Abstract
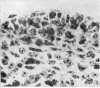
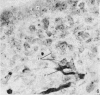
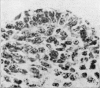
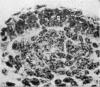
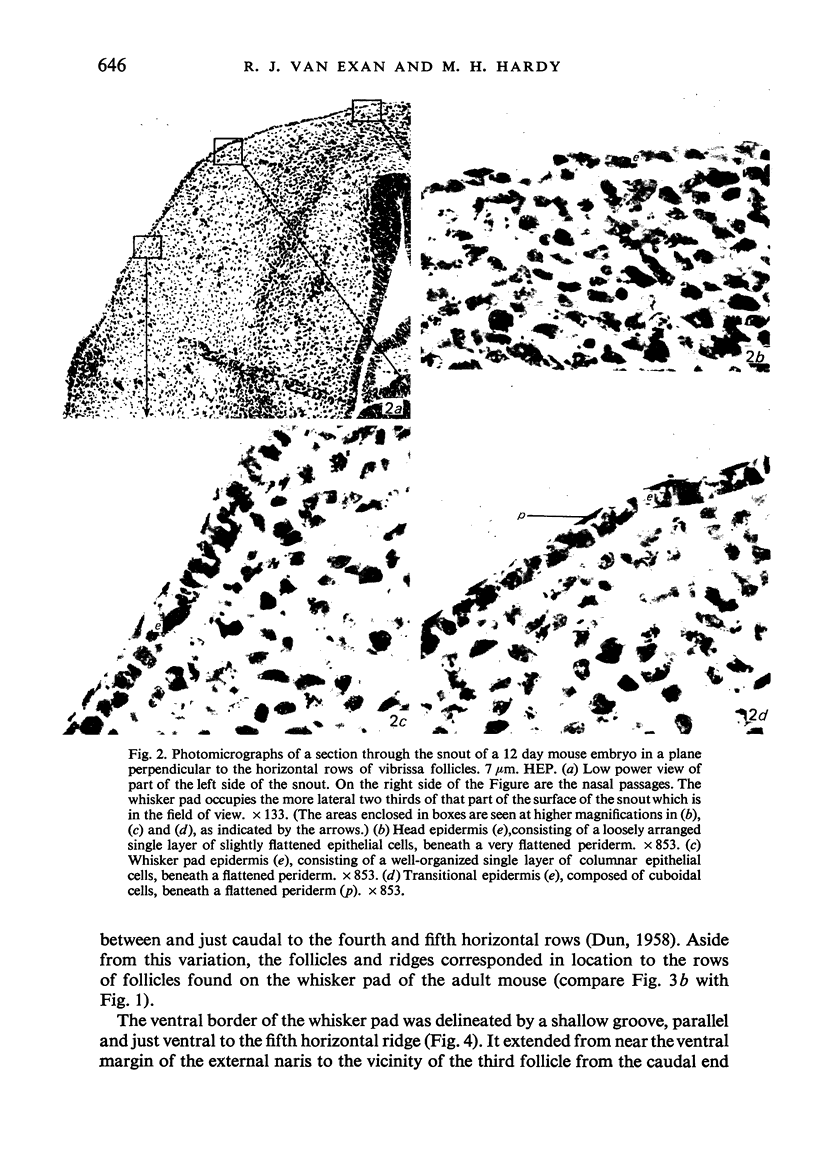
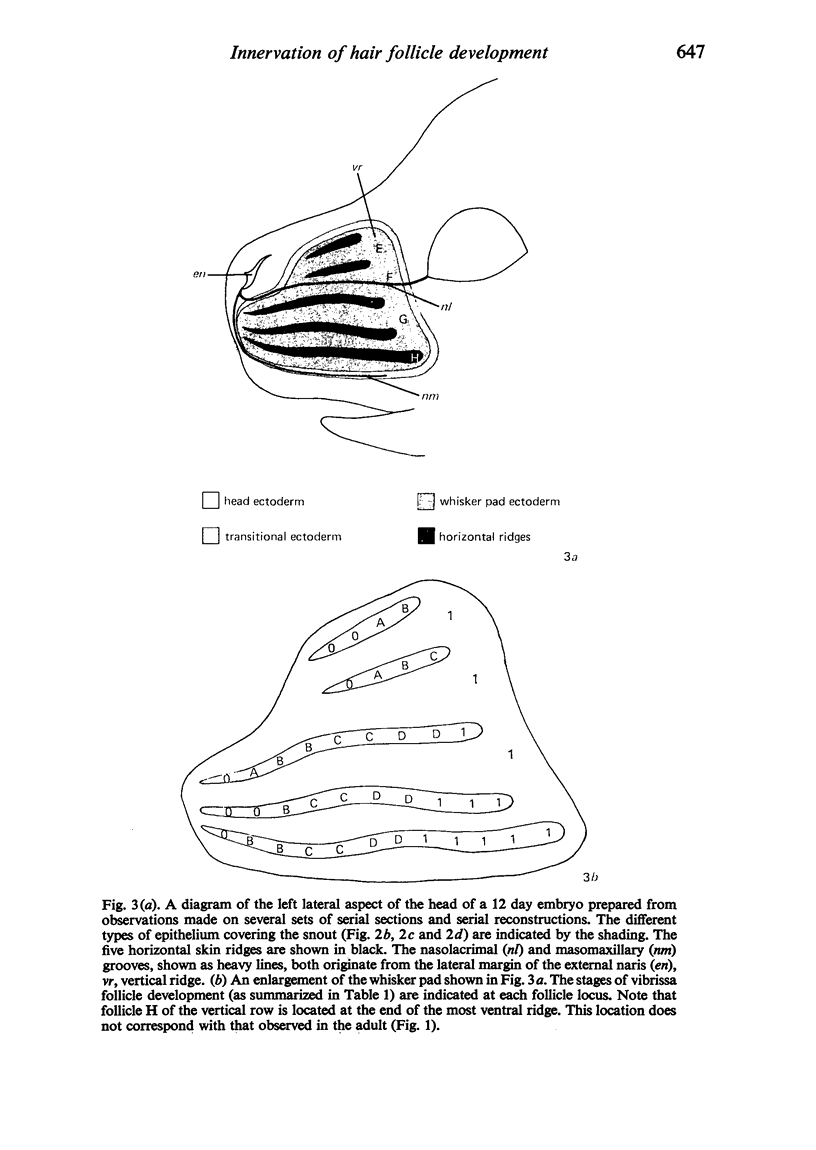
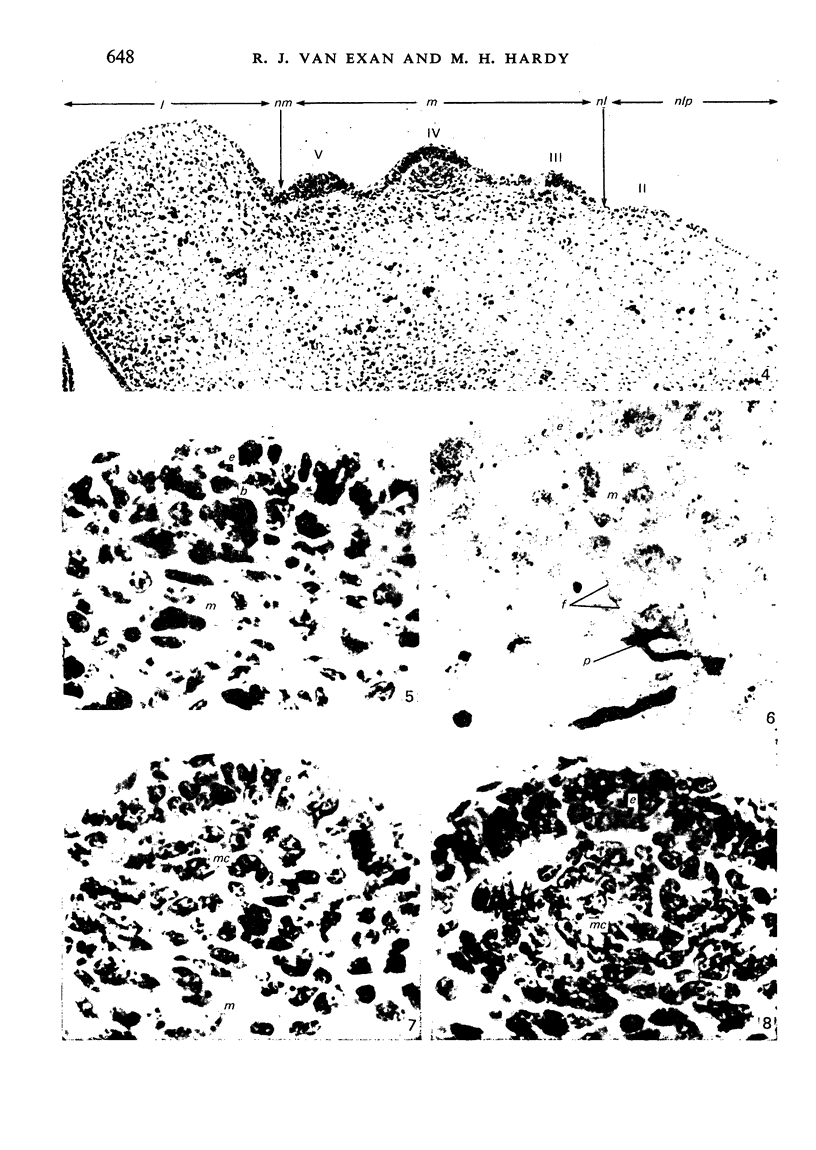
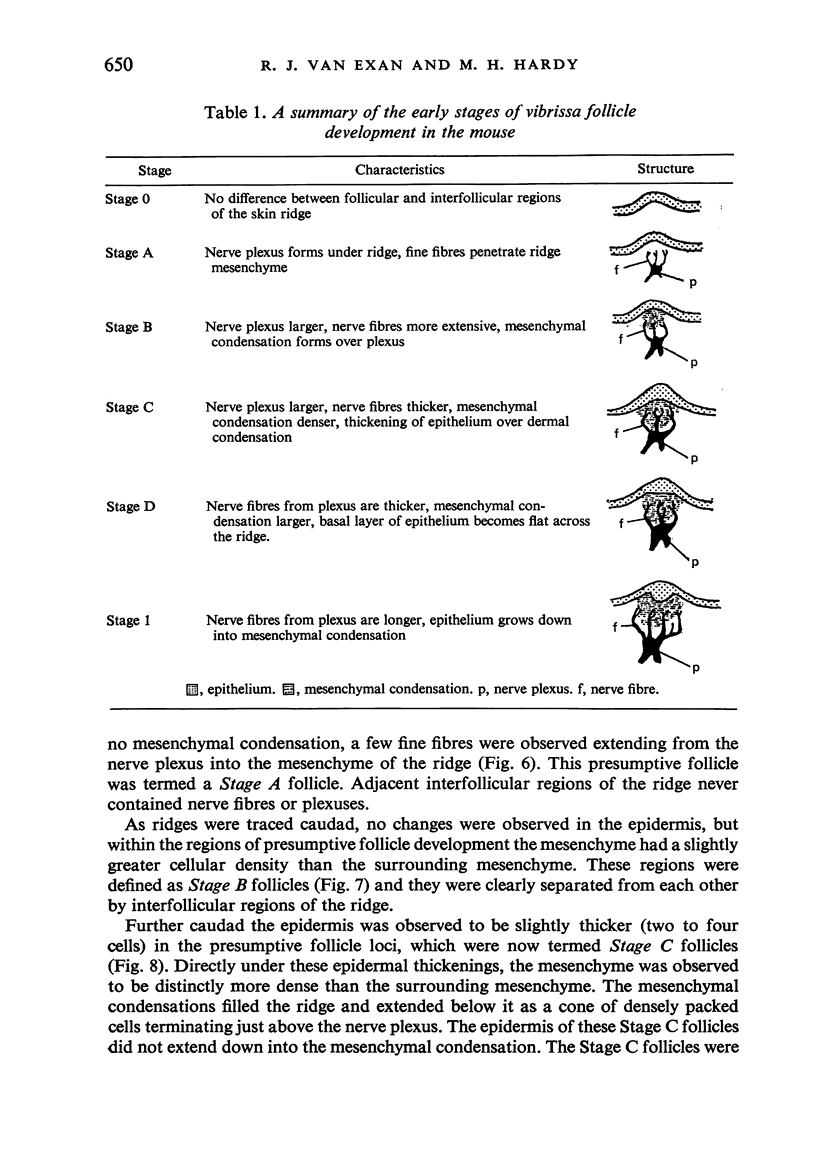
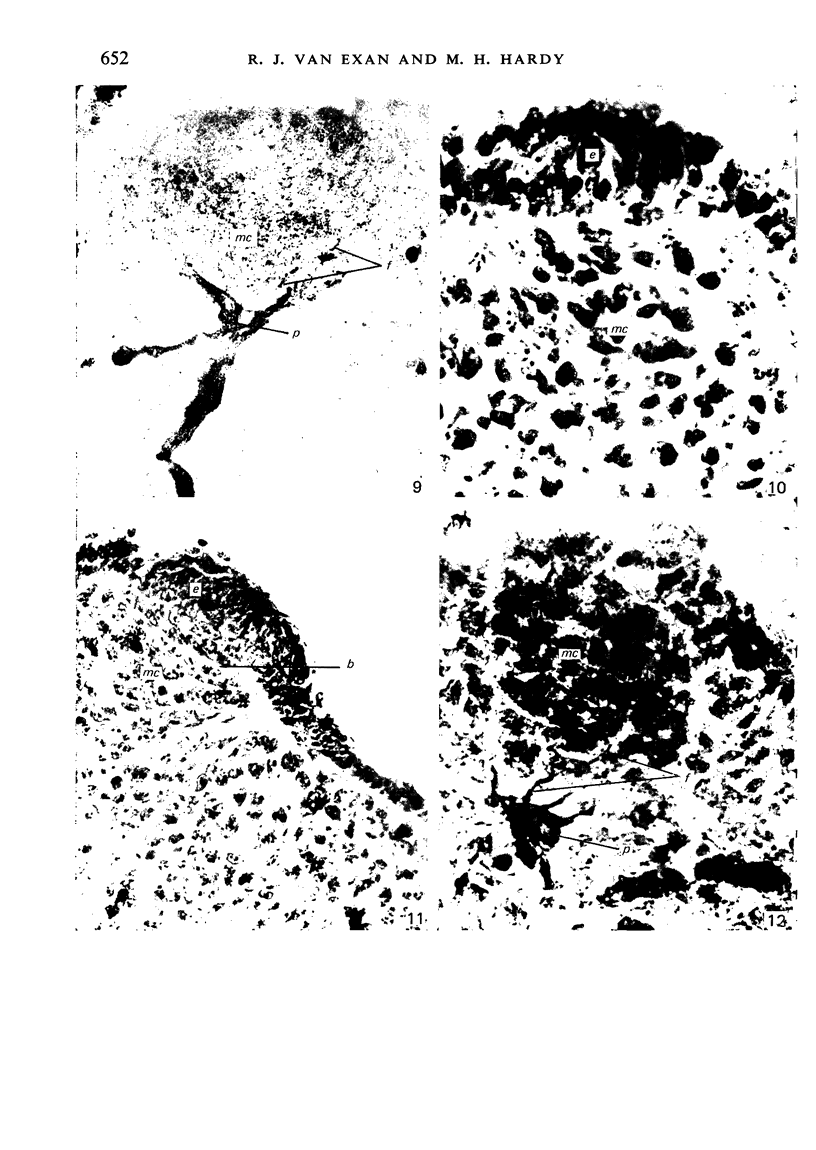
The present study has demonstrated that the mystacial vibrissae of the mouse began to develop at about 12 days of gestation on two plates of thickened ectoderm called the 'whisker pads' which were located on either side of the snout above the margin of the upper lip. Each whisker pad was traversed by five rostrocaudal skin ridges. The individual vibrissae developed along the ridges in a caudorostral sequence. Four new sub-stages of vibrissa follicle development which occurred prior to Stage 1 of Davidson & Hardy (1952) were described. The first of these, Stage A, was the formation of a small nerve plexus under the skin ridge. Stage B was then characterized by the formation of a dermal condensation above the nerve plexus. The epithelium over the dermal condensation began to thicken at Stage C and grow down into the dermal condensation at Stage D. The early morphogenesis of the mystacial vibrissa follicles of the mouse was compared to that of teeth and mammary glands. The possibility of nerve involvement in determining the pattern of follicle array on the snout was discussed. The sequence of the morphological changes in the dermal and epidermal components of the early follicles was related to the present knowledge of epithelial-mesenchymal interactions which occur during this phase of follicle development.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIDSON P., HARDY M. H. The development of mouse vibrissae in vivo and in vitro. J Anat. 1952 Oct;86(4):342–356. [PMC free article] [PubMed] [Google Scholar]
- Jacobson C. M. A comparative study of the mechanisms by which X-irradiation and genetic mutation cause loss of vibrissae in embryo mice. J Embryol Exp Morphol. 1966 Oct;16(2):369–379. [PubMed] [Google Scholar]
- Killackey H. P., Belford G., Ryugo R., Ryugo D. K. Anomalous organization of thalamocortical projections consequent to vibrissae removal in the newborn rat and mouse. Brain Res. 1976 Mar 12;104(2):309–315. doi: 10.1016/0006-8993(76)90623-5. [DOI] [PubMed] [Google Scholar]
- Kollar E. J., Lumsden A. G. Tooth morphogenesis: the role of the innervation during induction and pattern formation. J Biol Buccale. 1979 Mar;7(1):49–60. [PubMed] [Google Scholar]
- Kollar E. J. The induction of hair follicles by embryonic dermal papillae. J Invest Dermatol. 1970 Dec;55(6):374–378. doi: 10.1111/1523-1747.ep12260492. [DOI] [PubMed] [Google Scholar]
- Lee K. J., Woolsey T. A. A proportional relationship between peripheral innervation density and cortical neuron number in the somatosensory system of the mouse. Brain Res. 1975 Dec 5;99(2):349–353. doi: 10.1016/0006-8993(75)90035-9. [DOI] [PubMed] [Google Scholar]
- MELARAGNO H. P., MONTAGNA W. The tactile hair follicles in the mouse. Anat Rec. 1953 Feb;115(2):129–149. doi: 10.1002/ar.1091150202. [DOI] [PubMed] [Google Scholar]
- Park A. W. Morphological adaptation in developing vibrissae of rats. Acta Anat (Basel) 1970;75(1):67–78. doi: 10.1159/000143441. [DOI] [PubMed] [Google Scholar]
- SENGEL P., FEIGELSON M. SUR LES PROPRI'ET'ES BIOCHIMIQUES D'UN FACTEUR MORPHOG'ENE AGISSANT SUR LA DIFF'ERENCIATION DES GERMES PLUMAIRES. C R Hebd Seances Acad Sci. 1963 Dec 16;257:4024–4027. [PubMed] [Google Scholar]
- Van der Loos H., Woolsey T. A. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973 Jan 26;179(4071):395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- WINKELMANN R. K. The innervation of a hair follicle. Ann N Y Acad Sci. 1959 Nov 20;83:400–407. doi: 10.1111/j.1749-6632.1960.tb40915.x. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol. 1976 Mar 15;166(2):173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey T. A., Wann J. R. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976 Nov 1;170(1):53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Yamakado M., Yohro T. Subdivision of mouse vibrissae on an embryological basis, with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels in BALB/c (nu/+; nude, nu/nu) and hairless (hr/hr) strains. Am J Anat. 1979 Jun;155(2):153–173. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]
- Zalewski A. A. Trophic functions of the neuron. VI. Other trophic systems. Neuronal and tissue specifications involved in taste bud formation. Ann N Y Acad Sci. 1974 Mar 22;228(0):344–349. doi: 10.1111/j.1749-6632.1974.tb20523.x. [DOI] [PubMed] [Google Scholar]