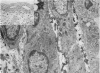Abstract
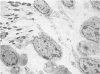
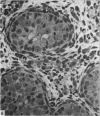
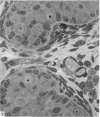
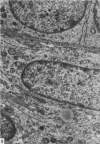
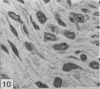
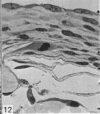
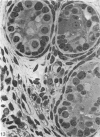
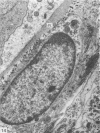
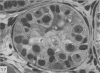
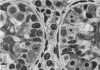
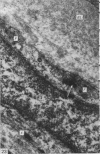
This study has been determined the postnatal development and differentiation of smooth muscle cells within the rabbit testicular capsule and within the peritubular tissue surrounding seminiferous tubules. Smooth muscle cells within the tunica albuginea are not identifiable at birth by light microscopy but by electron microscopy myocytes in early stages of development may be shown to be present. It is not until 42 to 49 days postnatum that smooth muscle cells can be identified by light microscopy. Differentiation of smooth muscle cells within the capsule is completed by 128 days postnatum. At this time, the muscle is arranged in two organized layers, a superficial layer of longitudinally oriented cells and a deeper layer of circularly arranged cells. At birth, the peritubular tissue consists of two to four layers of undifferentiated cells and, during the first postnatal week, the tissue becomes more condensed and generally is arranged in two cellular layers. Cells of the inner layer contain small bundles of microfibrils whereas cells of the outer layer are fibroblast in nature. Differentiation of the peritubular tissue is completed by 112 days postnatum. At this stage, it consists of four layers, two acellular and two cellular. The inner cellular layer, composed of attentuated myoid cells, possesses a basal lamina on both surfaces and is surrounded by two delicate connective tissue lamellae. The myoid cells of the peritubular tissue thus achieve structural maturity at approximately the same time postnatally as do those within the testicular capsule, which corresponds to the time when spermatogenesis becomes established. The relative contributions of the myoid cells in the peritubular tissue and within the testicular capsule to the movement of non-motile spermatozoa out of the testis and the possible significance of the peritubular tissue as a component of the permeability barrier are discussed in relation to the present findings.
Full text
PDF


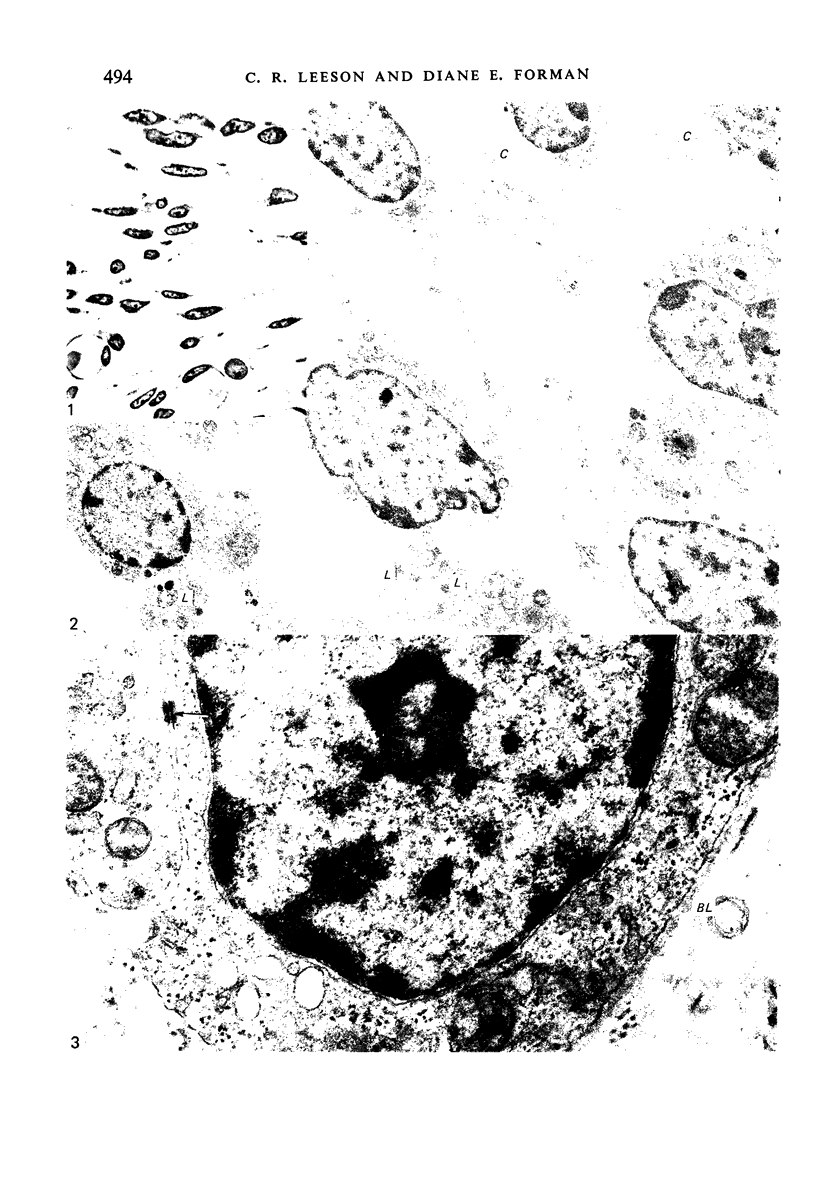

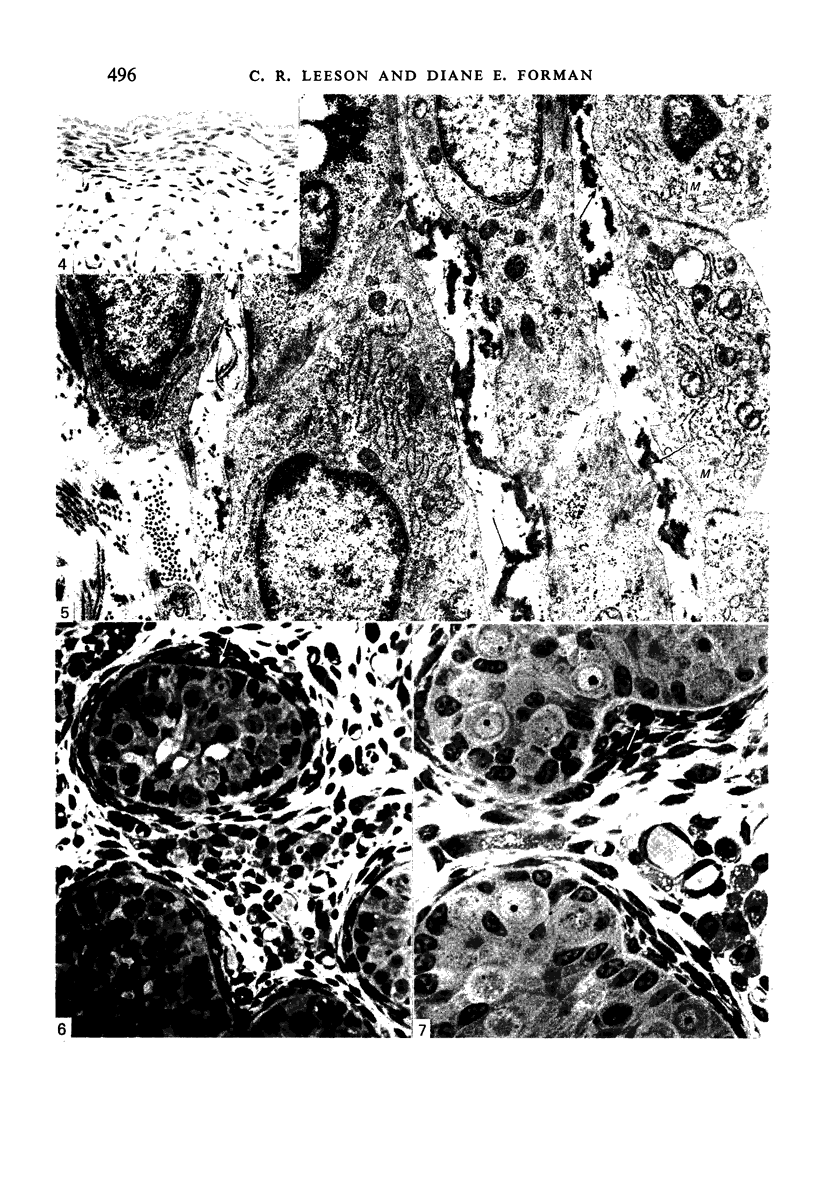












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustos-Obregón E., Courot M. Ultrastructure of the lamina propria in the ovine seminiferous tubule. Development and some endocrine considerations. Cell Tissue Res. 1974;150(4):481–492. doi: 10.1007/BF00225971. [DOI] [PubMed] [Google Scholar]
- CLERMONT Y. Contractile elements in the limiting membrane of the seminiferous tubules of the rat. Exp Cell Res. 1958 Oct;15(2):438–440. doi: 10.1016/0014-4827(58)90052-1. [DOI] [PubMed] [Google Scholar]
- CRABO B. FINE STRUCTURE OF THE INTERSTITIAL CELLS OF THE RABBIT TESTES. Z Zellforsch Mikrosk Anat. 1963 Dec 3;61:587–604. doi: 10.1007/BF00344002. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Uehara Y., Mark G., Burnstock G. Fine structure of smooth muscle cells grown in tissue culture. J Cell Biol. 1971 Apr;49(1):21–34. doi: 10.1083/jcb.49.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierichs R., Wrobel K. H. Licht- und elektronenmikroskopische Untersuchungen an den peritubulären Zellen des Schweinehodens während der postnatalen Entwicklung. Z Anat Entwicklungsgesch. 1973 Dec 31;143(1):49–64. [PubMed] [Google Scholar]
- Dym M., Cavicchia J. C. Functional morphology of the testis. Biol Reprod. 1978 Feb;18(1):1–15. doi: 10.1095/biolreprod18.1.1. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Leak L. V., Heidger P. M., Jr Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–122. [PubMed] [Google Scholar]
- Gondos B., Renston R. H., Conner L. A. Ultrastructure of germ cells and Sertoli cells in the postnatal rabbit testis. Am J Anat. 1973 Apr;136(4):427–439. doi: 10.1002/aja.1001360404. [DOI] [PubMed] [Google Scholar]
- Hafs H. D., Louis T. M., Stellflug J. N. Increased sperm numbers in the deferent duct after prostaglandin F2alpha in rabbits. Proc Soc Exp Biol Med. 1974 Mar;145(3):1120–1124. doi: 10.3181/00379727-145-37964. [DOI] [PubMed] [Google Scholar]
- Hargrove J. L., Ellis L. C. Autonomic nerves versus prostaglandins in the control of rat and rabbit testicular capsular contractions in vivo and in vitro. Biol Reprod. 1976 Jun;14(5):651–657. doi: 10.1095/biolreprod14.5.651. [DOI] [PubMed] [Google Scholar]
- Hargrove J. L., MacIndoe J. H., Ellis L. C. Testicular contractile cells and sperm transport. Fertil Steril. 1977 Nov;28(11):1146–1157. [PubMed] [Google Scholar]
- Hargrove J. L., Seeley R. R., Johnson J. M., Ellis L. C. Prostaglandin-like substances: initiation and maintenance of rabbit testicular contraction in vitro. Proc Soc Exp Biol Med. 1973 Jan;142(1):205–209. doi: 10.3181/00379727-142-36989. [DOI] [PubMed] [Google Scholar]
- Hermo L., Lalli M., Clermont Y. Arrangement of connective tissue components in the walls of seminiferous tubules of man and monkey. Am J Anat. 1977 Apr;148(4):433–445. doi: 10.1002/aja.1001480402. [DOI] [PubMed] [Google Scholar]
- Hodson N. Sympathetic nerves and reproductive organs in the male rabbit. J Reprod Fertil. 1965 Oct;10(2):209–220. doi: 10.1530/jrf.0.0100209. [DOI] [PubMed] [Google Scholar]
- Holstein A. F. Muklatur und Motilität des Nebenhodens beim Kaninchen. Z Zellforsch Mikrosk Anat. 1967;76(4):498–510. [PubMed] [Google Scholar]
- Holstein A. F., Weiss C. Uber die Wirkung der glatten Muskulatur in der Tunica Albuginea im Hoden des Kaninchens; Messungen des interstitiellen Druckes. Z Gesamte Exp Med. 1967;142(4):334–337. [PubMed] [Google Scholar]
- LEESON C. R., LEESON T. S. THE POSTNATAL DEVELOPMENT AND DIFFERENTIATION OF THE BOUNDARY TISSUE OF THE SEMINIFEROUS TUBULE OF THE RAT. Anat Rec. 1963 Oct;147:243–259. doi: 10.1002/ar.1091470208. [DOI] [PubMed] [Google Scholar]
- Langford G. A., Heller C. G. Fine structure of muscle cells of the human testicular capsule: basis of testicular contractions. Science. 1973 Feb 9;179(4073):573–575. doi: 10.1126/science.179.4073.573. [DOI] [PubMed] [Google Scholar]
- Leeson T. S. Smooth muscle cells in the rat testicular capsule: a developmental study. J Morphol. 1975 Oct;147(2):171–185. doi: 10.1002/jmor.1051470205. [DOI] [PubMed] [Google Scholar]
- McCord R. G., Jr Fine structural observations of the peritubular cell layer in the hamster testis. Brief report. Protoplasma. 1970;69(2):283–289. doi: 10.1007/BF01280728. [DOI] [PubMed] [Google Scholar]
- RISLEY P. L., SKREPETOS C. N. HISTOCHEMICAL DISTRIBUTION OF CHOLINESTERASES IN THE TESTIS, EPIDIDYMIS AND VAS DEFERENS OF THE RAT. Anat Rec. 1964 Feb;148:231–249. doi: 10.1002/ar.1091480213. [DOI] [PubMed] [Google Scholar]
- Ross M. H., Long I. R. Contractile cells in human seminiferous tubules. Science. 1966 Sep 9;153(3741):1271–1273. doi: 10.1126/science.153.3741.1271. [DOI] [PubMed] [Google Scholar]
- Ross M. H. The fine structure and development of the peritubular contractile cell component in the seminiferous tubules of the mouse. Am J Anat. 1967 Nov;121(3):523–557. doi: 10.1002/aja.1001210307. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. D. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978 Jan;190(1):99–111. doi: 10.1002/ar.1091900109. [DOI] [PubMed] [Google Scholar]
- Setchell B. P. The blood-testicular fluid barrier in sheep. J Physiol. 1967 Apr;189(2):63P–65P. [PubMed] [Google Scholar]
- Uehara Y., Campbell G. R., Burnstock G. Cytoplasmic filaments in developing and adult vertebrate smooth muscle. J Cell Biol. 1971 Aug;50(2):484–497. doi: 10.1083/jcb.50.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr J. K. Output of spermatozoa and fluid by the testis of the ram and its response to oxytocin. J Reprod Fertil. 1975 Apr;43(1):119–122. doi: 10.1530/jrf.0.0430119. [DOI] [PubMed] [Google Scholar]
- Willson J. T., Jones N. A., Katsh S., Smith S. W. Penetration of the testicular-tubular barrier by horseradish peroxidase induced by adjuvant. Anat Rec. 1973 May;176(1):83–100. doi: 10.1002/ar.1091760106. [DOI] [PubMed] [Google Scholar]