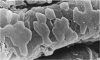
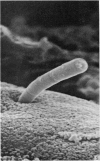
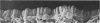Abstract
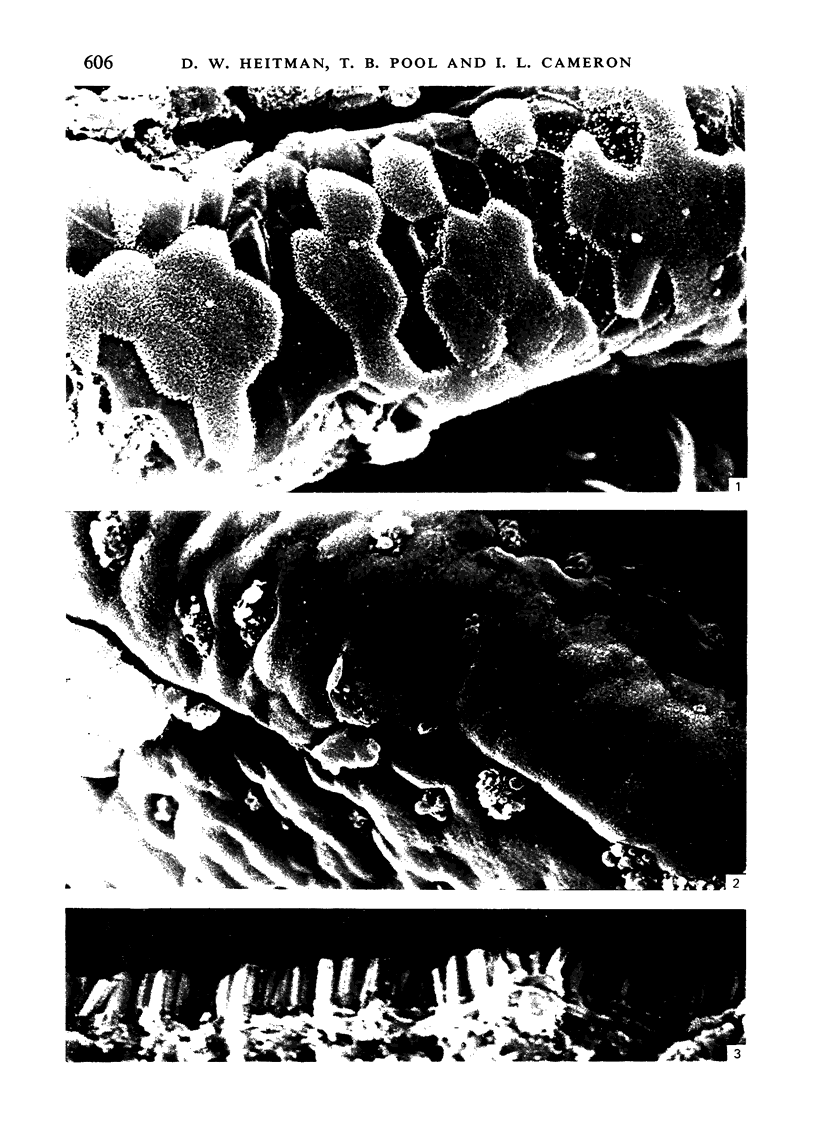
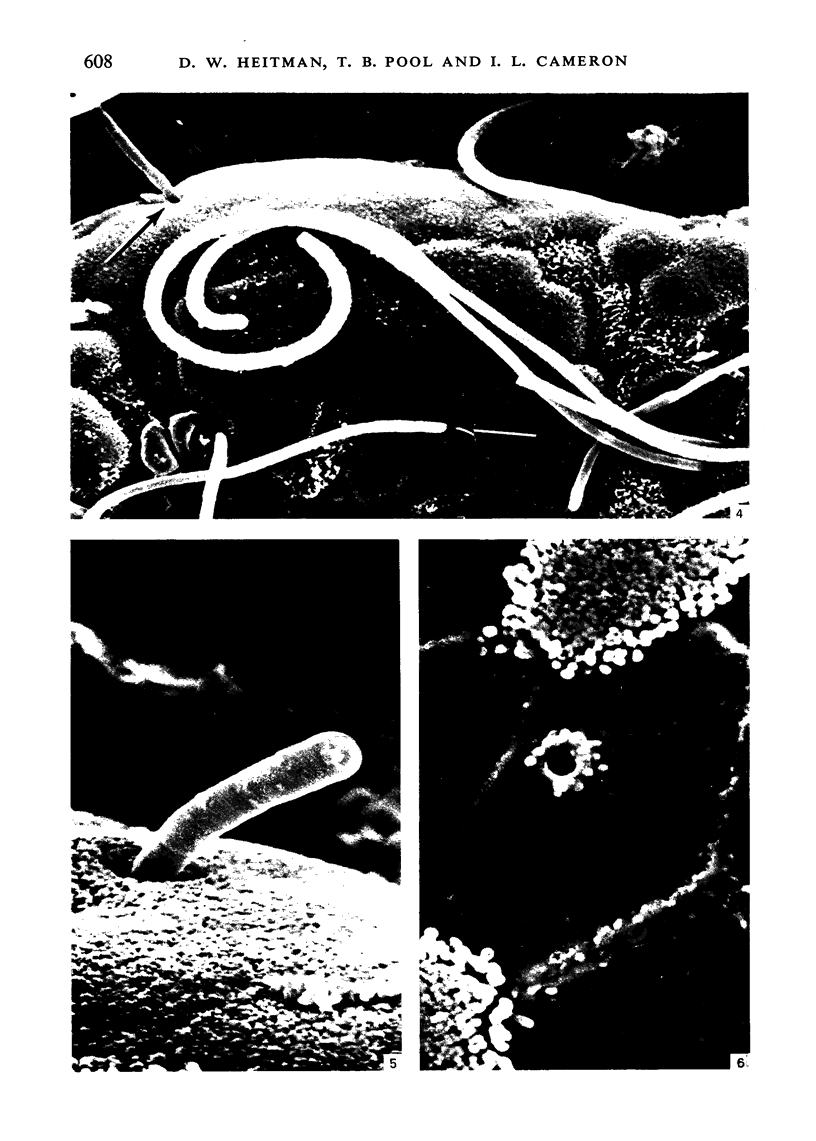
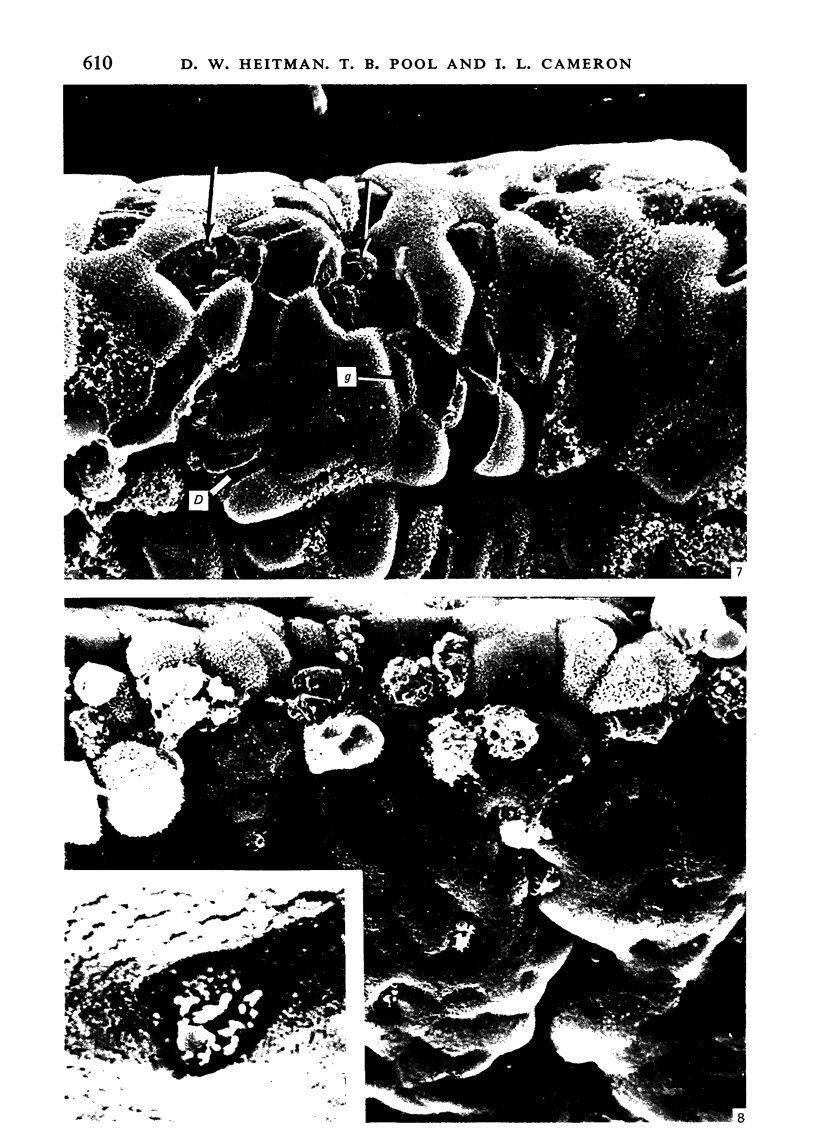
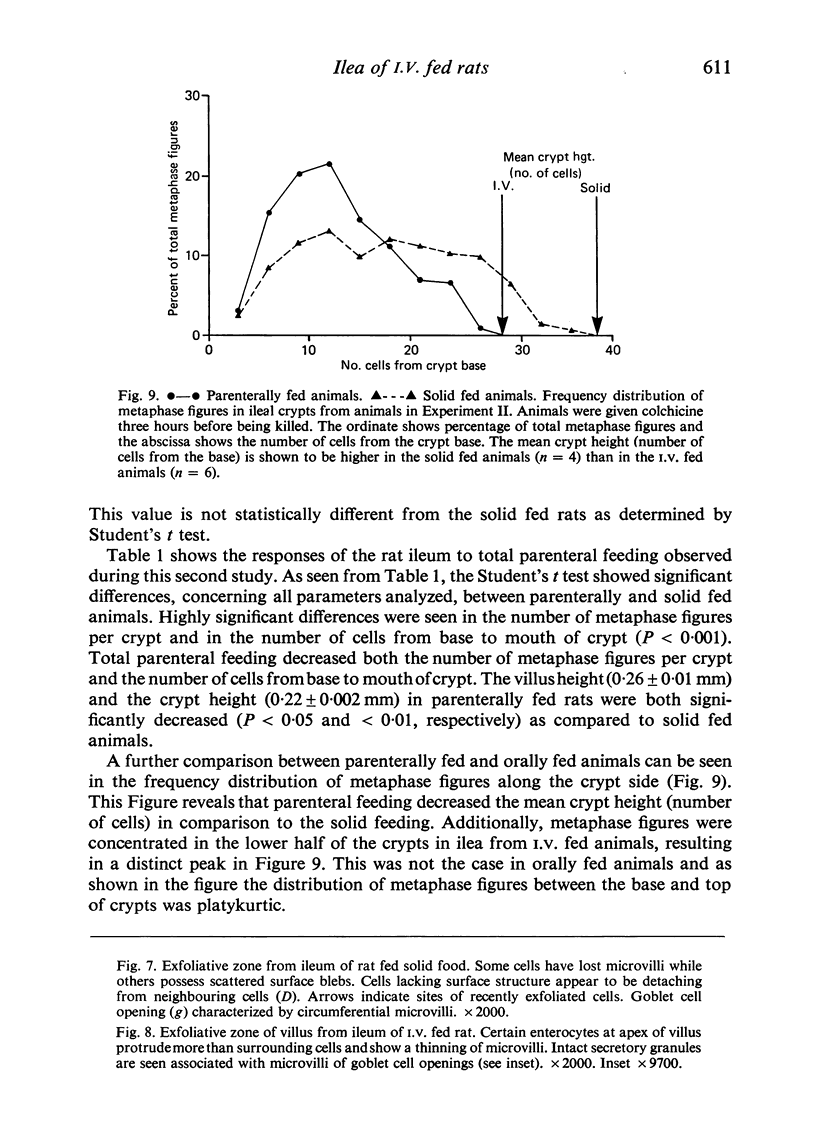
To find out how the ileum adapts to total parenteral feeding, two experiments were performed. In the first experiment rats were given total intravenous feeding for 10 days. The animals were injected with tritiated thymidine (1 muCi/g body weight) 1 hour before being killed. Portions of the ileum were used for (1) radioautography, (2) analysis of the tissue DNA content, (3) specific activity of the DNA, and (4) scanning electron microscopy. The DNA content of ileum was decreased 72% while the specific activity of DNA was increased 289% in the i.v. fed rats. In the second experiment rats were given total intravenous feeding for 10 days. The animal were injected with colchicine (1 mg/kg body weight) 3 hours before being killed. The number of labelled cell nuclei per ileal crypt section was significantly decreased by parenteral feeding as was the number of colchicine collected metaphase figures. Light microscopy revealed the crypt and the villus height to be shorter and the number of goblet cells per unit surface area to be increased in parenterally fed rats as compared to those fed solid food orally. Enterocytes of the exfoliative zone from the ileal villi of rats fed solid food showed three distinct types of surface architecture whereas those from i.v. fed rats all possessed abundant microvilli. No bacteria were seen in ilea of i.v. fed animals but many were seen embedded in enterocytes from orally fed rats. Because the amount of DNA per cell is known to be constant, we concluded that the overall number of cells in the lieum decreased about 72% in the i.v. fed rats and that cell proliferation in the crypts, although significantly deceased, was still supporting an epithelial renewal process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. The relation of size to the relative rates of degradation of intestinal brush border proteins. J Clin Invest. 1972 Oct;51(10):2621–2630. doi: 10.1172/JCI107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann G. G., Enesco M. Cell number as a measure of distribution and renewal of epithelial cells in the small intestine of growing and adult rats. Am J Anat. 1967 Sep;121(2):319–336. doi: 10.1002/aja.1001210210. [DOI] [PubMed] [Google Scholar]
- Altmann G. G. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. Am J Anat. 1971 Oct;132(2):167–177. doi: 10.1002/aja.1001320204. [DOI] [PubMed] [Google Scholar]
- Altmann G. G. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat. 1972 Apr;133(4):391–400. doi: 10.1002/aja.1001330403. [DOI] [PubMed] [Google Scholar]
- Altmann G. G., Leblond C. P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am J Anat. 1970 Jan;127(1):15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- Altmann G. G. Morphological effects of a large single dose of cycloheximide on the intestinal epithelium of the rat. Am J Anat. 1975 Jun;143(2):219–239. doi: 10.1002/aja.1001430205. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I. L., Pavlat W. A., Stevens M. D., Rogers W. Tumor-host responses to various nutritional feeding procedures in rats. J Nutr. 1979 Apr;109(4):671–684. doi: 10.1093/jn/109.4.671. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Pavlat W. A., Urban E. Adaptive responses to total intravenous feeding. J Surg Res. 1974 Jul;17(1):45–52. doi: 10.1016/0022-4804(74)90167-x. [DOI] [PubMed] [Google Scholar]
- Clarke R. M., Ecknauer R., Feyerabend G. Analysis of the effects of food and of digestive secretions on the small intestine of the rat. 1. Mucosal morphology and epithelial replacement. Gut. 1976 Nov;17(11):895–899. doi: 10.1136/gut.17.11.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. The time-course of changes in mucosal architecture and epithelial cell production and cell shedding in the small intestine of the rat fed after fasting. J Anat. 1975 Nov;120(Pt 2):321–327. [PMC free article] [PubMed] [Google Scholar]
- Feldman E. J., Dowling R. H., McNaughton J., Peters T. J. Effects of oral versus intravenous nutrition on intestinal adaptation after small bowel resection in the dog. Gastroenterology. 1976 May;70(5 PT1):712–719. [PubMed] [Google Scholar]
- Hamilton R. F., Davis W. C., Stephenson D. V., Magee D. F. Effects of parenteral hyperalimentation on upper gastrointestinal tract secretions. Arch Surg. 1971 Apr;102(4):348–352. doi: 10.1001/archsurg.1971.01350040110022. [DOI] [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. R., Copeland E. M., Dudrick S. J., Lichtenberger L. M., Castro G. A. Structural and hormonal alterations in the gastrointestinal tract of parenterally fed rats. Gastroenterology. 1975 May;68(5 Pt 1):1177–1183. [PubMed] [Google Scholar]
- LIPKIN M., QUASTLER H. Studies of protein metabolism in intestinal epithelial cells. J Clin Invest. 1962 Mar;41:646–653. doi: 10.1172/JCI104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine G. M., Deren J. J., Yezdimir E. Small-bowel resection. Oral intake is the stimulus for hyperplasia. Am J Dig Dis. 1976 Jul;21(7):542–546. doi: 10.1007/BF01464760. [DOI] [PubMed] [Google Scholar]
- McManus J. P., Isselbacher K. J. Effect of fasting versus feeding on the rat small intestine. Morphological, biochemical, and functional differences. Gastroenterology. 1970 Aug;59(2):214–221. [PubMed] [Google Scholar]
- Nakajima S., Magee D. F. Inhibition of exocrine pancreatic secretion by glucagon and D-glucose given intravenously. Can J Physiol Pharmacol. 1970 May;48(5):299–305. doi: 10.1139/y70-049. [DOI] [PubMed] [Google Scholar]
- Pavlat W. A., Rogers W., Cameron I. L. Morphometric analysis of pancreatic acinar cells from orally fed and intravenously fed rats. J Surg Res. 1975 Oct;19(4):267–276. doi: 10.1016/0022-4804(75)90091-8. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977 Aug;60(2):272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- Steiger E., Oram-Smith J., Miller E., Kuo L., Vars H. M. Effects of nutrition on tumor growth and tolerance to chemotherapy. J Surg Res. 1975 Apr;18(4):455–466. doi: 10.1016/0022-4804(75)90109-2. [DOI] [PubMed] [Google Scholar]
- Steiger E., Vars H. M., Dudrick S. J. A technique for long-term intravenous feeding in unrestrained rats. Arch Surg. 1972 Mar;104(3):330–332. doi: 10.1001/archsurg.1972.04180030076017. [DOI] [PubMed] [Google Scholar]
- Towne J. B., Hamilton R. F., Stephenson D. V., Jr Mechanism of hyperalimentation in the suppression of upper gastrointestinal secretions. Am J Surg. 1973 Dec;126(6):714–716. doi: 10.1016/s0002-9610(73)80055-8. [DOI] [PubMed] [Google Scholar]
- Tutton P. J. Control of epithelial cell proliferation in the small intestinal crypt. Cell Tissue Kinet. 1973 May;6(3):211–216. doi: 10.1111/j.1365-2184.1973.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Williams R. P., Cameron I. L., Adrian E. K. Effects of intestinally absorbed thymidine on tritiated thymidine utilization. Cell Tissue Kinet. 1979 Jul;12(4):405–410. doi: 10.1111/j.1365-2184.1979.tb00163.x. [DOI] [PubMed] [Google Scholar]