Abstract
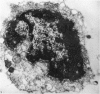
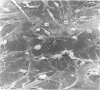
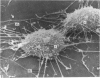
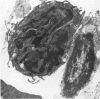
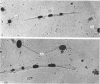
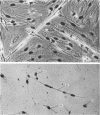
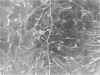
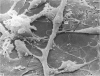
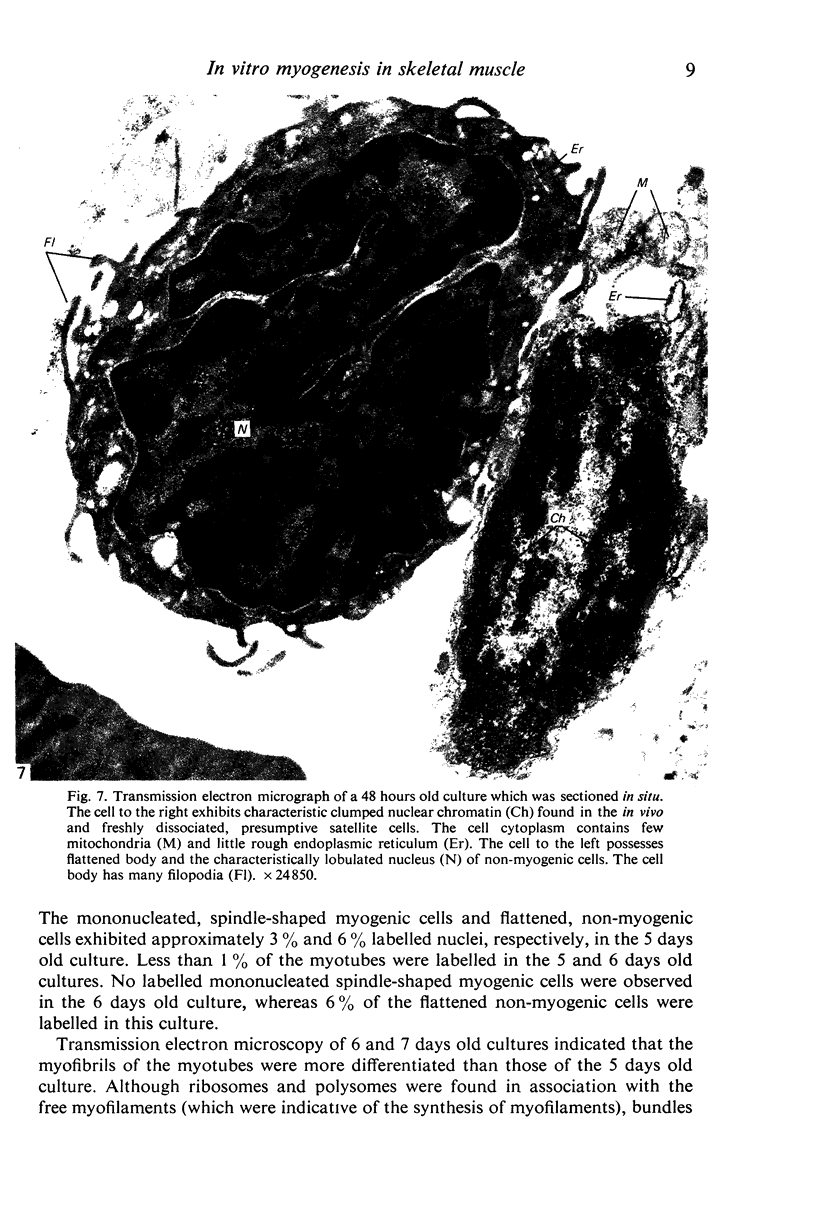
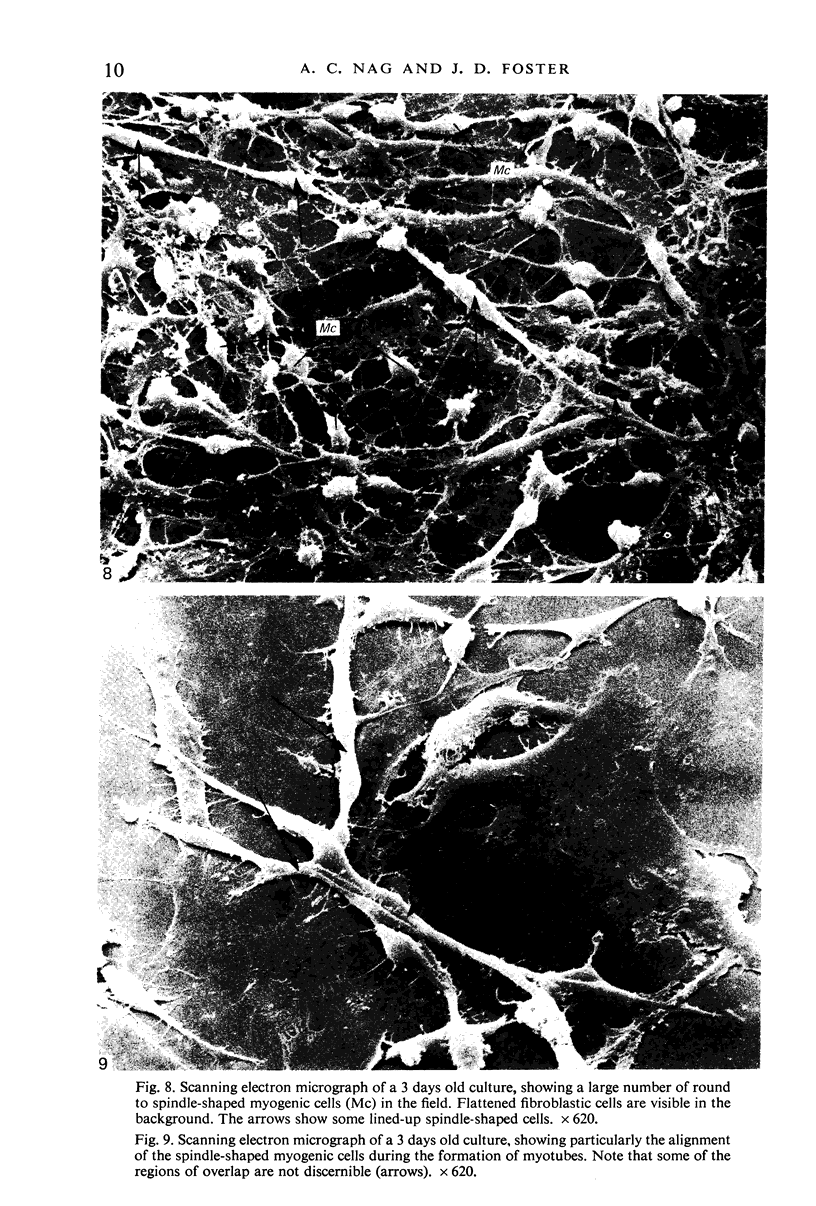
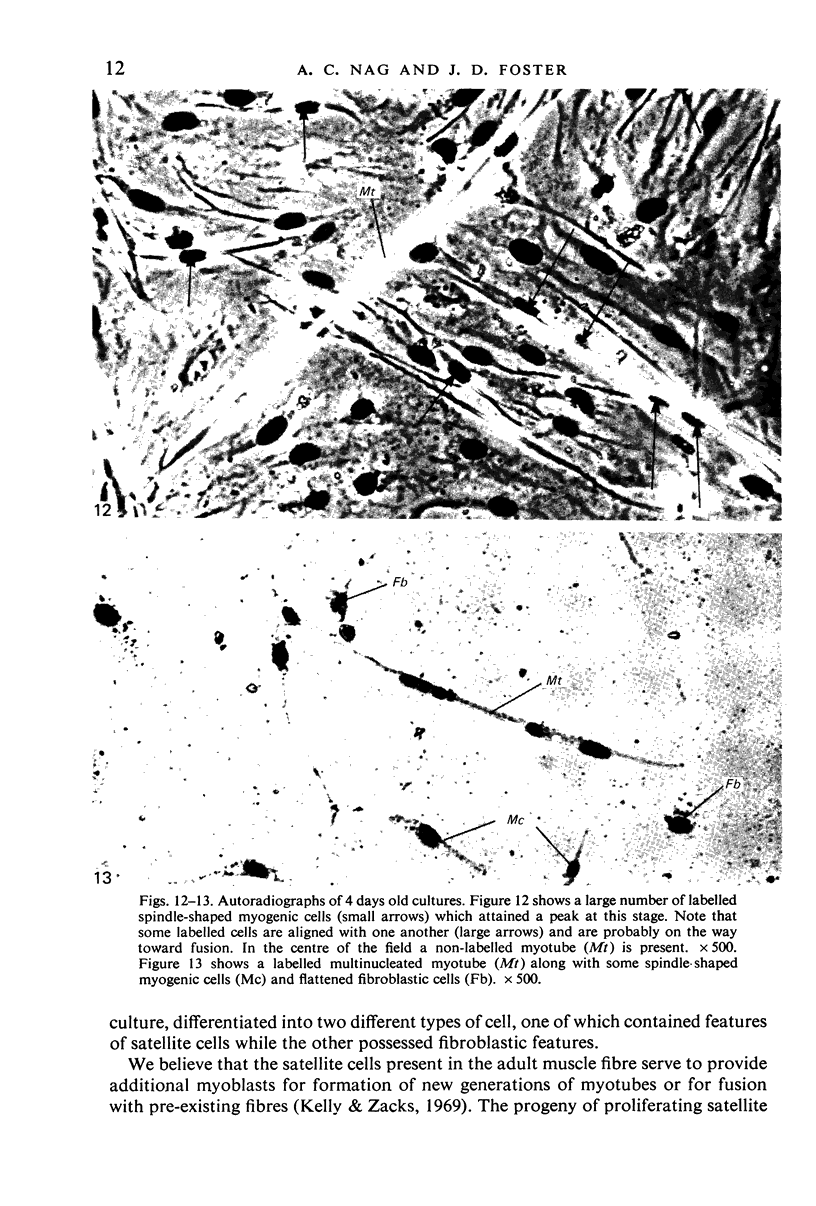
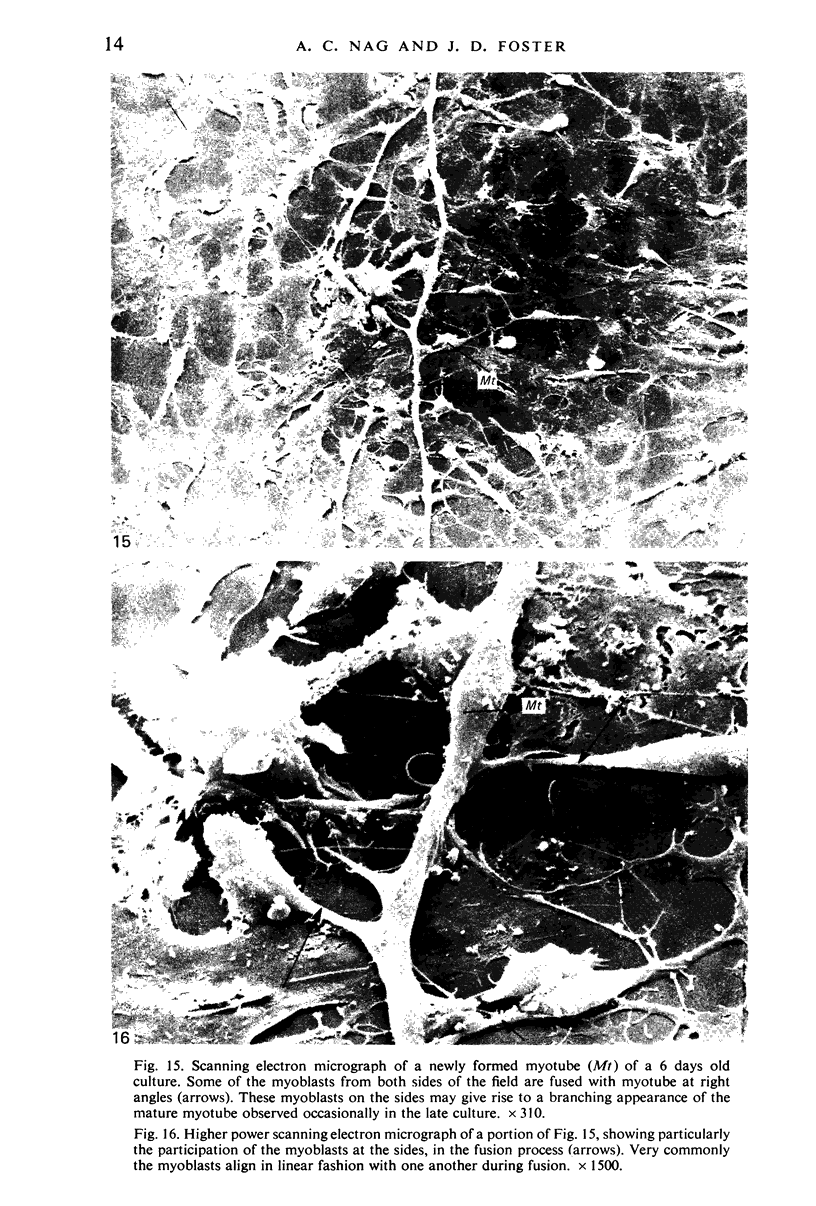
An injury to adult mammalian skeletal muscle is followed by regeneration, which involves a process believed to be similar to the differentiation of muscle fibres in the embryo. The origin of these differentiating myogenic cells is conjectural. The aim of the present study was to examine the source of myogenic cells and the process of myogenesis in adult skeletal muscle. Mononucleated cells were released from adult rat leg muscle mince after incubation with 0.1% pronase for 50-60 minutes at 37 degrees C. The ultrastructural studies revealed that the freshly dissociated mononucleated cells consisted of at least two populations of cells: myogenic satellite cells and non-myogenic fibroblastic cells. These cells were plated in growth media at various densities in cell culture dishes and incubated for 3 weeks in a balanced air atmosphere at 37 degrees C. The culture was routinely examined with a phase contrasted microscope for evidence of myogenic activities of the plated cells. At selected time intervals, the cell cultures were processed for autoradiography and scanning and transmission electron microscopy (SEM and TEM). Attachment of cells to the dish began soon after plating, with flattening of some non-muscle cells. The round- to spindle-shaped cells, indicative of myoblasts, began to appear within 24 hours. DNA synthesis and cell proliferation were observed in myogenic and non-myogenic cells within 24 hours of culture. SEM revealed that at 72 hours some myoblasts aligned and fused with one another, forming myotubes. Quantitation of autoradiographs indicated that the maximum number of labelled myotubes were present in the 3 days old culture, and thereafter, the labelled myotubes decreased in number and were absent in the 7 days old culture. Within 5-7 days the myotubes became larger and showed contractility. TEM of 6 to 21 day culture revealed that the myotubes contained well differentiated myofibrils, T-tubules and sarcoplasmic reticulum. It was evident from our studies that the mononucleated cells, having satellite cell morphology, were capable of differentiating into fully formed muscle fibres. This study lends support to the satellite cell hypothesis for regeneration of the skeletal muscle.
Full text
PDF



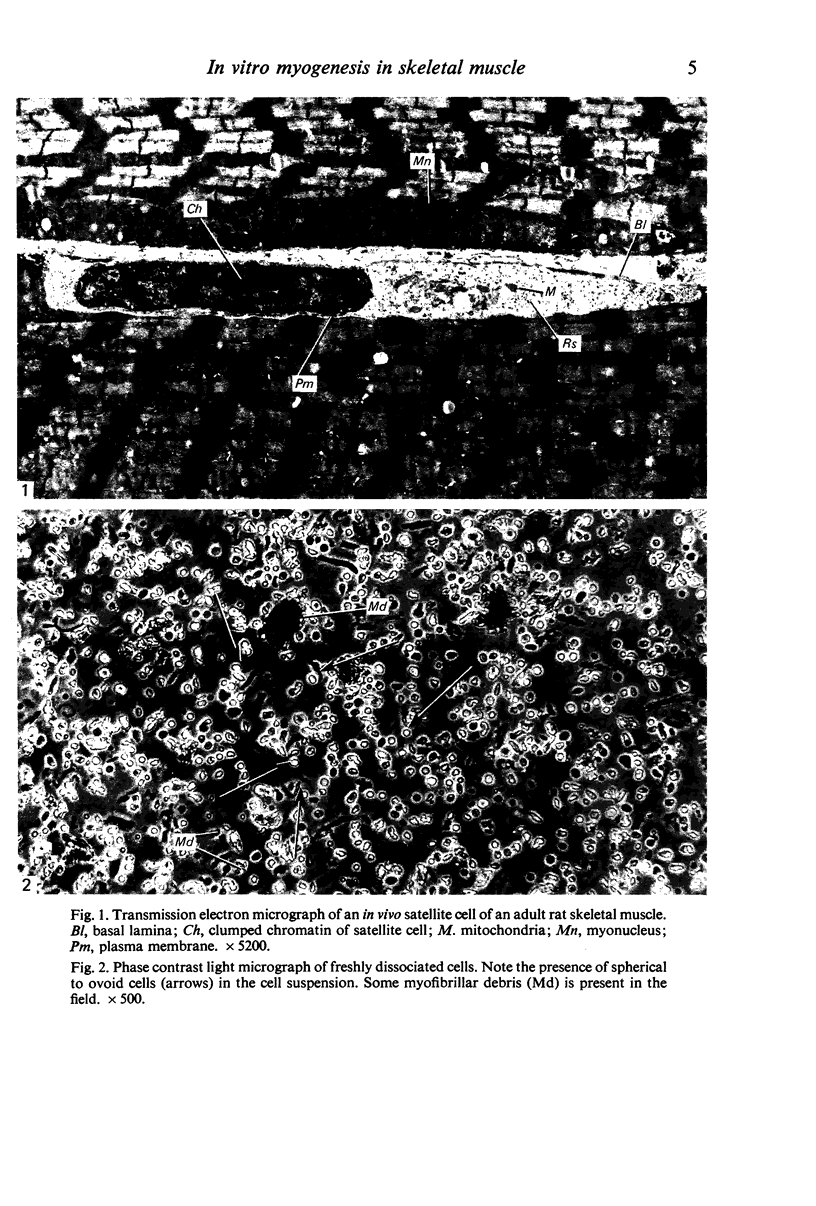
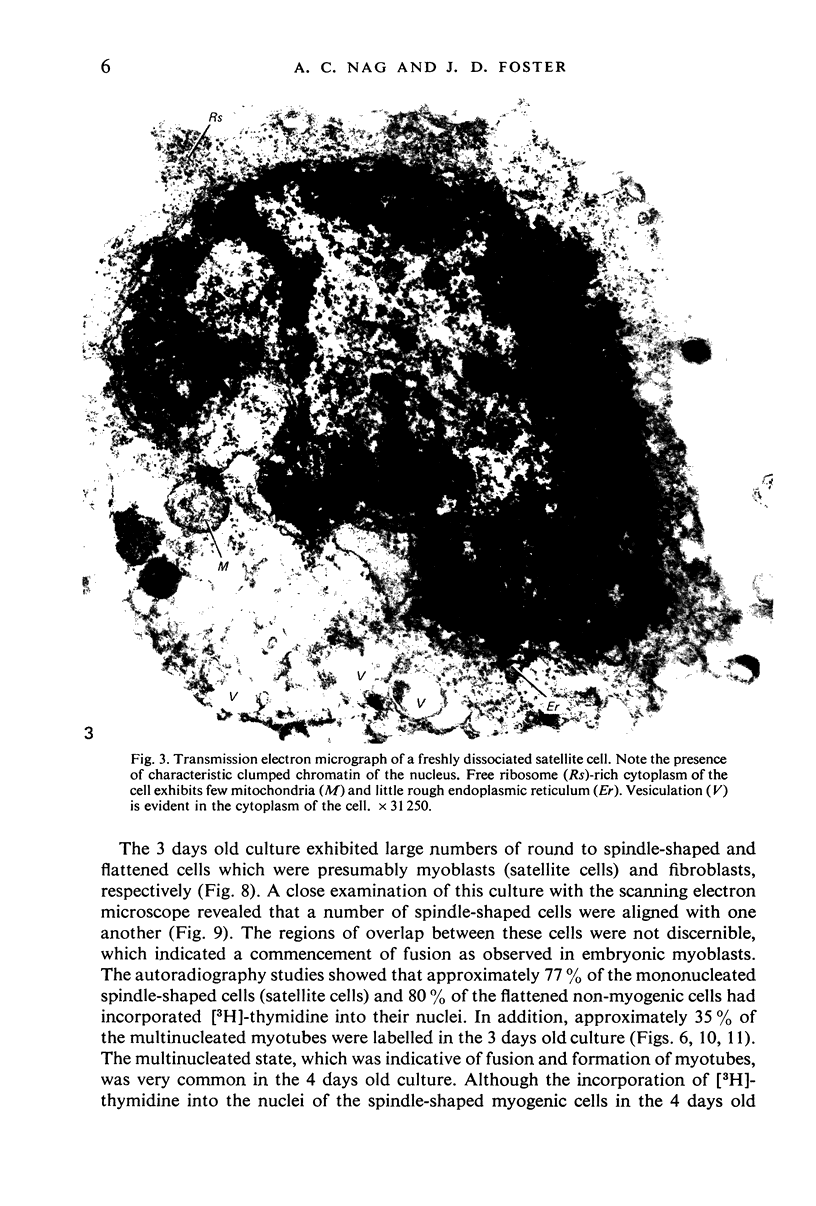
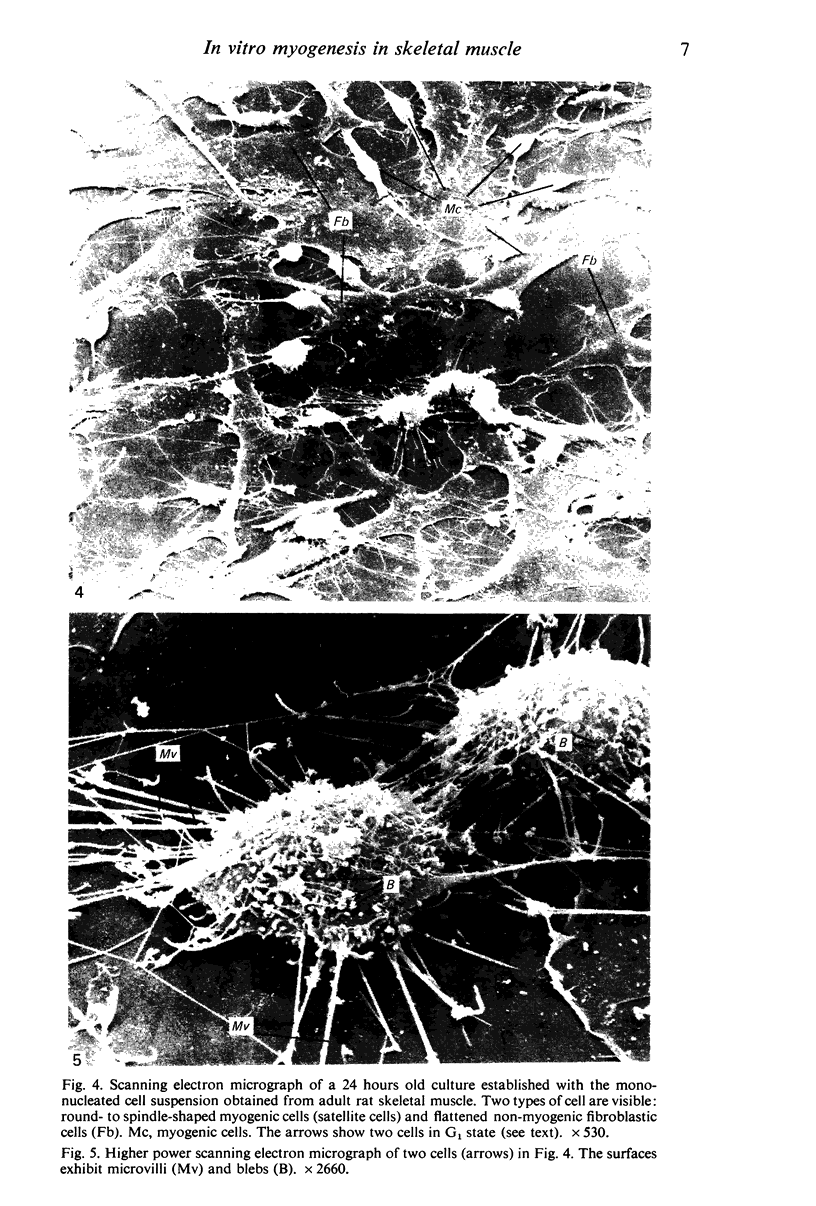
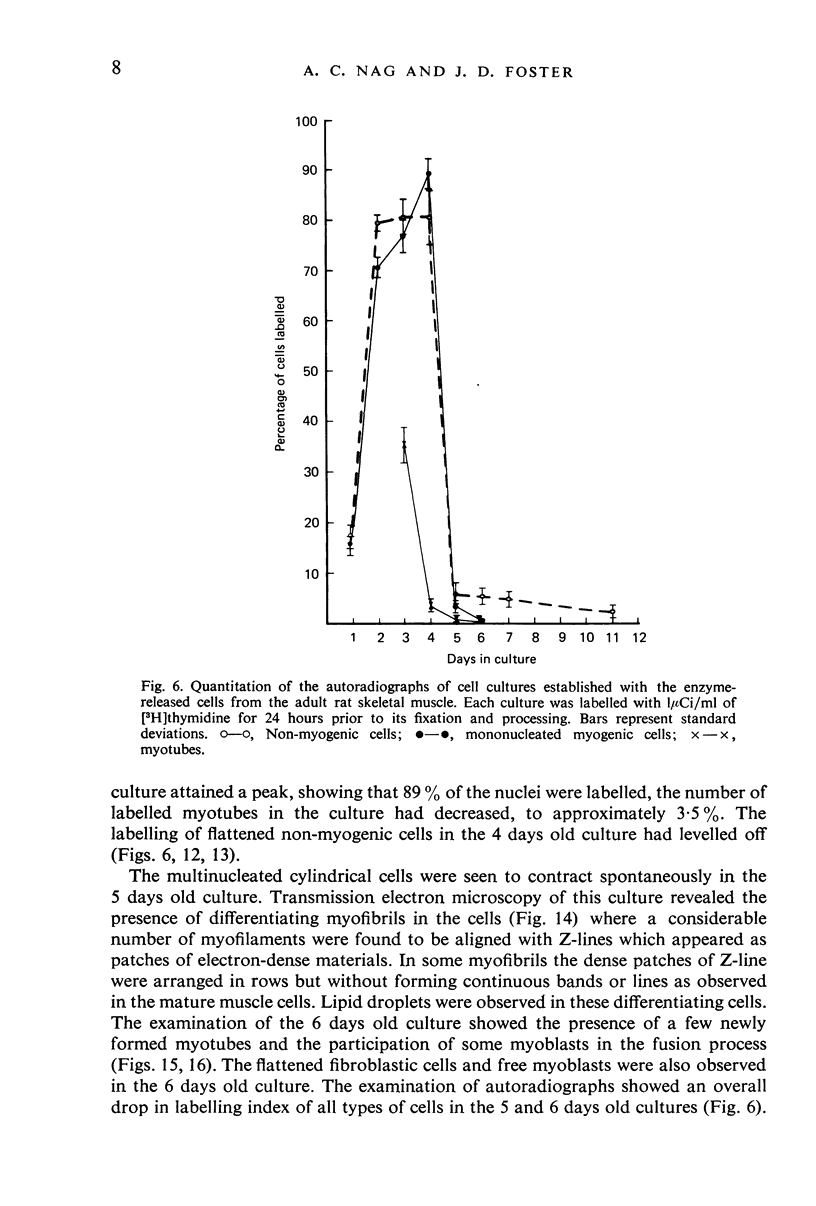






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbrook D. B., Han M. F., Hellmuth A. E. Population of muscle satellite cells in relation to age and mitotic activity. Pathology. 1971 Jul;3(3):223–243. doi: 10.3109/00313027109073739. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec. 1974 Dec;180(4):645–661. doi: 10.1002/ar.1091800410. [DOI] [PubMed] [Google Scholar]
- Carlson B. M. The regeneration of skeletal muscle. A review. Am J Anat. 1973 Jun;137(2):119–149. doi: 10.1002/aja.1001370202. [DOI] [PubMed] [Google Scholar]
- Hess A., Rosner S. The satellite cell bud and myoblast in denervated mammalian muscle fibers. Am J Anat. 1970 Sep;129(1):21–39. doi: 10.1002/aja.1001290103. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Electron microscopic observations of satellite cells with special reference to the development of mammalian skeletal muscles. Z Anat Entwicklungsgesch. 1966;125(1):43–63. doi: 10.1007/BF00521974. [DOI] [PubMed] [Google Scholar]
- Kelley R. O., Dekker R. A., Bluemink J. G. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J Ultrastruct Res. 1973 Nov;45(3):254–258. doi: 10.1016/s0022-5320(73)80051-6. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The histogenesis of rat intercostal muscle. J Cell Biol. 1969 Jul;42(1):135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg U. R., Lipton B. H., Konigsberg I. R. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol. 1975 Aug;45(2):260–275. doi: 10.1016/0012-1606(75)90065-2. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971 Aug;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Ontell M. Muscle satellite cells: a validated technique for light microscopic identification and a quantitative study of changes in their population following denervation. Anat Rec. 1974 Feb;178(2):211–227. doi: 10.1002/ar.1091780206. [DOI] [PubMed] [Google Scholar]
- Porter K., Prescott D., Frye J. Changes in surface morphology of Chinese hamster ovary cells during the cell cycle. J Cell Biol. 1973 Jun;57(3):815–836. doi: 10.1083/jcb.57.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik M. Origin of myoblasts during skeletal muscle regeneration. Electron microscopic observations. Lab Invest. 1969 Apr;20(4):353–363. [PubMed] [Google Scholar]
- Schultz E. Fine structure of satellite cells in growing skeletal muscle. Am J Anat. 1976 Sep;147(1):49–70. doi: 10.1002/aja.1001470105. [DOI] [PubMed] [Google Scholar]