Abstract
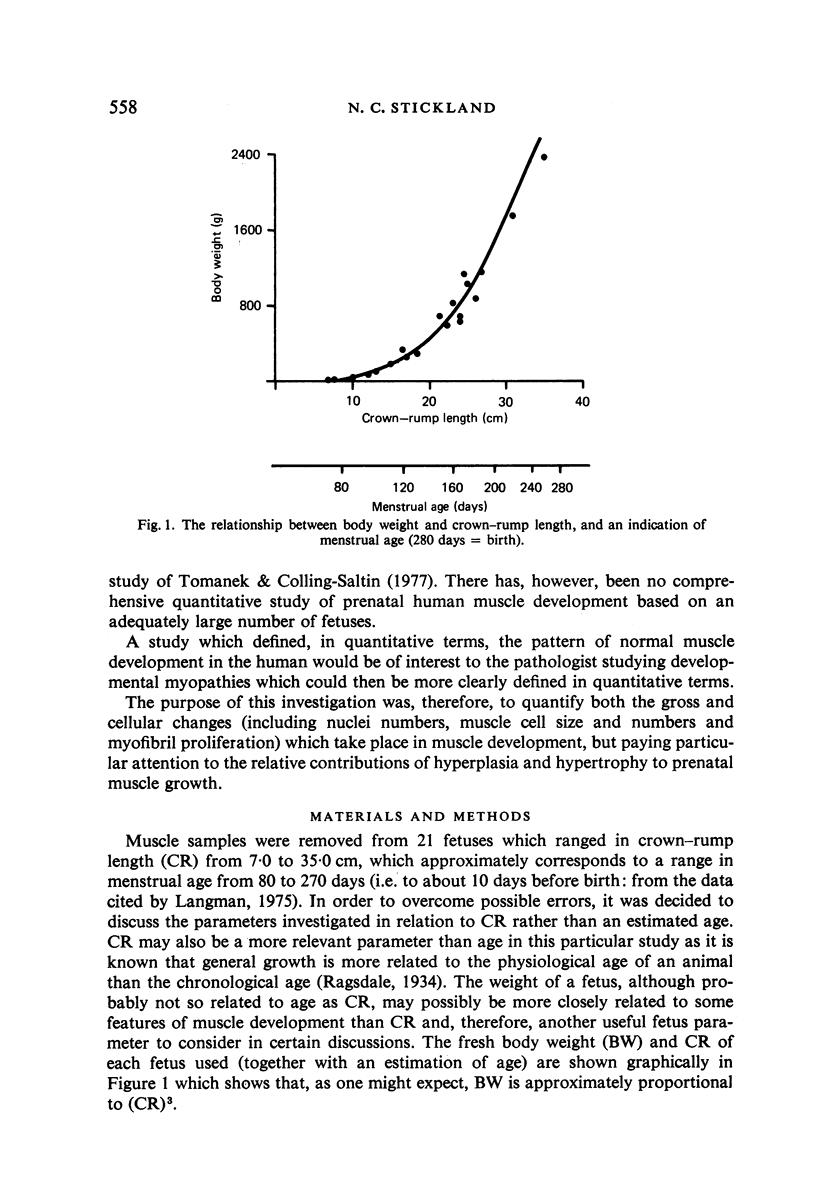
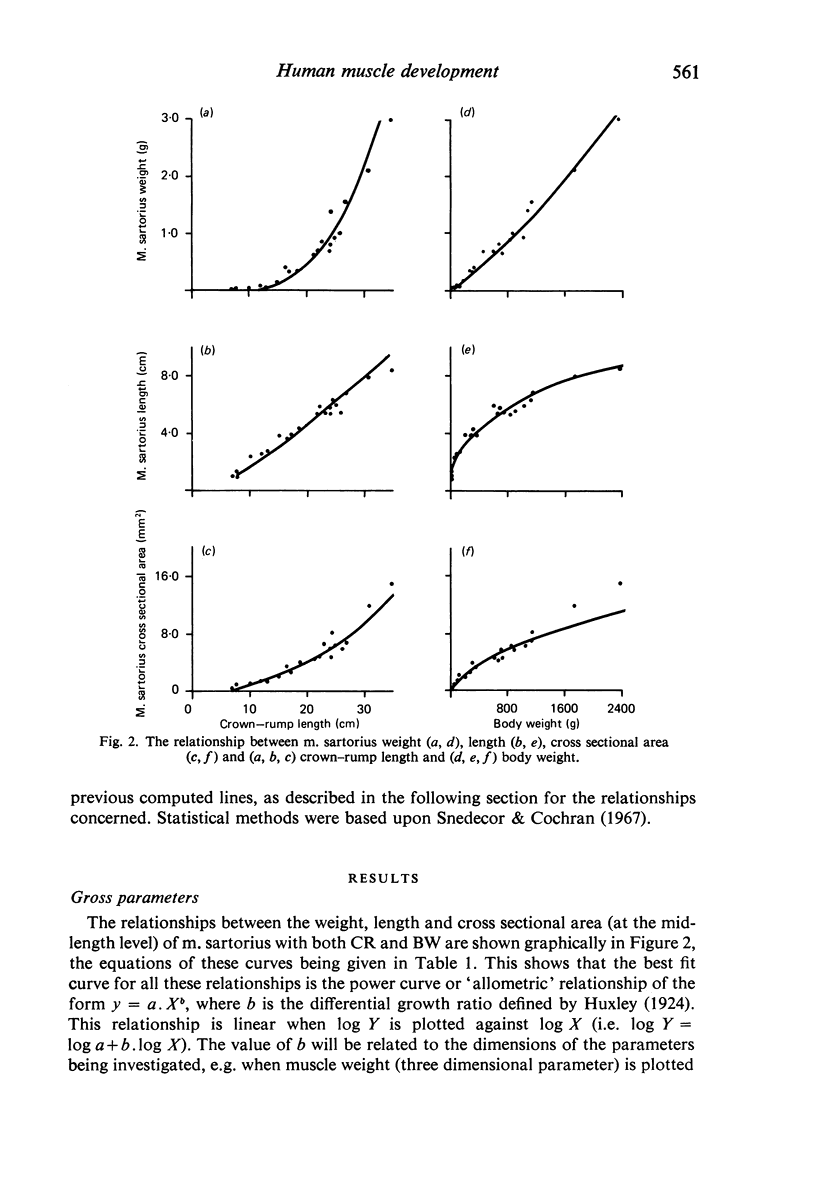
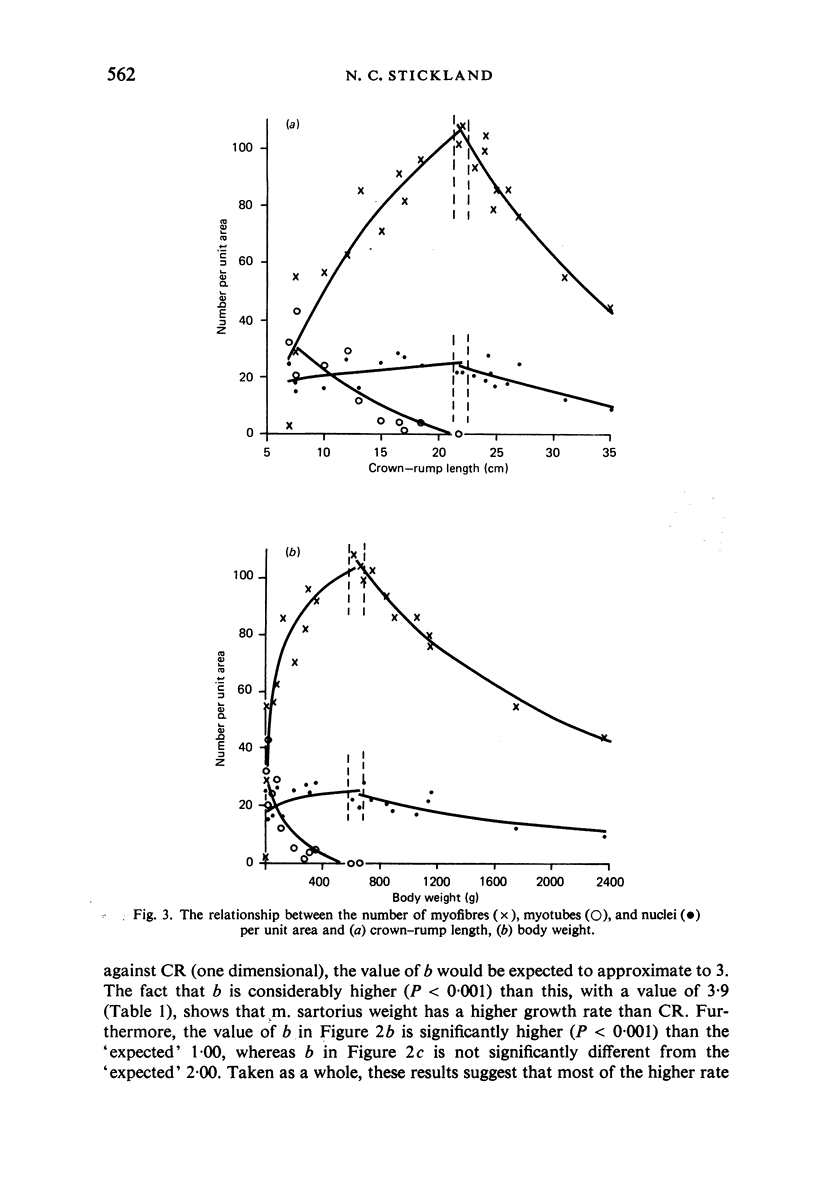
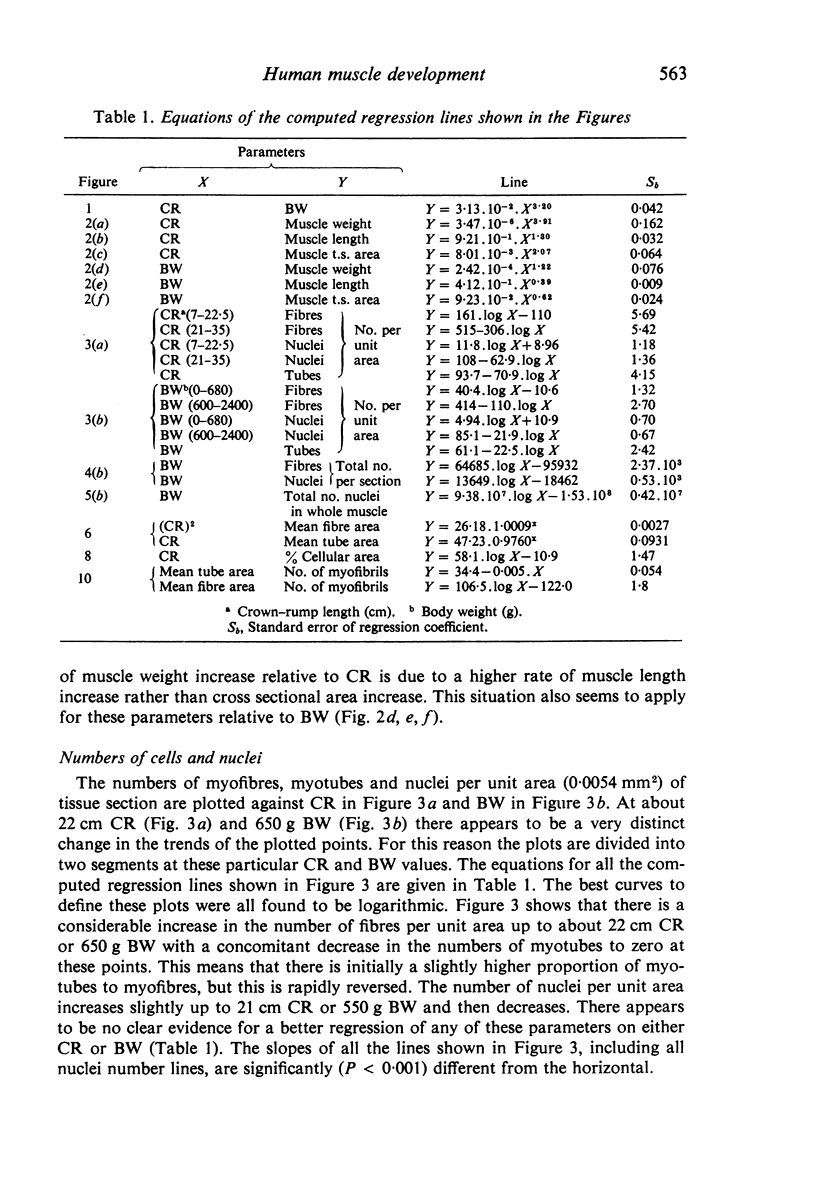
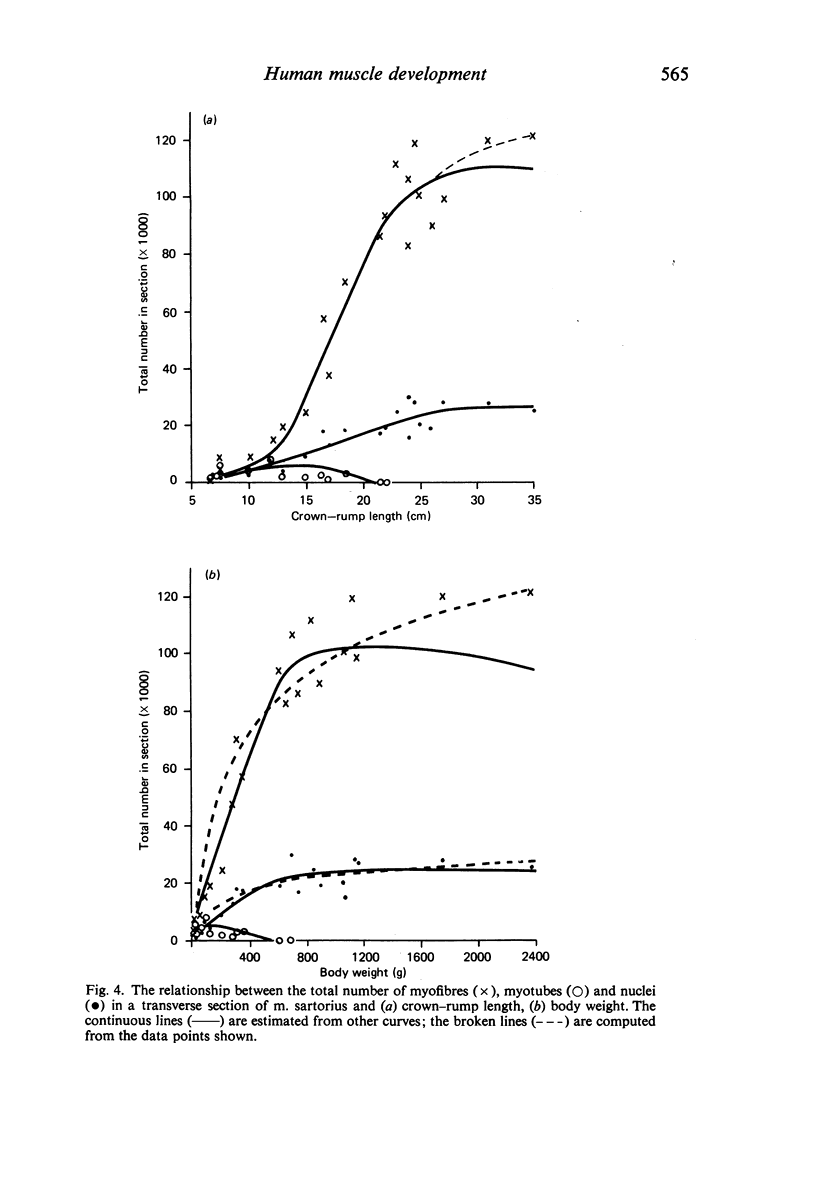
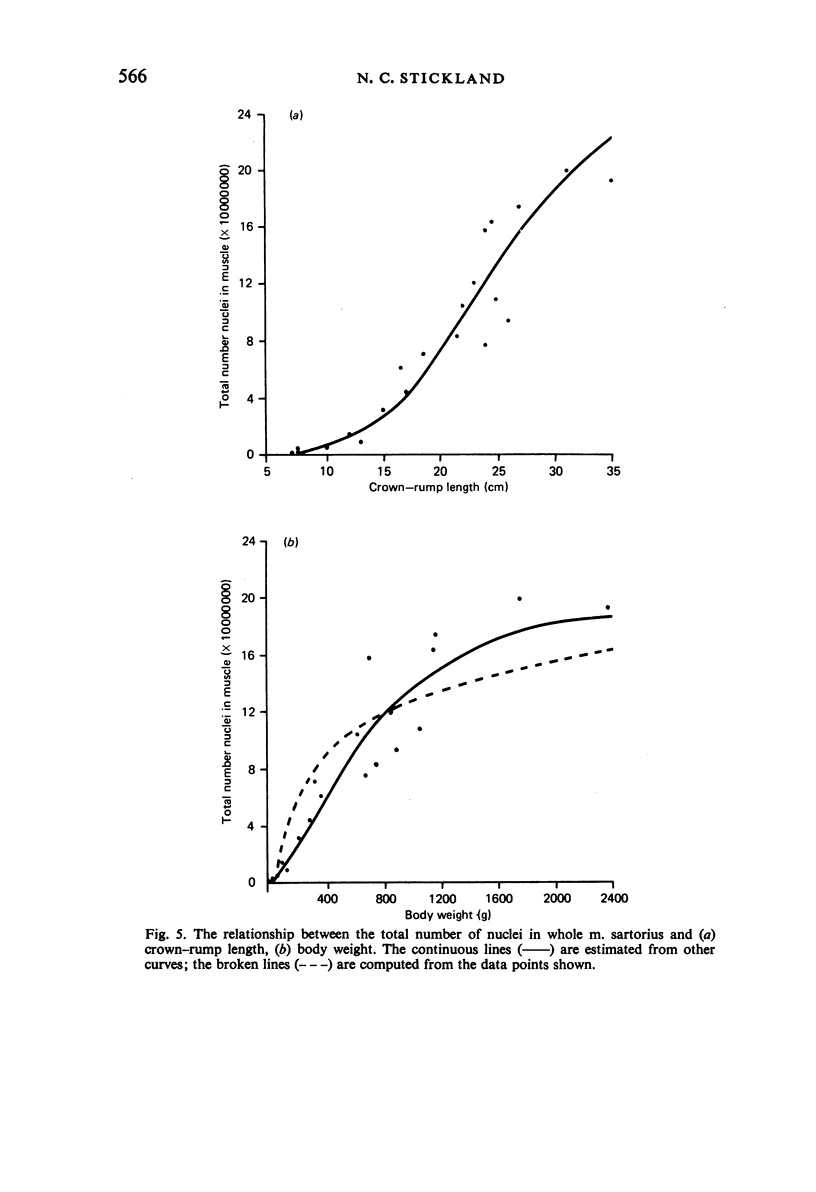
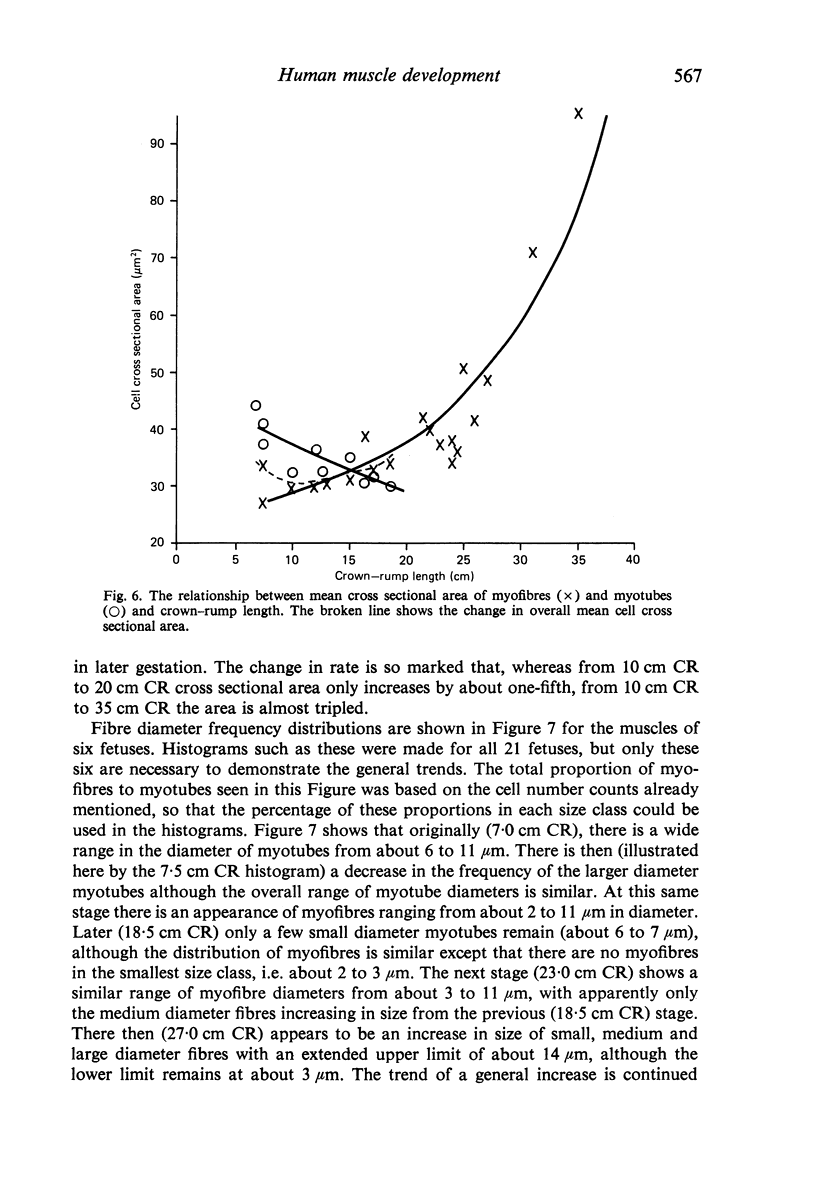
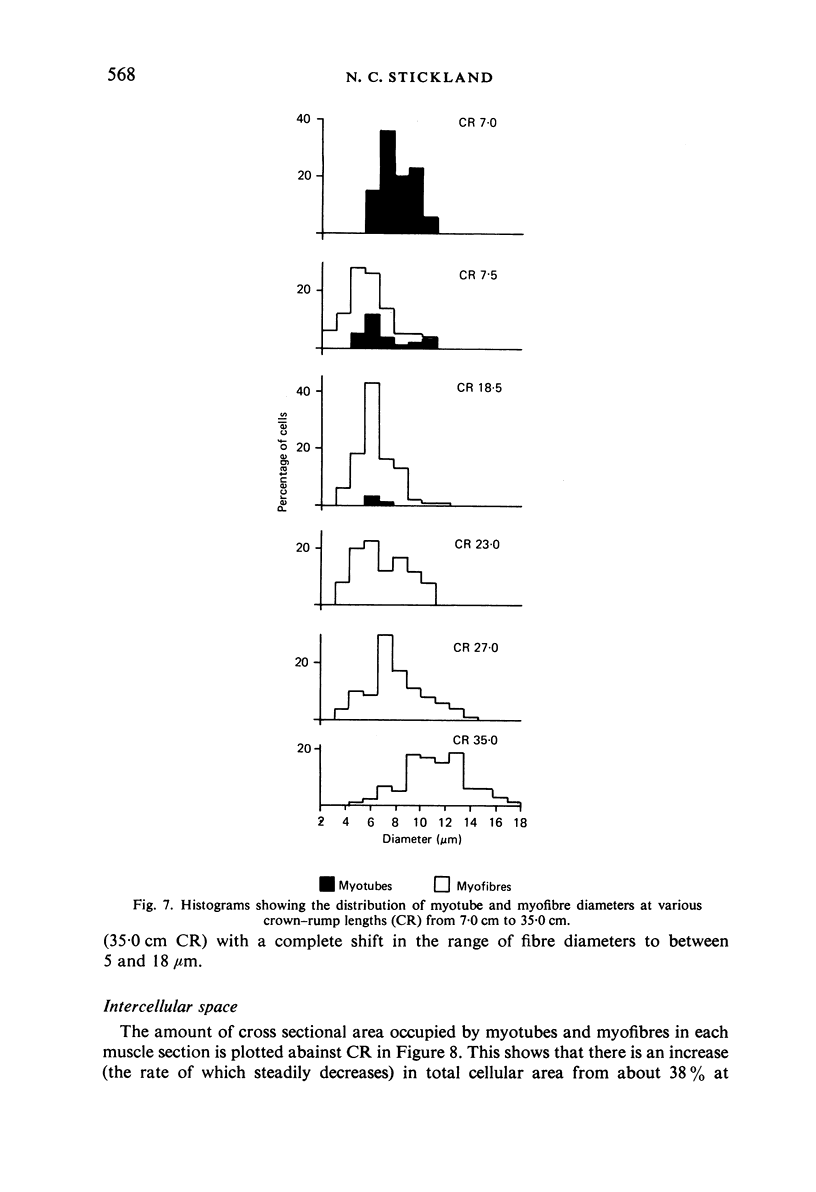
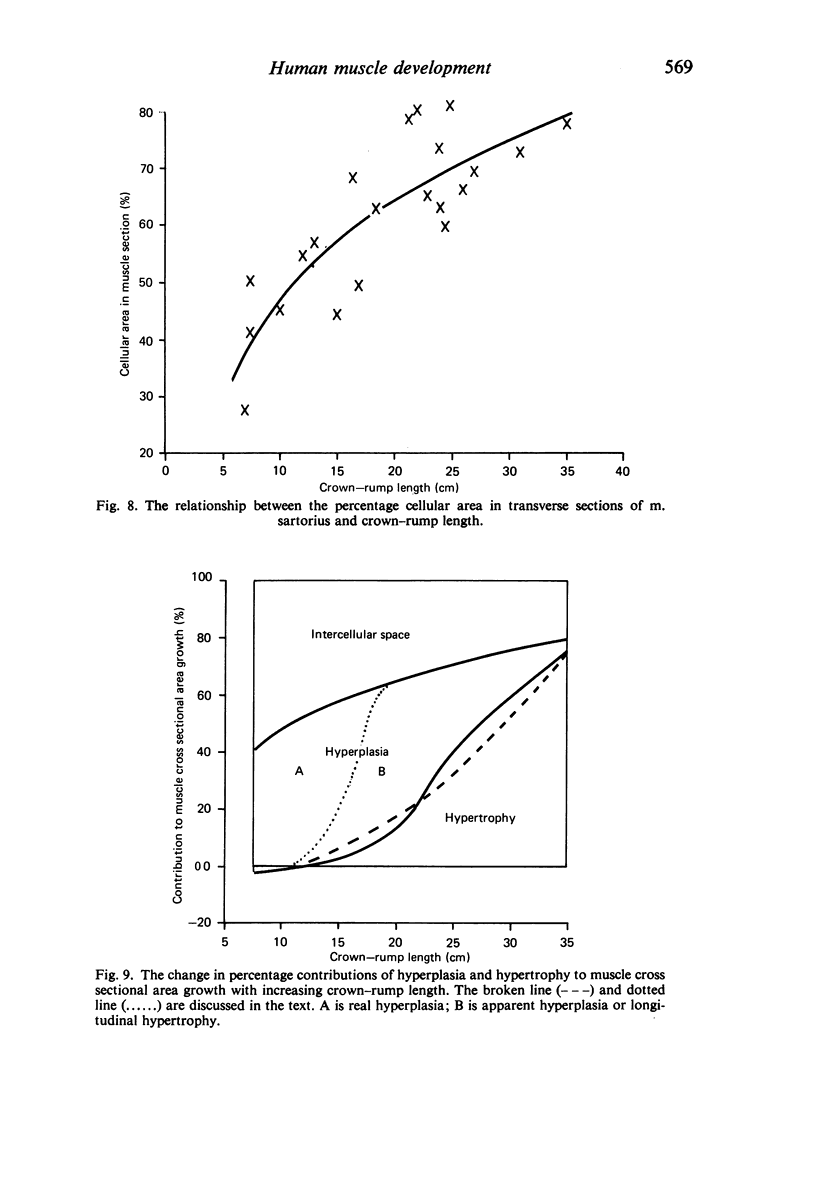
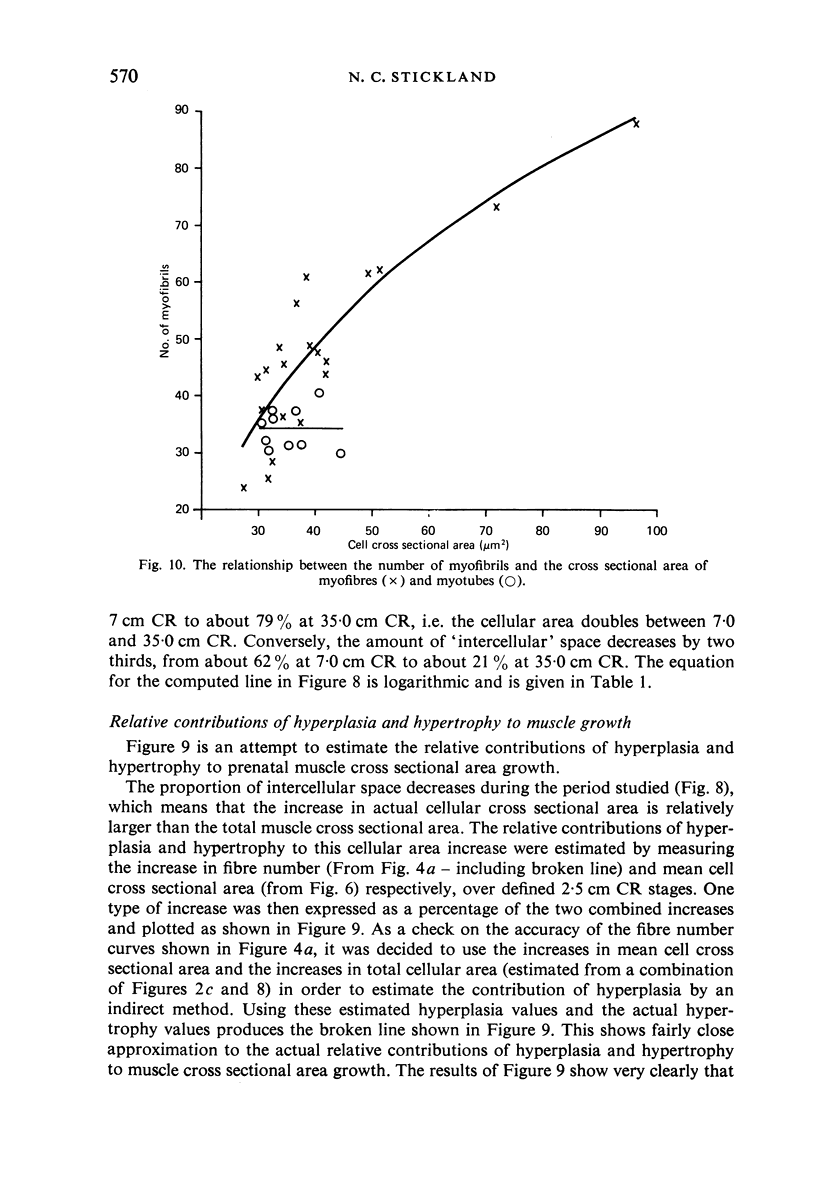
M. sartorius was removed from 21 human fetuses ranging from 7.0 to 35.0 cm crown-rump length (CR). Various gross amd cellular changes (as seen in a transverse section) which take place in developing human skeletal muscle were quantified. The weights, lengths and cross sectional areas (at mid-length level) of m. sartorius were found to exhibit allometric relationship with CR and body weight (BW). Initially (7.5 cm CR) myotubes were more numerous and larger (40 micrometer 2 cross sectional area) than the myofibres (about 26 micrometer 2). This situation was soon reversed, however, so that at about 19 cm CR myotubes were only a very small proportion of the total muscle cell population and somewhat smaller than the myofibres in cross sectional area. At about 21 cm CR all myotube appearance was lost, whilst the total number of myofibres increased rapidly up to about 22.5 cm CR, and thereafter the rate slowed down. This stage (22.5 cm CR) seemed, in fact, to be about the time when hypertrophy of myofibres started to markedly replace hyperplasia as the main factor contributing to total muscle cross sectional area increase, although there was still a 6% contribution from hyperplasia at 35 cm CR. At 18 to 22 cm CR there were no more myofibres in the smallest size class (2-3 micrometer diameter). This may be an indication that real hyperplasia had ceased at this point so that beyond this the hyperplasia seen was only apparent and represented longitudinal growth of existing myofibres. Throughout the period studied the amount of intercellular space decreased (at a declining rate) from about 62% at 7 cm CR to about 21% at 35 cm CR. Results on counts of nuclei suggested that total muscle nuclear proliferation slowed down in later gestation. Myofibril number was not related to myotube size but increased, though at a declining rate, with myofibre size. All the muscle parameters mentioned were plotted against CR, and sometimes BW, and regression equations given wherever possible.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore C. R., Addis P. B., Doerr L. Development of muscle fibers in the fetal pig. J Anim Sci. 1973 Jun;36(6):1088–1093. doi: 10.2527/jas1973.3661088x. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Robinson D. W., Rattray P., Doerr L. Biphasic development of muscle fibers in the fetal lamb. Exp Neurol. 1972 Nov;37(2):241–255. doi: 10.1016/0014-4886(72)90071-4. [DOI] [PubMed] [Google Scholar]
- Beermann D. H., Cassens R. G., Hausman G. J. A second look at fiber type differentiation in porcine skeletal muscle. J Anim Sci. 1978 Jan;46(1):125–132. doi: 10.2527/jas1978.461125x. [DOI] [PubMed] [Google Scholar]
- DICKERSON J. W., WIDDOWSON E. M. Chemical changes in skeletal muscle during development. Biochem J. 1960 Feb;74:247–257. doi: 10.1042/bj0740247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. S. Postnatal changes in the histochemical fibre types of procine skeletal muscle. J Anat. 1972 Nov;113(Pt 2):213–240. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Mobley B. A. Size changes in single muscle fibers during fixation and embedding. Tissue Cell. 1975;7(2):383–387. doi: 10.1016/0040-8166(75)90013-0. [DOI] [PubMed] [Google Scholar]
- Fidziańska A. Electron microscopic study of the development of human foetal muscle, motor end-plate and nerve. Preliminary report. Acta Neuropathol. 1971;17(3):234–247. doi: 10.1007/BF00685057. [DOI] [PubMed] [Google Scholar]
- GOLDSPINK G. THE COMBINED EFFECTS OF EXERCISE AND REDUCED FOOD INTAKE ON SKELETAL MUSCLE FIBERS. J Cell Physiol. 1964 Apr;63:209–216. doi: 10.1002/jcp.1030630211. [DOI] [PubMed] [Google Scholar]
- Goldspink G. The proliferation of myofibrils during muscle fibre growth. J Cell Sci. 1970 Mar;6(2):593–603. doi: 10.1242/jcs.6.2.593. [DOI] [PubMed] [Google Scholar]
- Hewer E. E. The Development of Muscle in the Human Foetus. J Anat. 1927 Oct;62(Pt 1):72–78. [PMC free article] [PubMed] [Google Scholar]
- JOUBERT D. M. Growth of muscle fibre in the foetal sheep. Nature. 1955 May 28;175(4465):936–937. doi: 10.1038/175936a0. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The histogenesis of rat intercostal muscle. J Cell Biol. 1969 Jul;42(1):135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTGOMERY R. D., DICKERSON J. W., MCCANCE R. A. SEVERE UNDERNUTRITION IN GROWING AND ADULT ANIMALS. 13. THE MORPHOLOGY AND CHEMISTRY OF DEVELOPMENT AND UNDERNUTRITION IN THE SARTORIUS MUSCLE OF THE FOWL. Br J Nutr. 1964;18:587–593. doi: 10.1079/bjn19640052. [DOI] [PubMed] [Google Scholar]
- MONTGOMERY R. D. Growth of human striated muscle. Nature. 1962 Jul 14;195:194–195. doi: 10.1038/195194a0. [DOI] [PubMed] [Google Scholar]
- Ontell M., Dunn R. F. Neonatal muscle growth: a quantitative study. Am J Anat. 1978 Aug;152(4):539–555. doi: 10.1002/aja.1001520408. [DOI] [PubMed] [Google Scholar]
- Ontell M. Neonatal muscle: an electron microscopic study. Anat Rec. 1977 Dec;189(4):669–690. doi: 10.1002/ar.1091890410. [DOI] [PubMed] [Google Scholar]
- Rayne J., Crawford G. N. Increase in fibre numbers of the rat pterygoid muscles during postnatal growth. J Anat. 1975 Apr;119(Pt 2):347–357. [PMC free article] [PubMed] [Google Scholar]
- Robinson D. W. The cellular response of porcine skeletal muscle to prenatal and neonatal nutritional stress. Growth. 1969 Sep;33(3):231–240. [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. J Anat. 1969 May;104(Pt 3):519–530. [PMC free article] [PubMed] [Google Scholar]
- Spiro A. J., Shy G. M., Gonatas N. K. Myotubular myopathy. Persistence of fetal muscle in an adolescent boy. Arch Neurol. 1966 Jan;14(1):1–14. doi: 10.1001/archneur.1966.00470070005001. [DOI] [PubMed] [Google Scholar]
- Stickland N. C. A detailed analysis of the effects of various fixatives on animal tissue with particular reference to muscle tissue. Stain Technol. 1975 Jul;50(4):255–264. doi: 10.3109/10520297509117068. [DOI] [PubMed] [Google Scholar]
- Stickland N. C. A quantitative study of muscle development in the bovine foetus (Bos indicus). Anat Histol Embryol. 1978 Sep;7(3):193–205. doi: 10.1111/j.1439-0264.1978.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Stickland N. C., Widdowson E. M., Goldspink G. Effects of severe energy and protein deficiencies on the fibres and nuclei in skeletal muscle of pigs. Br J Nutr. 1975 Nov;34(3):421–428. doi: 10.1017/s0007114575000487. [DOI] [PubMed] [Google Scholar]
- Stromer M. H., Goll D. E., Young R. B., Robson R. M., Parrish F. C., Jr Ultrastructural features of skeletal muscle differentiation and development. J Anim Sci. 1974 May;38(5):1111–1141. doi: 10.2527/jas1974.3851111x. [DOI] [PubMed] [Google Scholar]
- Swatland H. J., Cassens R. G. Prenatal development, histochemistry and innervation of porcine muscle. J Anim Sci. 1973 Feb;36(2):343–354. doi: 10.2527/jas1973.362343x. [DOI] [PubMed] [Google Scholar]
- Swatland H. J. Muscle growth in the fetal and neonatal pig. J Anim Sci. 1973 Aug;37(2):536–545. doi: 10.2527/jas1973.372536x. [DOI] [PubMed] [Google Scholar]
- Thurley D. C. Increase in diameter of muscle fibres in the foetal pig. Br Vet J. 1972 Jul;128(7):355–358. doi: 10.1016/s0007-1935(17)36885-9. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J., Colling-Saltin A. S. Cytological differentiation of human fetal skeletal muscle. Am J Anat. 1977 Jun;149(2):227–245. doi: 10.1002/aja.1001490207. [DOI] [PubMed] [Google Scholar]
- Webb J. N. The development of human skeletal muscle with particular reference to muscle cell death. J Pathol. 1972 Apr;106(4):221–228. doi: 10.1002/path.1711060403. [DOI] [PubMed] [Google Scholar]
- Widdowson E. M. Changes in the extracellular compartment of muscle and skin during normal and retarded development. Bibl Nutr Dieta. 1969;13:60–68. doi: 10.1159/000385152. [DOI] [PubMed] [Google Scholar]
- Widdowson E. M., Crabb D. E., Milner R. D. Cellular development of some human organs before birth. Arch Dis Child. 1972 Aug;47(254):652–655. doi: 10.1136/adc.47.254.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson E. M. Intra-uterine growth retardation in the pig. I. Organ size and cellular development at birth and after growth to maturity. Biol Neonate. 1971;19(4):329–340. doi: 10.1159/000240427. [DOI] [PubMed] [Google Scholar]
- Winick M., Noble A. Cellular response in rats during malnutrition at various ages. J Nutr. 1966 Jul;89(3):300–306. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]


