Abstract
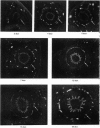
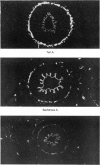
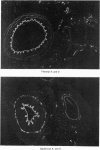
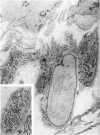
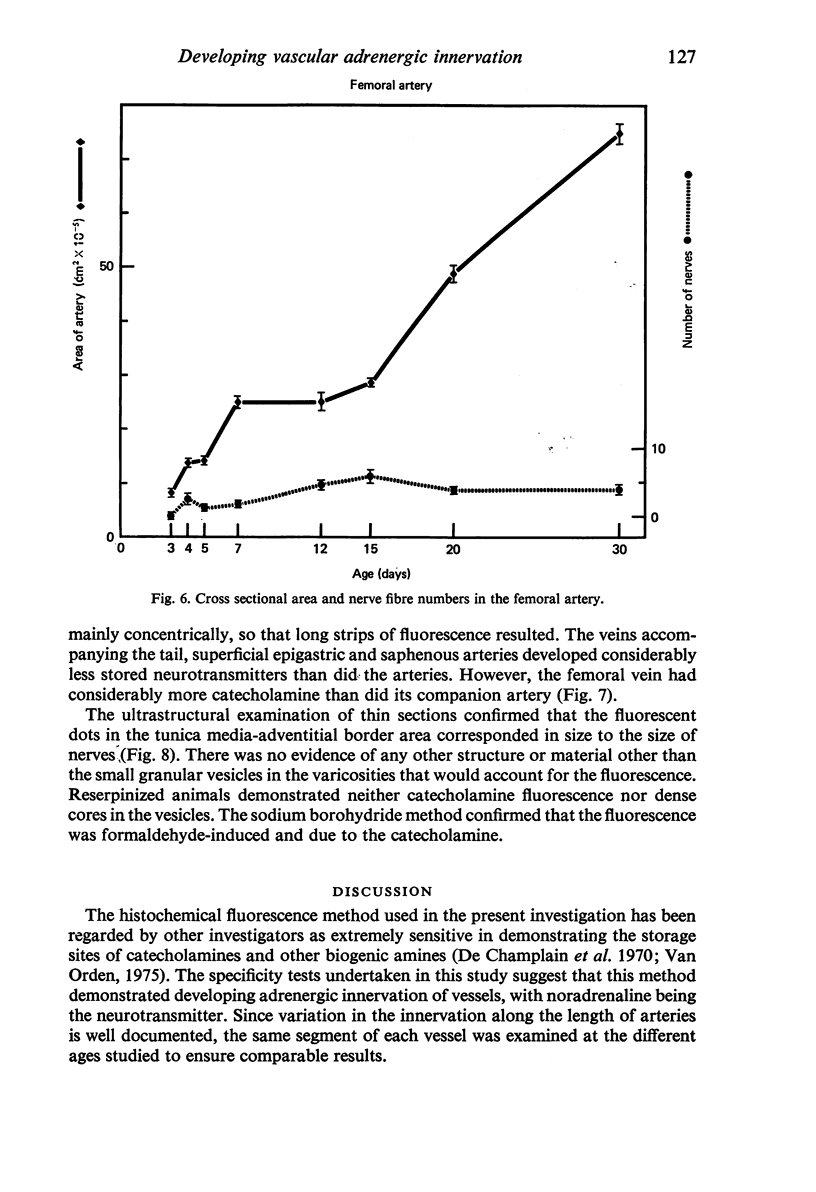
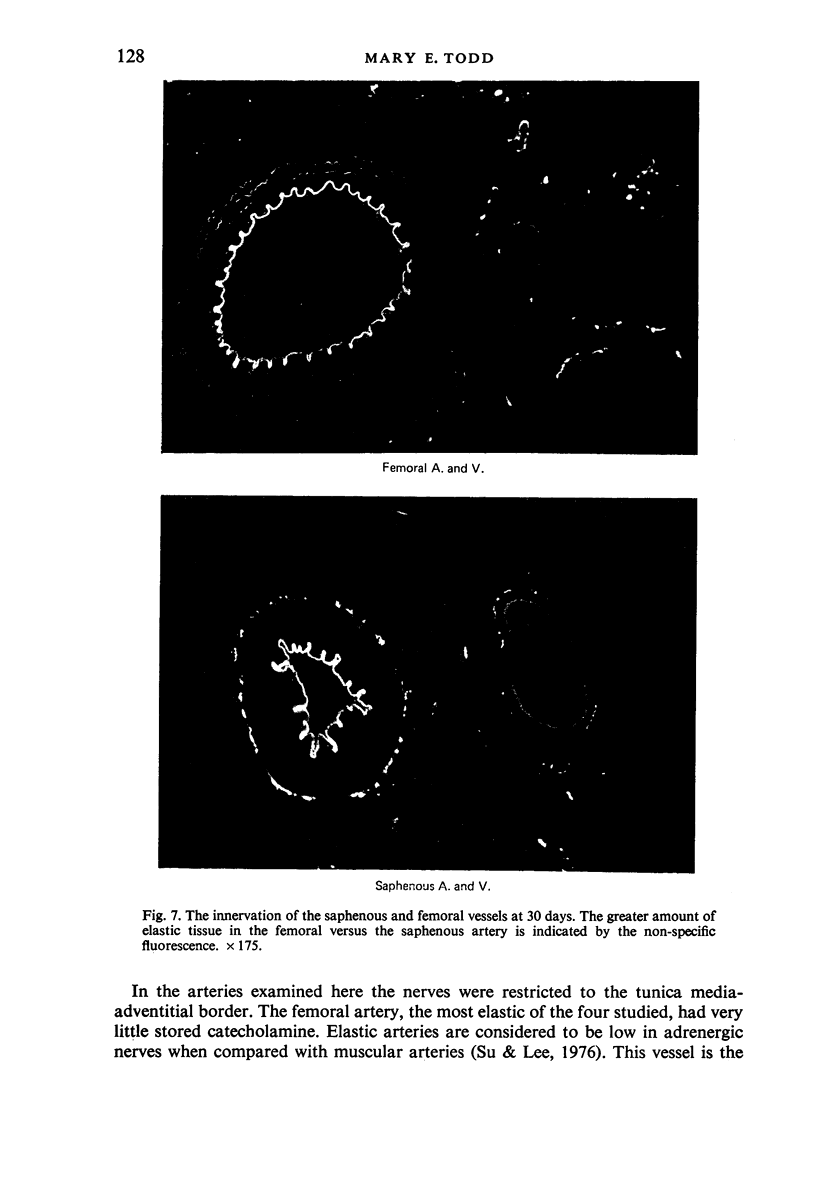
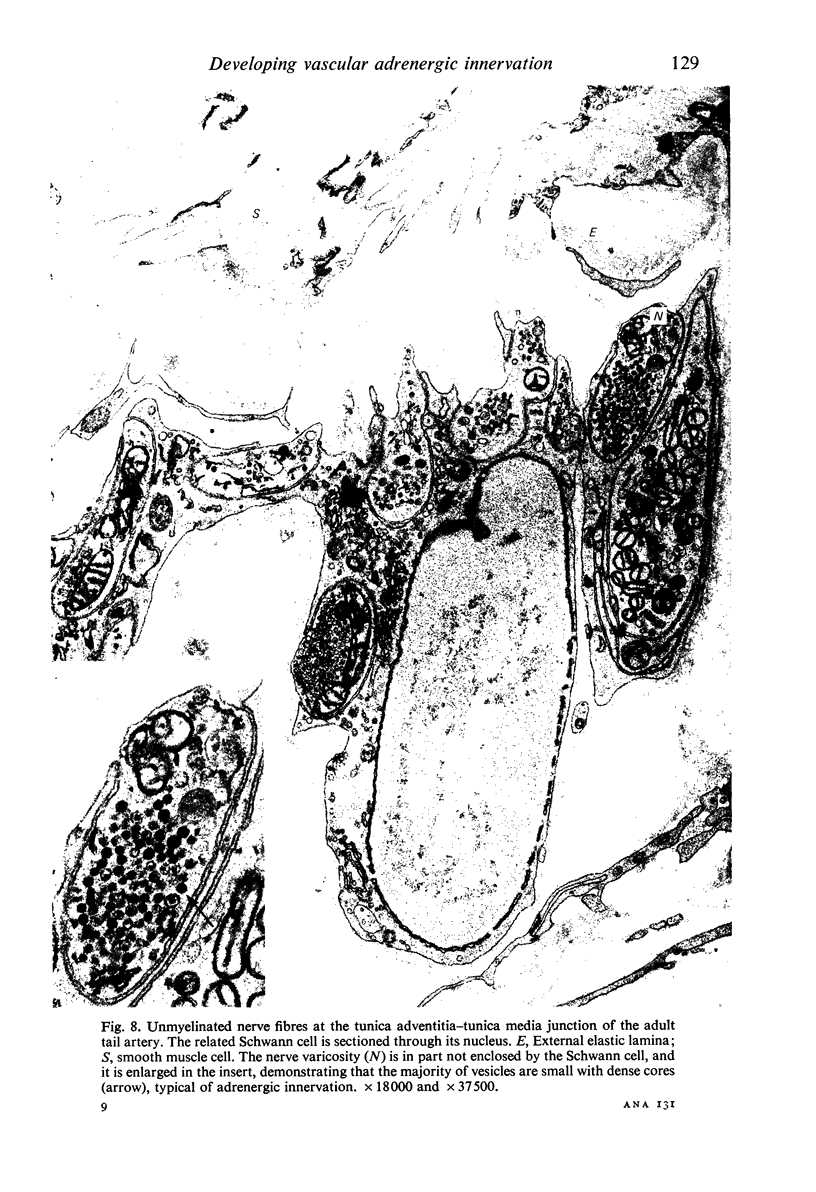
The postnatal development of adrenergic innervation was followed in peripheral blood vessels of Wistar rats. The femoral vessels and their branches, the superficial epigastric and saphenous vessels, and the tail artery, were investigated from birth to maturity. The proximal ends of the vessels were studied with fluorescence microscopy after the catecholamine was converted to a fluorophore by hot formaldehyde vapour, and ultrastructural morphology confirmed that the nerve varicosities mainly contained small vesicles with dense cores, typical of adrenergic innervation. Further confirmation was obtained with reserpine pre-pretreatment, the sodium borohydride specificity test, and experiments to alter the non-specific fluorescence of elastin. The nerves in the arteries were immediately adjacent to the external elastic lamina, and they retained this position throughout postnatal development. Of the three muscular arteries, the development of innervation was earlier and more intense in the saphenous and superficial epigastric arteries than in the tail artery. However, the tail artery surpassed the other two both in the total number of nerves and in the density of innervation per unit area beyond 12 days of age, and maintained this lead to maturity. The superficial epigastric artery had the smallest total number of nerves but had a greater density of innervation than the saphenous. The femoral artery did not develop any appreciable innervation. The femoral vein demonstrated the greatest amount of fluorescence of any of the veins, the others having considerably less innervation than their companion arteries.
Full text
PDF

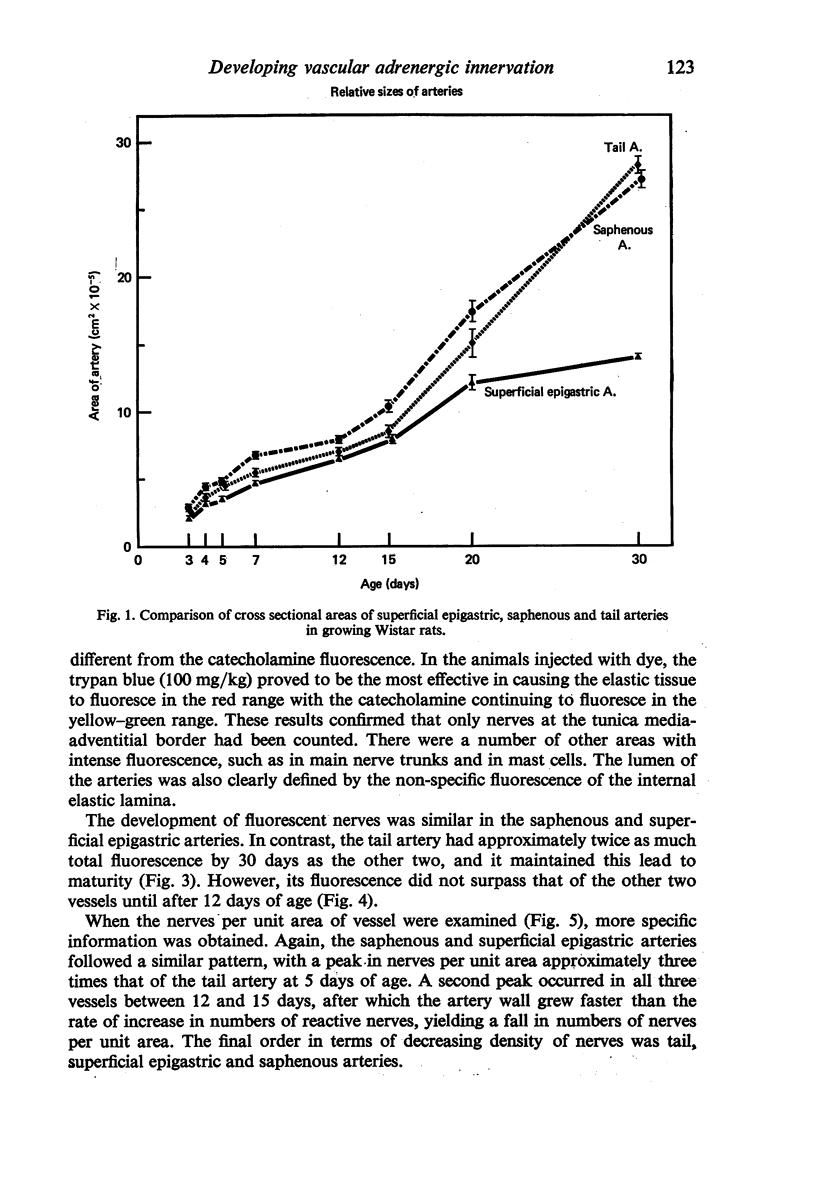
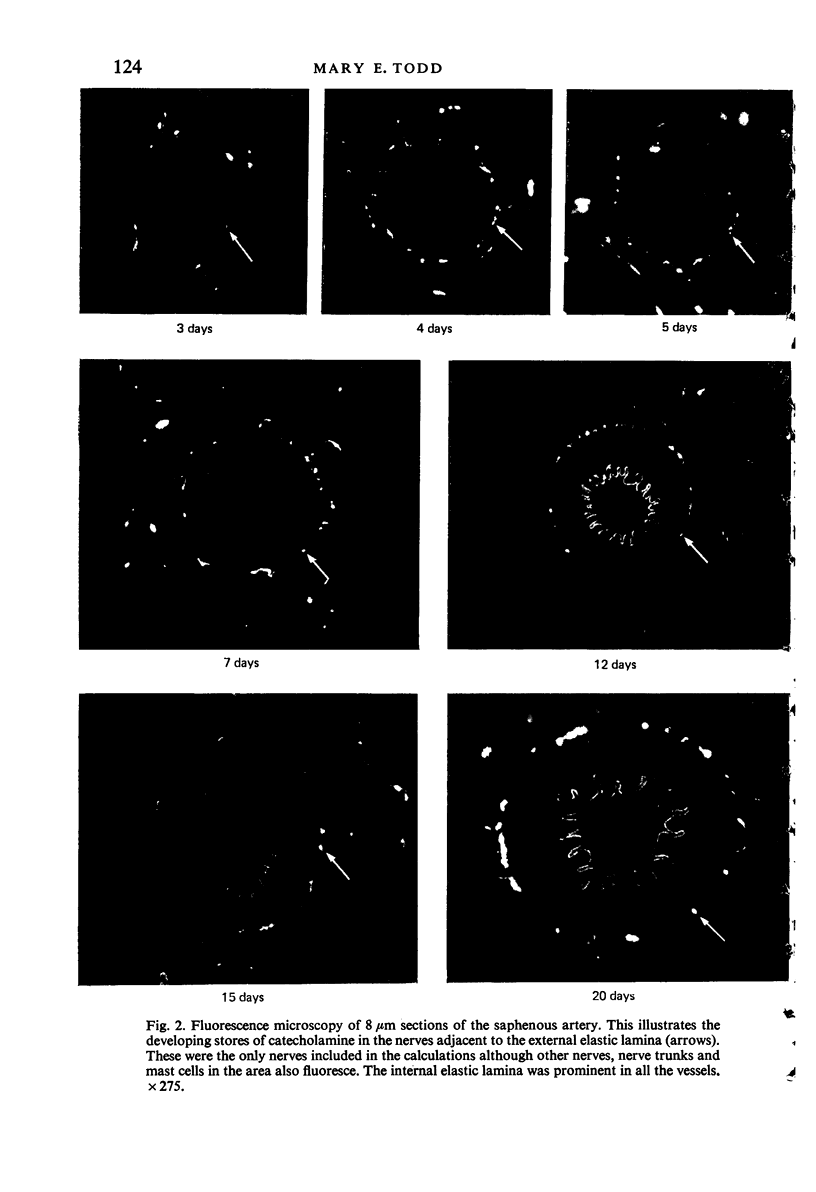
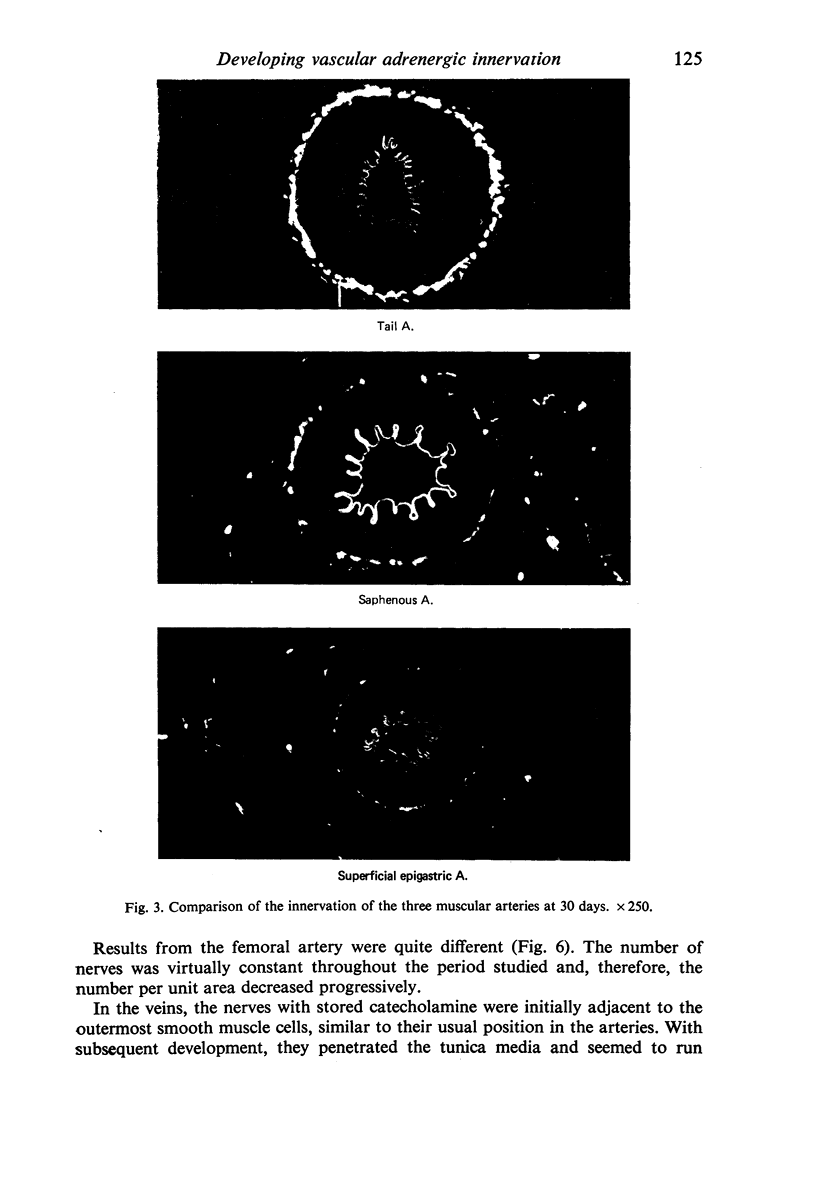
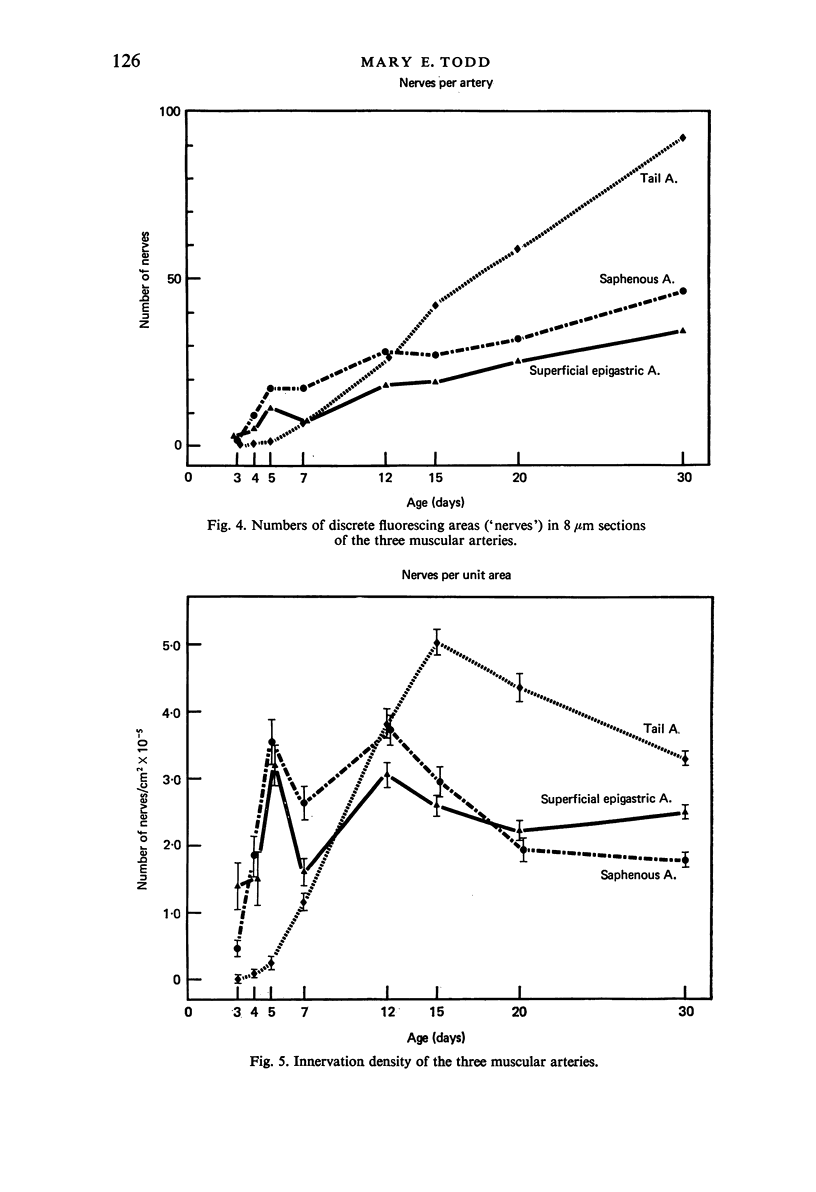




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan J. A., Hosmer D. W., Ljung B., Pegram B. L., Su C. Innervation pattern and neurogenic response of rabbit veins. Blood Vessels. 1974;11(3):172–182. doi: 10.1159/000158010. [DOI] [PubMed] [Google Scholar]
- Bevan J. A., Purdy R. E. Variations in adrenergic innervation and contractile responses of the rabbit saphenous artery. Circ Res. 1973 Jun;32(6):746–751. doi: 10.1161/01.res.32.6.746. [DOI] [PubMed] [Google Scholar]
- Bevan J. A., Su C. Sympathetic mechanisms in blood vessels: nerve and muscle relationships. Annu Rev Pharmacol. 1973;13:269–285. doi: 10.1146/annurev.pa.13.040173.001413. [DOI] [PubMed] [Google Scholar]
- Borchard F. The adrenergic nerves of the normal and the hypertrophied heart. Norm Pathol Anat (Stuttg) 1978;33:1–68. [PubMed] [Google Scholar]
- CORRODI H., HILLARP N. A., JONSSON G. FLUORESCENCE METHODS FOR THE HISTOCHEMICAL DEMONSTRATION OF MONOAMINES. 3. SODIUM BOROHYDRIDE REDUCTION OF THE FLUORESCENT COMPOUNDS AS A SPECIFICITY TEST. J Histochem Cytochem. 1964 Aug;12:582–586. doi: 10.1177/12.8.582. [DOI] [PubMed] [Google Scholar]
- De Champlain J., Malmfors T., Olson L., Sachs C. Ontogenesis of peripheral adrenergic neurons in the rat: pre- and postnatal observations. Acta Physiol Scand. 1970 Oct;80(2):276–288. doi: 10.1111/j.1748-1716.1970.tb04791.x. [DOI] [PubMed] [Google Scholar]
- De la Lande I. S., Waterson J. G. Modification of autofluorescence in the formaldehyde-treated rabbit ear artery by Evans blue. J Histochem Cytochem. 1968 Apr;16(4):281–282. doi: 10.1177/16.4.281. [DOI] [PubMed] [Google Scholar]
- FUXE K., SEDVALL G. THE DISTRIBUTION OF ADRENERGIC NERVE FIBRES TO THE BLOOD VESSELS IN SKELETAL MUSCLE. Acta Physiol Scand. 1965 May-Jun;64:75–86. doi: 10.1111/j.1748-1716.1965.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Furness J. B. Arrangement of blood vessels and their relation with adrenergic nerves in the rat mesentery. J Anat. 1973 Sep;115(Pt 3):347–364. [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., McLean J. R., Burnstock G. Distribution of adrenergic nerves and changes in neuromuscular transmission in the mouse vas deferens during postnatal development. Dev Biol. 1970 Apr;21(4):491–505. doi: 10.1016/0012-1606(70)90074-6. [DOI] [PubMed] [Google Scholar]
- Furness J. B. The adrenergic innervation of the vessels supplying and draining the gastrointestinal tract. Z Zellforsch Mikrosk Anat. 1971;113(1):67–82. doi: 10.1007/BF00331202. [DOI] [PubMed] [Google Scholar]
- Ljung B., Stage D., Carlsson C. Postnatal ontogenetic development of neurogenic and myogenic control in the rat portal vein. Acta Physiol Scand. 1975 May;94(1):112–127. doi: 10.1111/j.1748-1716.1975.tb05867.x. [DOI] [PubMed] [Google Scholar]
- McInnes A. Modification of the Falck-Hillarp technique with intravital trypan blue to differentiate elastic fibres from noradrenergic endings [proceedings]. J Physiol. 1977 Jun;268(1):22P–23P. [PubMed] [Google Scholar]
- Mellander S., Johansson B. Control of resistance, exchange, and capacitance functions in the peripheral circulation. Pharmacol Rev. 1968 Sep;20(3):117–196. [PubMed] [Google Scholar]
- Osborne-Pellegrin M. J. Some ultrastructural characteristics of the renal artery and abdominal aorta in the rat. J Anat. 1978 Mar;125(Pt 3):641–652. [PMC free article] [PubMed] [Google Scholar]
- Palatý V., Todd M. E. Some effects of the ionophore X-537A on the isolated rat tail artery. Can J Physiol Pharmacol. 1978 Jun;56(3):474–482. doi: 10.1139/y78-071. [DOI] [PubMed] [Google Scholar]
- Rickenbacher J., Ruflin G. Zur Entwicklung der Innervation der Extremitätengefässe beim Hühnchen. Vasa. 1974;3(1):5–9. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Somlyo A. P. Structural characteristics, mechanisms of contraction, innervation and proliferation of smooth muscle cells. Ultrastructure and function of vascular smooth muscle. Adv Exp Med Biol. 1975;57:1–80. doi: 10.1007/978-1-4613-4476-6_1. [DOI] [PubMed] [Google Scholar]
- Su C., Bevan J. A., Assali N. S., Brinkman C. R., 3rd Development of neuroeffector mechanisms in the carotid artery of the fetal lamb. Blood Vessels. 1977;14(1):12–24. doi: 10.1159/000158111. [DOI] [PubMed] [Google Scholar]