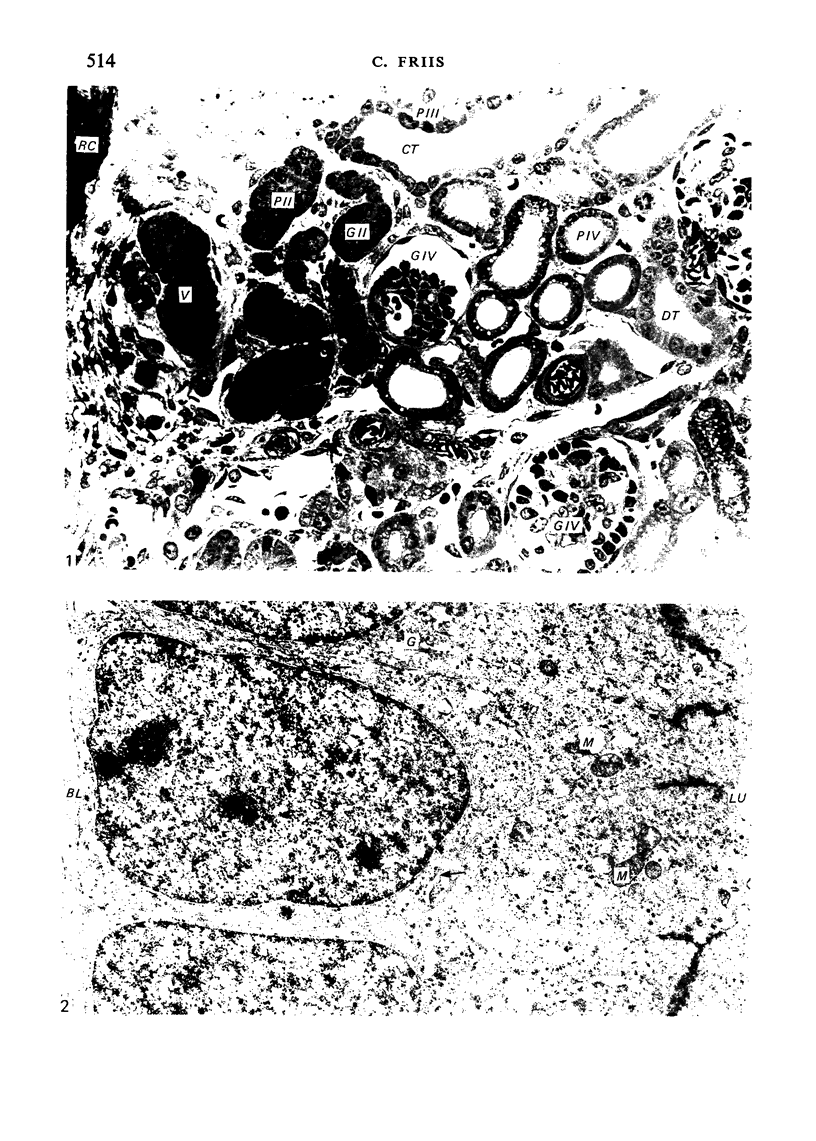

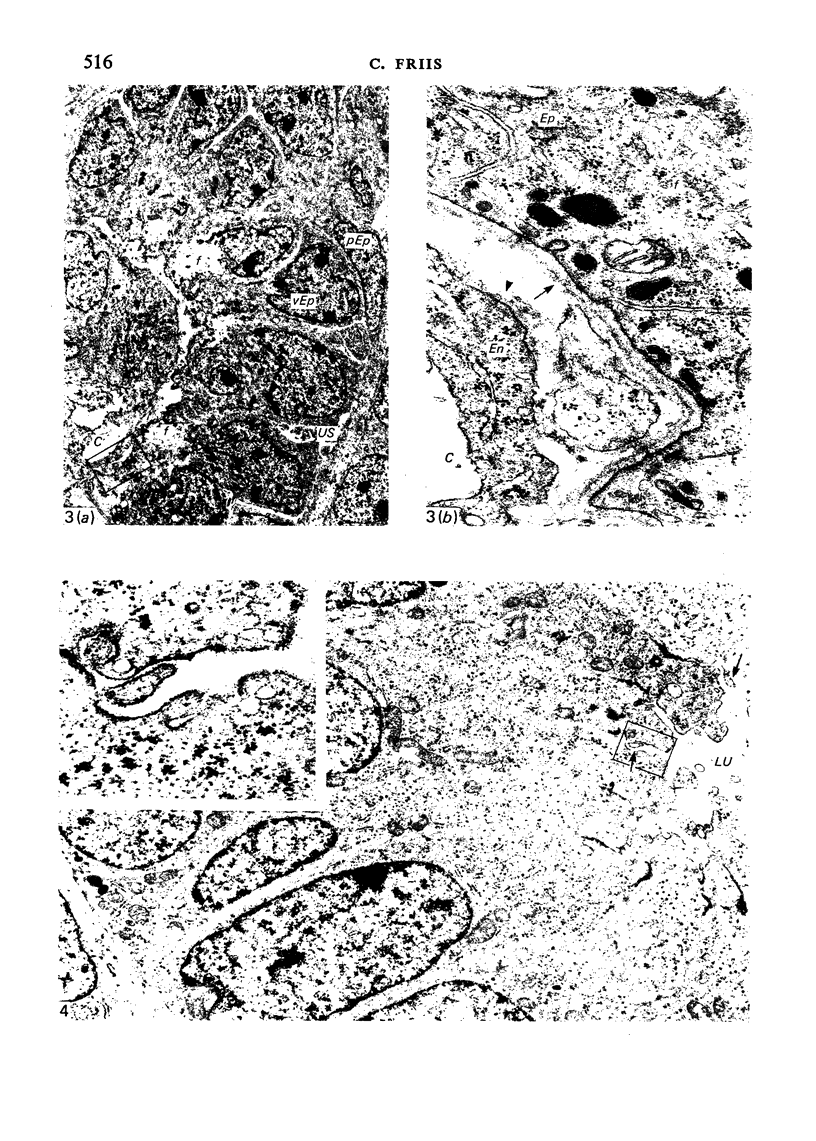

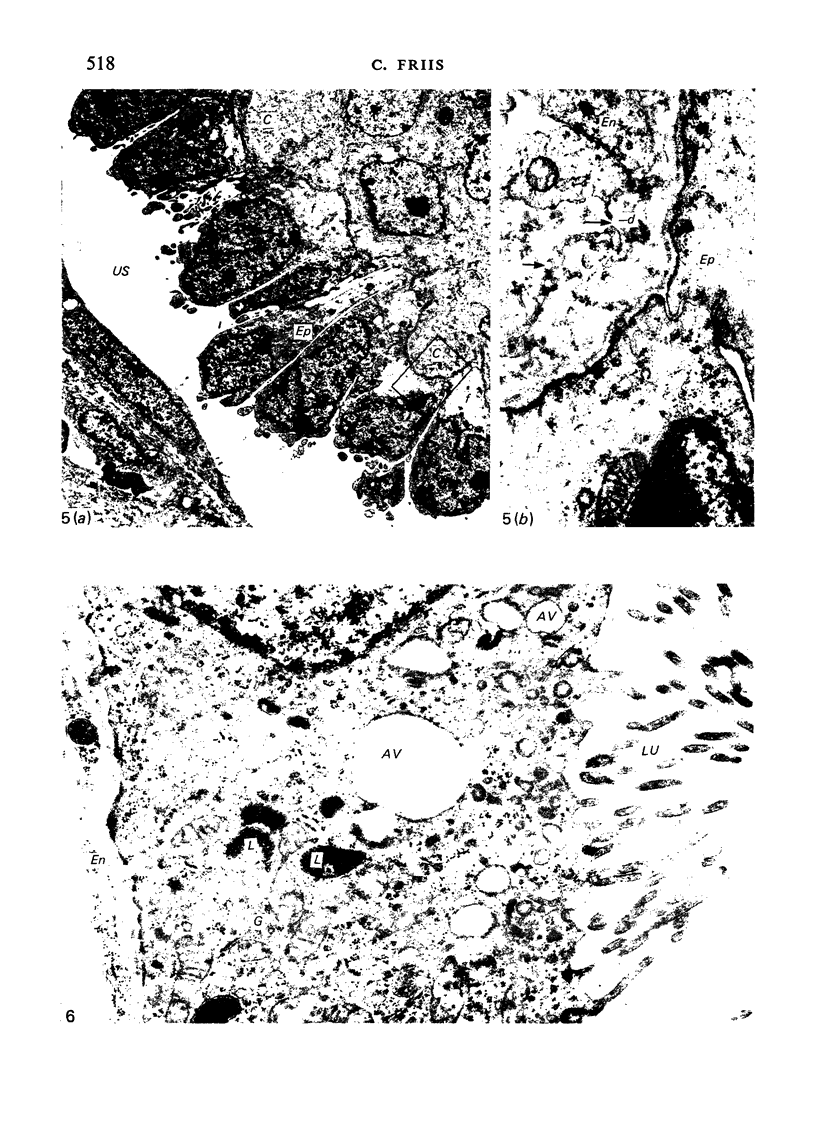






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki A. Development of the human renal glomerulus. I. Differentiation of the filtering membrane. Anat Rec. 1966 Jul;155(3):339–351. doi: 10.1002/ar.1091550307. [DOI] [PubMed] [Google Scholar]
- Arturson G., Groth T., Grotte G. Human glomerular membrane porosity and filtration pressure: dextran clearance data analysed by theoretical models. Clin Sci. 1971 Feb;40(2):137–158. doi: 10.1042/cs0400137. [DOI] [PubMed] [Google Scholar]
- Bergelin I. S., Karlsson B. W. Functional structure of the glomerular filtration barrier and the proximal tubuli in the developing foetal and neonatal pig kidney. Anat Embryol (Berl) 1975 Dec 31;148(3):223–234. doi: 10.1007/BF00319845. [DOI] [PubMed] [Google Scholar]
- Elling F., Hasselager E., Friis C. Perfusion fixation of kidneys in adult pigs for electron microscopy. Acta Anat (Basel) 1977;98(3):340–342. doi: 10.1159/000144810. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., WISSIG S. L., PALADE G. E. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med. 1961 Jan 1;113:47–66. doi: 10.1084/jem.113.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Editorial: The primary glomerular filtration barrier--basement membrane or epithelial slits? Kidney Int. 1975 Oct;8(4):197–211. doi: 10.1038/ki.1975.103. [DOI] [PubMed] [Google Scholar]
- Friis C. Postnatal development of renal function in piglets: glomerular filtration rate, clearance of PAH and PAH extraction. Biol Neonate. 1979;35(3-4):180–187. doi: 10.1159/000241170. [DOI] [PubMed] [Google Scholar]
- Hay D. A., Evan A. P. Maturation of the glomerular visceral epithelium and capillary endothelium in the puppy kidney. Anat Rec. 1979 Jan;193(1):1–21. doi: 10.1002/ar.1091930102. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Fitzpatrick T. B. Changes in distribution pattern of cytoplasmic filaments in human melanocytes during ultraviolet-mediated melanin pigmentation. The role of the 100-Angstrom filaments in the elongation of melanocytic dendrites and in the movement and transfer of melanosomes. J Cell Biol. 1975 May;65(2):481–488. doi: 10.1083/jcb.65.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. Effects of different fixatives on the ultrastructure of the developing proximal tubule in the rat kidney. J Ultrastruct Res. 1975 Apr;51(1):140–151. doi: 10.1016/s0022-5320(75)80014-1. [DOI] [PubMed] [Google Scholar]
- Larsson L., Maunsbach A. B. Differentiation of the vacuolar apparatus in cells of the developing proximal tubule in the rat kidney. J Ultrastruct Res. 1975 Nov;53(2):254–270. doi: 10.1016/s0022-5320(75)80142-0. [DOI] [PubMed] [Google Scholar]
- Larsson L. The ultrastructure of the developing proximal tubule in the rat kidney. J Ultrastruct Res. 1975 Apr;51(1):119–139. doi: 10.1016/s0022-5320(75)80013-x. [DOI] [PubMed] [Google Scholar]
- Larsson L. Ultrastructure and permeability of intercellular contacts of developing proximal tubule in rat kidney. J Ultrastruct Res. 1975 Jul;52(1):100–113. doi: 10.1016/s0022-5320(75)80025-6. [DOI] [PubMed] [Google Scholar]
- Loggie J. M., Kleinman L. I., Van Maanen E. F. Renal function and diuretic therapy in infants and children. Part I. J Pediatr. 1975 Apr;86(4):485–496. doi: 10.1016/s0022-3476(75)80136-3. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Lentz T. L. Microfilaments in epidermal cancer cells. J Cell Biol. 1974 Feb;60(2):473–482. doi: 10.1083/jcb.60.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C., Holmes R. Peroxisomes: new aspects of cell physiology and biochemistry. Physiol Rev. 1977 Oct;57(4):816–882. doi: 10.1152/physrev.1977.57.4.816. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Miyoshi M., Fujita T., Tokunaga J. The differentiation of renal podocytes. A combined scanning and transmission electron microscope study in rats. Arch Histol Jpn. 1971 Jun;33(2):161–178. doi: 10.1679/aohc1950.33.161. [DOI] [PubMed] [Google Scholar]
- RHODIN J. A. The diaphragm of capillary endothelial fenestrations. J Ultrastruct Res. 1962 Apr;6:171–185. doi: 10.1016/s0022-5320(62)90052-7. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Axline S. G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973 Oct;59(1):12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNIER R. L., BIRCH-ANDERSEN A. Studies of the human fetal kidney. I. Development of the glomerulus. J Pediatr. 1962 May;60:754–768. doi: 10.1016/s0022-3476(62)80103-6. [DOI] [PubMed] [Google Scholar]
- VERNIER R. L., BIRCH-ANDERSEN A. Studies of the human fetal kidney. II. Permeability characteristics of the developing glomerulus. J Ultrastruct Res. 1963 Feb;8:66–88. doi: 10.1016/s0022-5320(63)80021-0. [DOI] [PubMed] [Google Scholar]
- Webber W. A., Blackbourn J. The permeability of the immature glomerulus to large molecules. Lab Invest. 1970 Jul;23(1):1–7. [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nakamura M. Light and electron microscopy on the proximal convoluted tubules during the postnatal development. Okajimas Folia Anat Jpn. 1965 Aug;41(2):121–157. doi: 10.2535/ofaj1936.41.2-3_121. [DOI] [PubMed] [Google Scholar]