Abstract
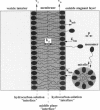
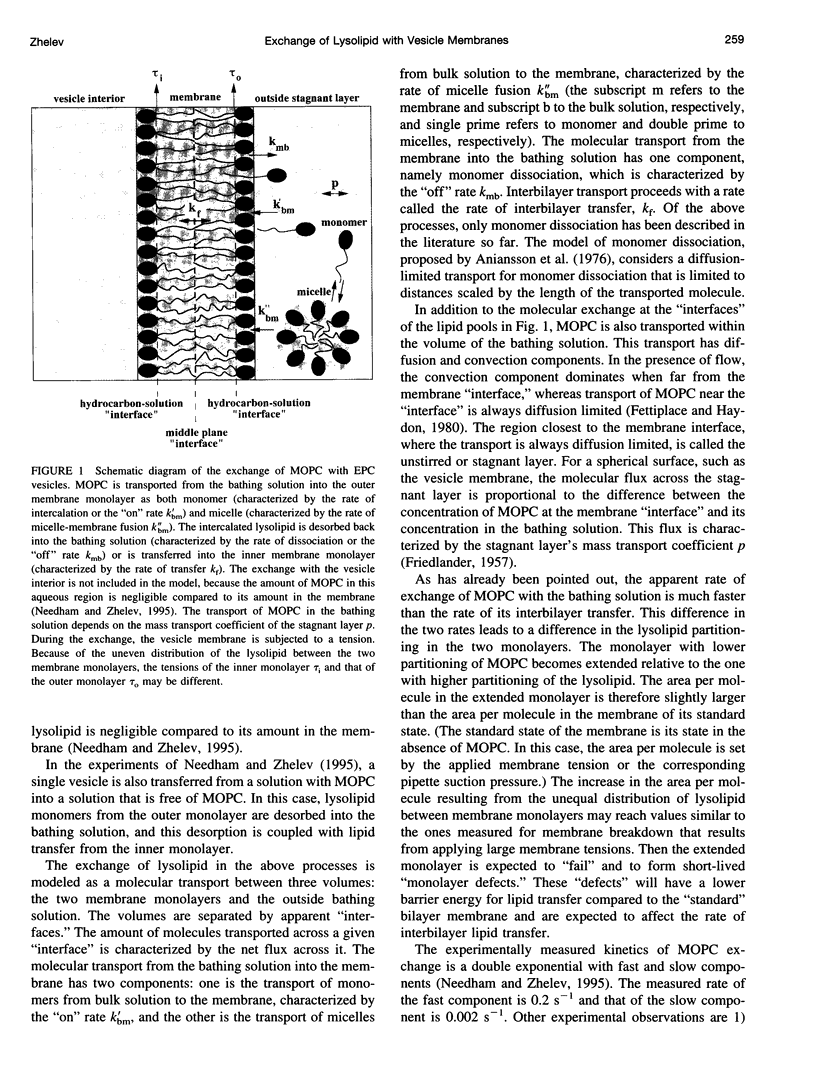
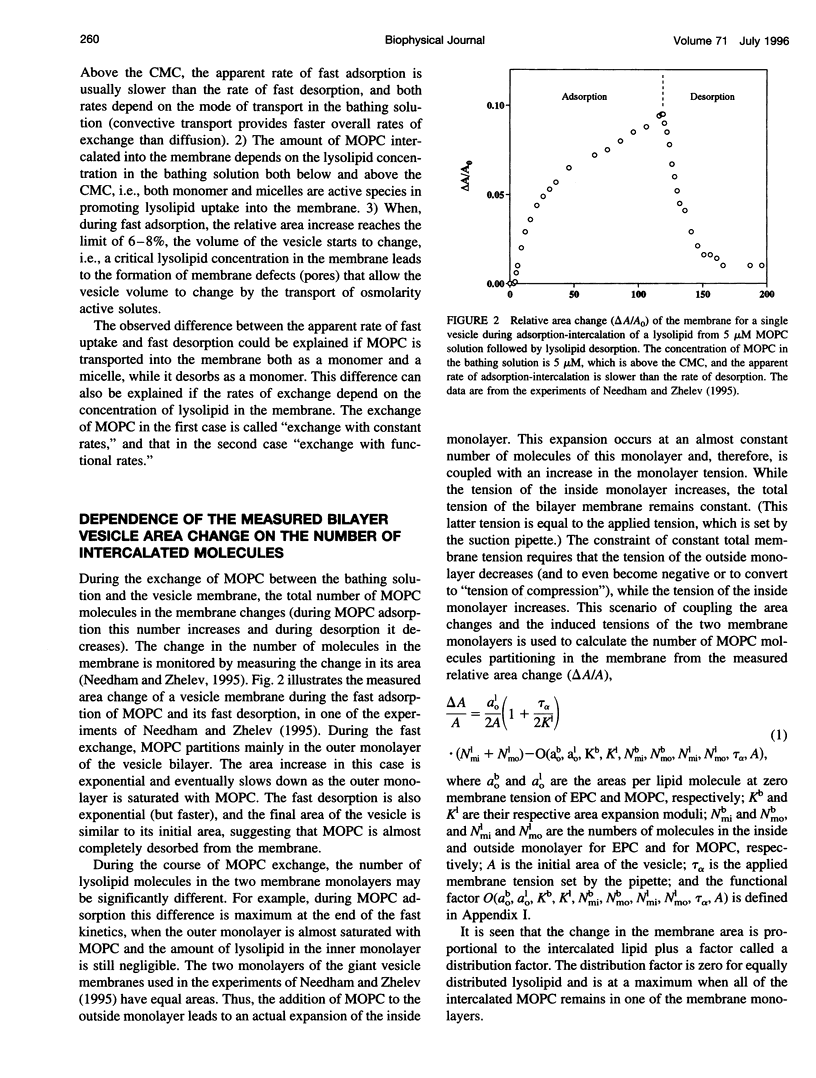
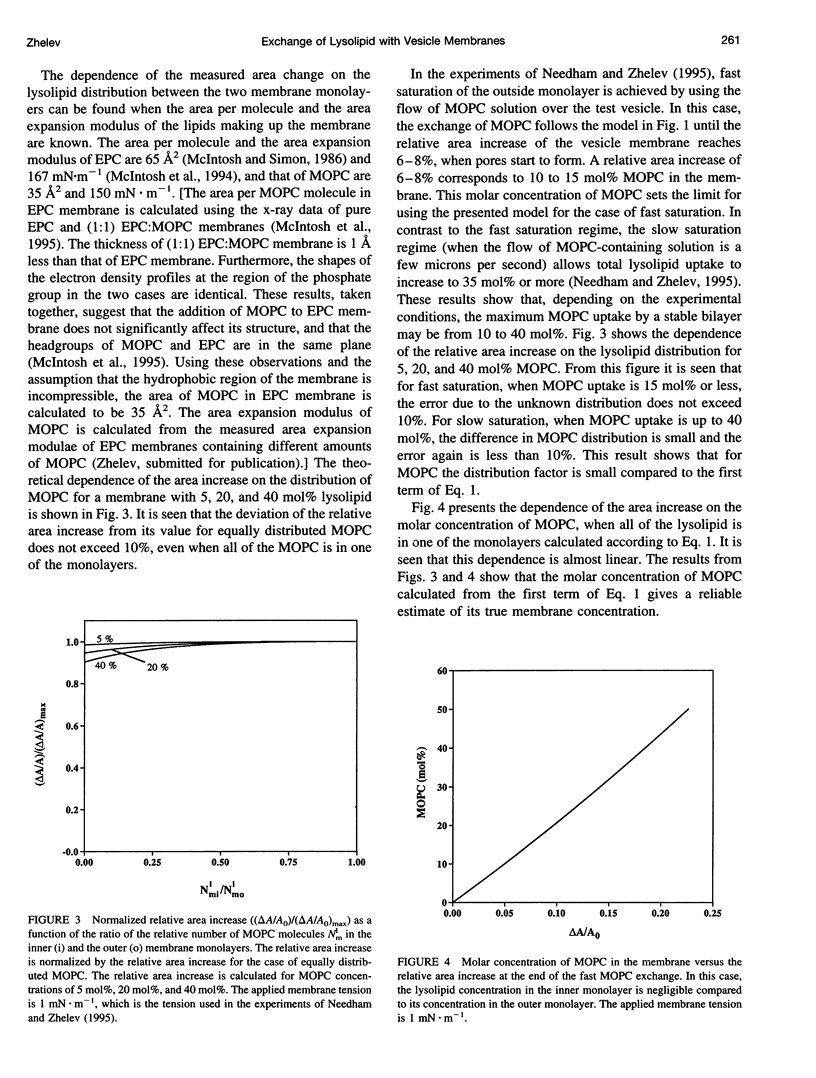
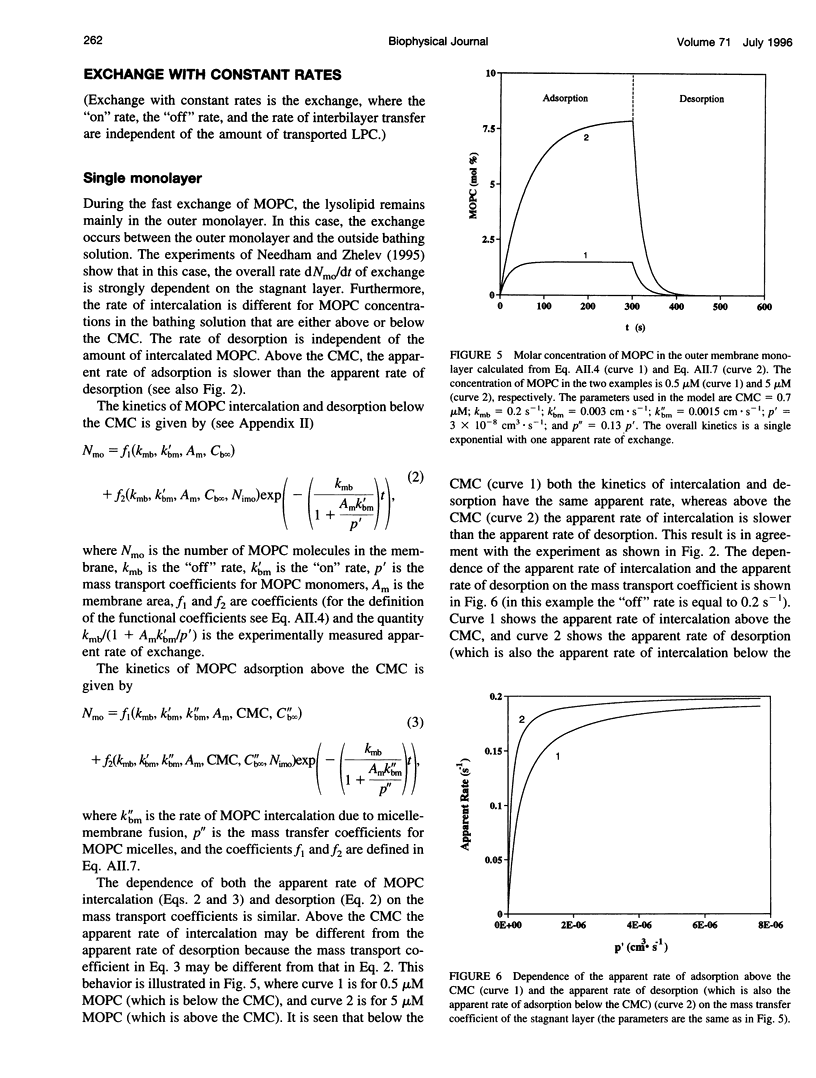
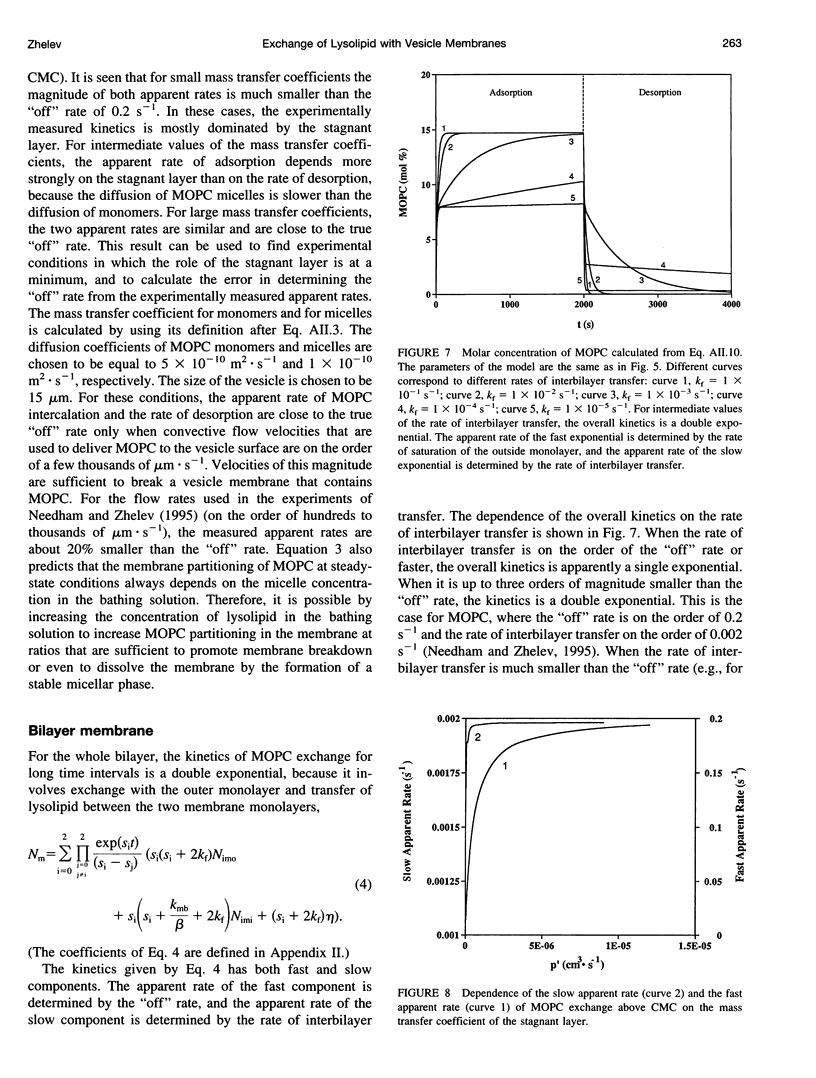
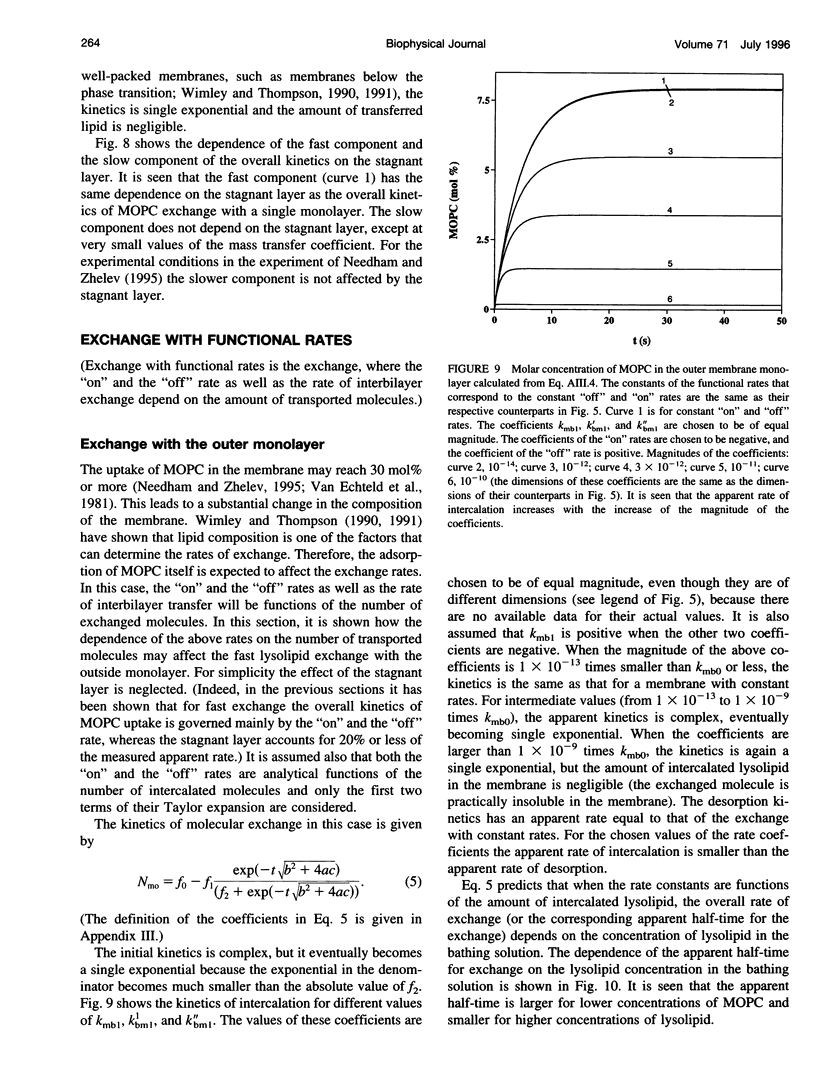
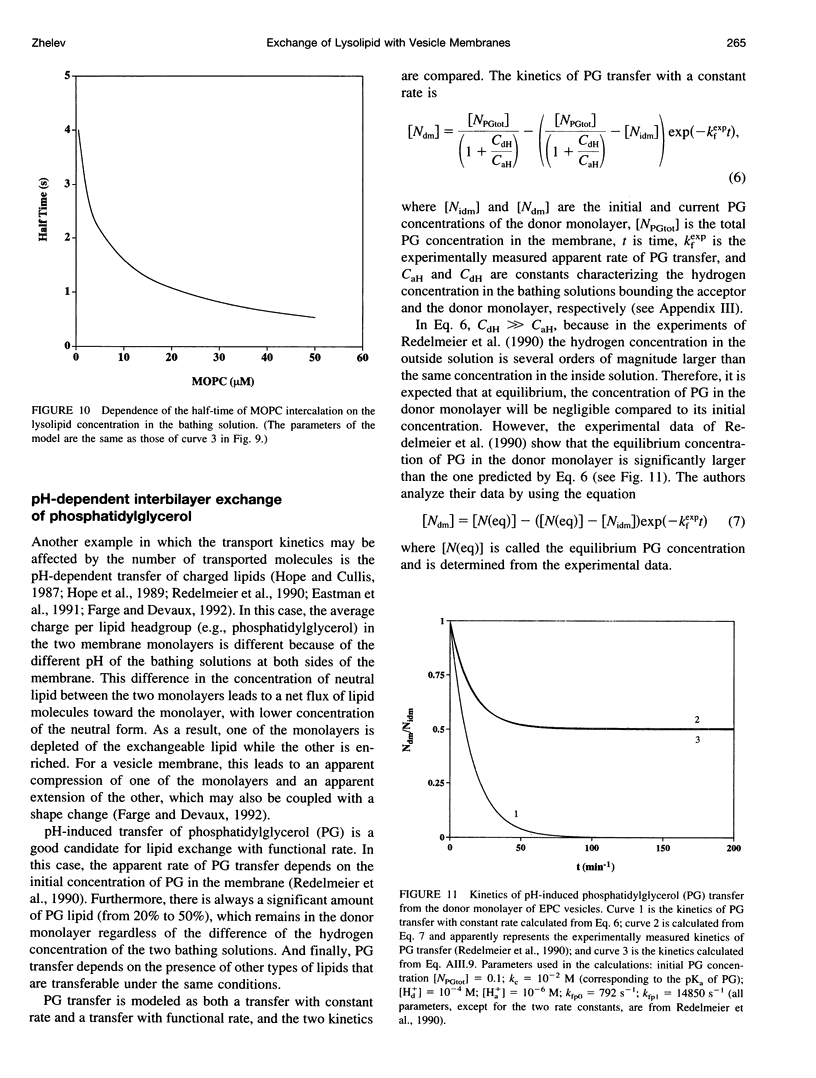
In a previous paper we described the experiments and the framework of a model for the exchange of monooleoylphosphatidylcholine with a single egg phosphatidylcholine membrane. In the present paper a model is presented that relates the experimentally measured apparent characteristics of the overall kinetics of lysolipid exchange to the true rates of lysolipid exchange and interbilayer transfer. It is shown that the adsorption of the lysolipid follows two pathways: one through the adsorption of lipid monomers and other through the fusion of micelles. The desorption of lysolipid follows a single pathway, namely, the desorption of monomers. The overall rate of fast desorption under convective flow conditions gives the true rate of monomer desorption from the outer membrane monolayer. The overall rate of both slow lysolipid uptake and slow desorption gives the rate of interbilayer transfer. Because of the uneven distribution of lysolipid between the two monolayers during its uptake, one of the membrane monolayers is apparently extended relative to the other. This relative extension of one of the monolayers induces a monolayer tension. The induced monolayer tension can increase up to 7 mN.m-1, when most of the intercalated lysolipid only partitions into the monolayer facing the lysolipid solution. This value is similar to the measured value for the critical monolayer tension of membrane failure, which is on the order of 5 mN.m-1. The similarity of the magnitudes of the induced monolayer tension during monooleoylphosphatidylcholine exchange and the monolayer tension of membrane failure suggests that the interbilayer lipid transfer may be affected by the formation of short living membrane defects. Furthermore, the pH-induced interbilayer exchange of phosphatidylglycerol is considered. In this case, it is shown that the rate of interbilayer transfer is a function of the phosphatidylglycerol concentration in the membrane.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson S., Dahlén B., Löfgren H., Pascher I. Lateral packing of hydrocarbon chains. Prog Chem Fats Other Lipids. 1978;16:125–143. doi: 10.1016/0079-6832(78)90039-3. [DOI] [PubMed] [Google Scholar]
- Asaoka Y., Yoshida K., Sasaki Y., Nishizuka Y., Murakami M., Kudo I., Inoue K. Possible role of mammalian secretory group II phospholipase A2 in T-lymphocyte activation: implication in propagation of inflammatory reaction. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):716–719. doi: 10.1073/pnas.90.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. L., Blaxall B. C., Reese D. A., Smith G. R., Bell J. D. Quantification of the interaction between lysolecithin and phospholipase A2. Biochim Biophys Acta. 1994 Mar 24;1211(3):289–300. doi: 10.1016/0005-2760(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Domanico P. L. Association and metabolism of exogenously-derived lysophosphatidylcholine by cultured mammalian cells: kinetics and mechanisms. Biochemistry. 1992 Feb 25;31(7):2046–2056. doi: 10.1021/bi00122a022. [DOI] [PubMed] [Google Scholar]
- Brown S. D., Baker B. L., Bell J. D. Quantification of the interaction of lysolecithin with phosphatidylcholine vesicles using bovine serum albumin: relevance to the activation of phospholipase A2. Biochim Biophys Acta. 1993 May 20;1168(1):13–22. doi: 10.1016/0005-2760(93)90260-g. [DOI] [PubMed] [Google Scholar]
- Eastman S. J., Hope M. J., Cullis P. R. Transbilayer transport of phosphatidic acid in response to transmembrane pH gradients. Biochemistry. 1991 Feb 19;30(7):1740–1745. doi: 10.1021/bi00221a002. [DOI] [PubMed] [Google Scholar]
- Elamrani K., Blume A. Incorporation kinetics of lysolecithin into lecithin vesicles. Kinetics of lysolecithin-induced vesicle fusion. Biochemistry. 1982 Feb 2;21(3):521–526. doi: 10.1021/bi00532a017. [DOI] [PubMed] [Google Scholar]
- Farge E., Devaux P. F. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys J. 1992 Feb;61(2):347–357. doi: 10.1016/S0006-3495(92)81841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Lee K. J., Huestis W. H. Lipid transfer between phosphatidylcholine vesicles and human erythrocytes: exponential decrease in rate with increasing acyl chain length. Biochemistry. 1985 Jun 4;24(12):2857–2864. doi: 10.1021/bi00333a007. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Lee K. J., Huestis W. H. Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry. 1985 Jun 4;24(12):2849–2857. doi: 10.1021/bi00333a006. [DOI] [PubMed] [Google Scholar]
- Fettiplace R., Haydon D. A. Water permeability of lipid membranes. Physiol Rev. 1980 Apr;60(2):510–550. doi: 10.1152/physrev.1980.60.2.510. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hoffman R. D., Kligerman M., Sundt T. M., Anderson N. D., Shin H. S. Stereospecific chemoattraction of lymphoblastic cells by gradients of lysophosphatidylcholine. Proc Natl Acad Sci U S A. 1982 May;79(10):3285–3289. doi: 10.1073/pnas.79.10.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan R., Pownall H. J. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim Biophys Acta. 1988 Feb 18;938(2):155–166. doi: 10.1016/0005-2736(88)90155-1. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Cullis P. R. Lipid asymmetry induced by transmembrane pH gradients in large unilamellar vesicles. J Biol Chem. 1987 Mar 25;262(9):4360–4366. [PubMed] [Google Scholar]
- Hope M. J., Redelmeier T. E., Wong K. F., Rodrigueza W., Cullis P. R. Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients. Biochemistry. 1989 May 16;28(10):4181–4187. doi: 10.1021/bi00436a009. [DOI] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin E. L., Jr, Kugiyama K., Nguy J. H., Kerns S. A., Henry P. D. Effects of lysolipids and oxidatively modified low density lipoprotein on endothelium-dependent relaxation of rabbit aorta. Circ Res. 1993 Jan;72(1):161–166. doi: 10.1161/01.res.72.1.161. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Advani S., Burton R. E., Zhelev D. V., Needham D., Simon S. A. Experimental tests for protrusion and undulation pressures in phospholipid bilayers. Biochemistry. 1995 Jul 11;34(27):8520–8532. doi: 10.1021/bi00027a002. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Nakano T., Raines E. W., Abraham J. A., Klagsbrun M., Ross R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1069–1073. doi: 10.1073/pnas.91.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraba H., Imai Y., Hayashi M., Oh-ishi S. Enhanced production of platelet-activating factor in stimulated rat leukocytes caused by the blockade of lysophospholipid acylation. Jpn J Pharmacol. 1993 Feb;61(2):109–113. doi: 10.1254/jjp.61.109. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., Zhelev D. V. Lysolipid exchange with lipid vesicle membranes. Ann Biomed Eng. 1995 May-Jun;23(3):287–298. doi: 10.1007/BF02584429. [DOI] [PubMed] [Google Scholar]
- Nichols J. W. Thermodynamics and kinetics of phospholipid monomer-vesicle interaction. Biochemistry. 1985 Nov 5;24(23):6390–6398. doi: 10.1021/bi00344a011. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelmeier T. E., Hope M. J., Cullis P. R. On the mechanism of transbilayer transport of phosphatidylglycerol in response to transmembrane pH gradients. Biochemistry. 1990 Mar 27;29(12):3046–3053. doi: 10.1021/bi00464a022. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- Saito T., Wolf A., Menon N. K., Saeed M., Alves C., Bing R. J. Lysolecithins as endothelium-dependent vascular smooth muscle relaxants that differ from endothelium-derived relaxing factor (nitric oxide) Proc Natl Acad Sci U S A. 1988 Nov;85(21):8246–8250. doi: 10.1073/pnas.85.21.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Baldwin J. H., Nilsen-Hamilton M., Hamilton R. T., Thanassi N. M. Regulation of guanylate and adenylate cyclase activities by lysolecithin. Proc Natl Acad Sci U S A. 1976 May;73(5):1586–1590. doi: 10.1073/pnas.73.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., Leventis R. Spontaneous interbilayer transfer of phospholipids: dependence on acyl chain composition. Biochemistry. 1993 Dec 7;32(48):13318–13326. doi: 10.1021/bi00211a045. [DOI] [PubMed] [Google Scholar]
- Storch J., Kleinfeld A. M. Transfer of long-chain fluorescent free fatty acids between unilamellar vesicles. Biochemistry. 1986 Apr 8;25(7):1717–1726. doi: 10.1021/bi00355a041. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Van Echteld C. J., De Kruijff B., Mandersloot J. G., De Gier J. Effects of lysophosphatidylcholines on phosphatidylcholine and phosphatidylcholine/cholesterol liposome systems as revealed by 31P-NMR, electron microscopy and permeability studies. Biochim Biophys Acta. 1981 Dec 7;649(2):211–220. doi: 10.1016/0005-2736(81)90408-9. [DOI] [PubMed] [Google Scholar]
- Vogel S. S., Leikina E. A., Chernomordik L. V. Lysophosphatidylcholine reversibly arrests exocytosis and viral fusion at a stage between triggering and membrane merger. J Biol Chem. 1993 Dec 5;268(34):25764–25768. [PubMed] [Google Scholar]
- Wimley W. C., Thompson T. E. Exchange and flip-flop of dimyristoylphosphatidylcholine in liquid-crystalline, gel, and two-component, two-phase large unilamellar vesicles. Biochemistry. 1990 Feb 6;29(5):1296–1303. doi: 10.1021/bi00457a027. [DOI] [PubMed] [Google Scholar]
- Wimley W. C., Thompson T. E. Transbilayer and interbilayer phospholipid exchange in dimyristoylphosphatidylcholine/dimyristoylphosphatidylethanolamine large unilamellar vesicles. Biochemistry. 1991 Feb 12;30(6):1702–1709. doi: 10.1021/bi00220a036. [DOI] [PubMed] [Google Scholar]
- Zhelev D. V., Needham D. Tension-stabilized pores in giant vesicles: determination of pore size and pore line tension. Biochim Biophys Acta. 1993 Apr 8;1147(1):89–104. doi: 10.1016/0005-2736(93)90319-u. [DOI] [PubMed] [Google Scholar]