Abstract
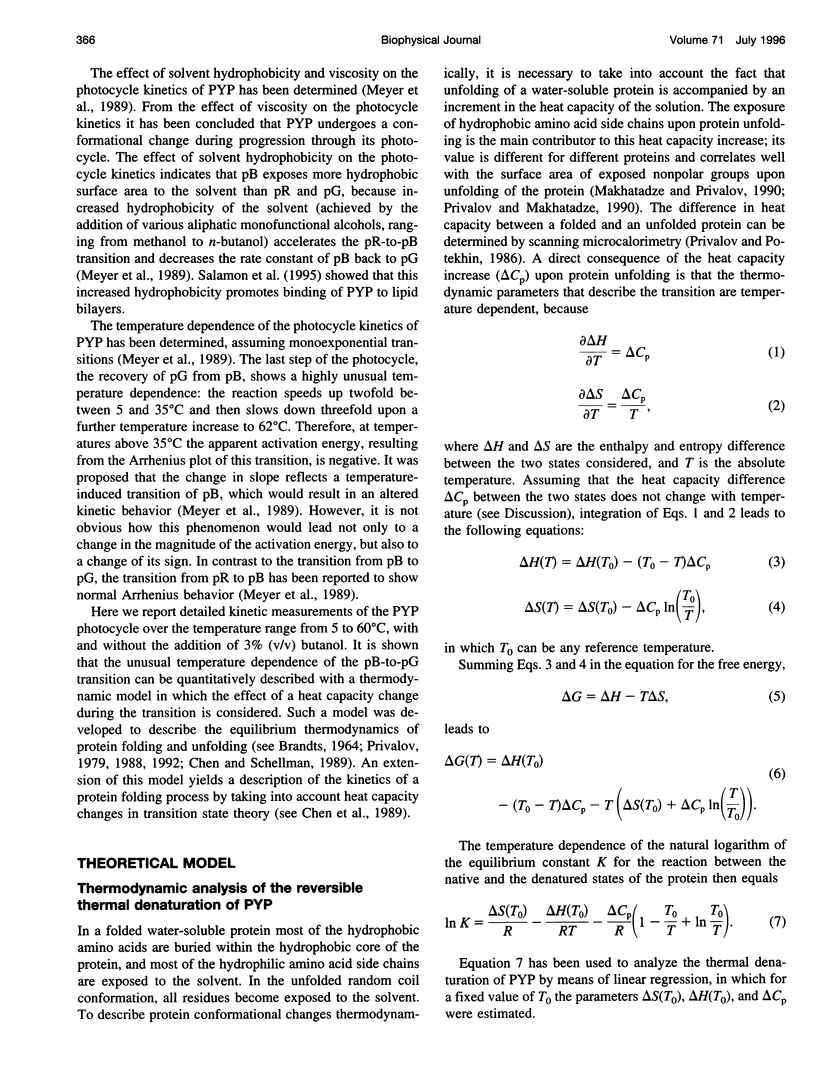
Two complementary aspects of the thermodynamics of the photoactive yellow protein (PYP), a new type of photoreceptor that has been isolated from Ectothiorhodospira halophila, have been investigated. First, the thermal denaturation of PYP at pH 3.4 has been examined by global analysis of the temperature-induced changes in the UV-VIS absorbance spectrum of this chromophoric protein. Subsequently, a thermodynamic model for protein (un)folding processes, incorporating heat capacity changes, has been applied to these data. The second aspect of PYP that has been studied is the temperature dependence of its photocycle kinetics, which have been reported to display an unexplained deviation from normal Arrhenius behavior. We have extended these measurements in two solvents with different hydrophobicities and have analyzed the number of rate constants needed to describe these data. Here we show that the resulting temperature dependence of the rate constants can be quantitatively explained by the application of a thermodynamic model which assumes that heat capacity changes are associated with the two transitions in the photocycle of PYP. This result is the first example of an enzyme catalytic cycle being described by a thermodynamic model including heat capacity changes. It is proposed that a strong link exists between the processes occurring during the photocycle of PYP and protein (un)folding processes. This permits a thermodynamic analysis of the light-induced, physiologically relevant, conformational changes occurring in this photoreceptor protein.
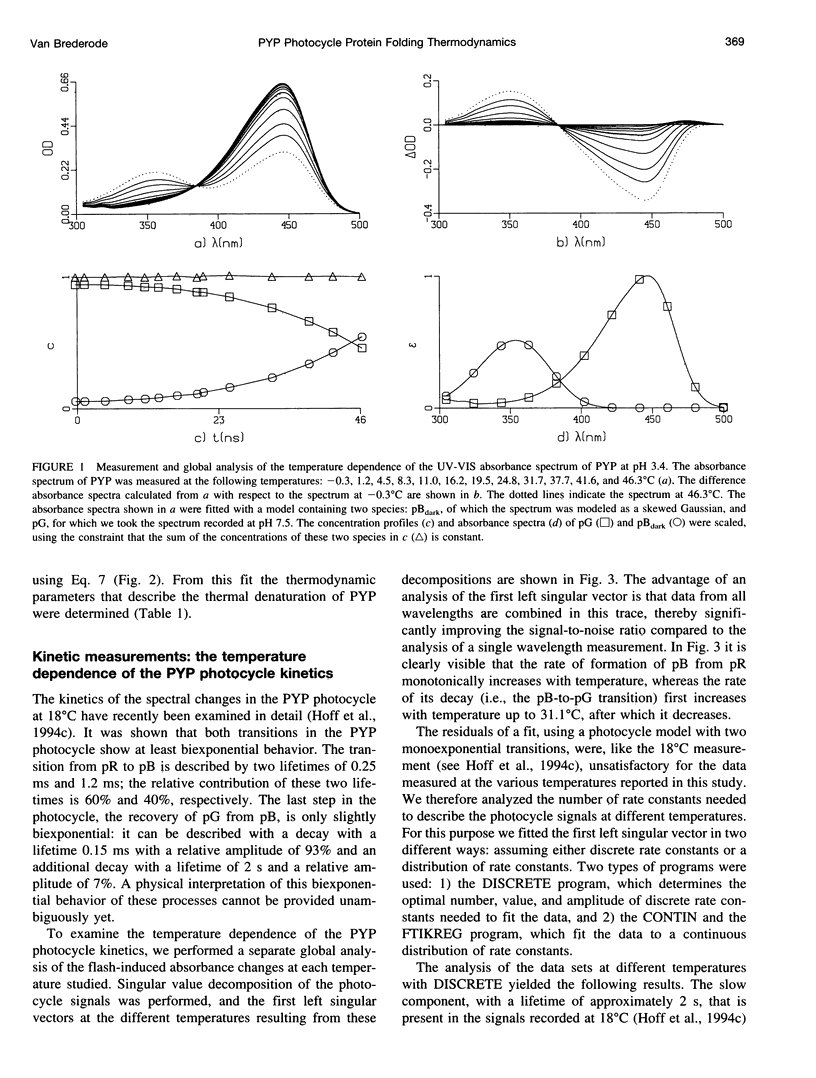
Full text
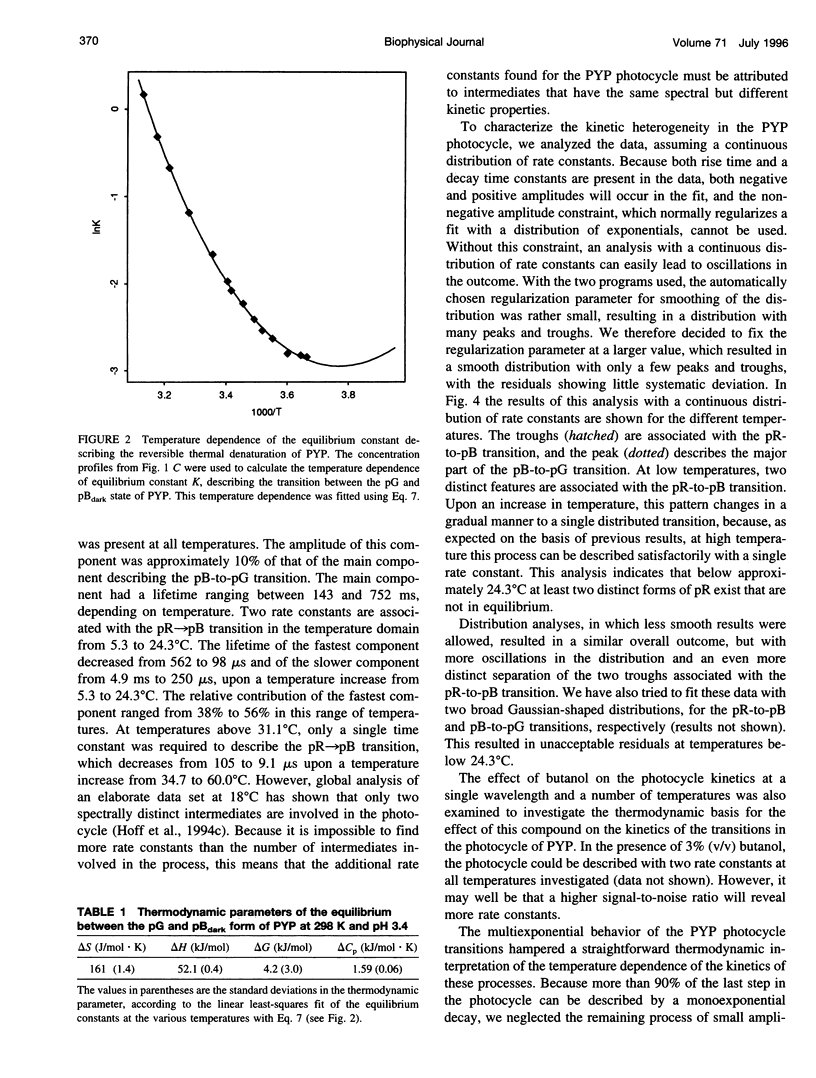
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baca M., Borgstahl G. E., Boissinot M., Burke P. M., Williams D. R., Slater K. A., Getzoff E. D. Complete chemical structure of photoactive yellow protein: novel thioester-linked 4-hydroxycinnamyl chromophore and photocycle chemistry. Biochemistry. 1994 Dec 6;33(48):14369–14377. doi: 10.1021/bi00252a001. [DOI] [PubMed] [Google Scholar]
- Borgstahl G. E., Williams D. R., Getzoff E. D. 1.4 A structure of photoactive yellow protein, a cytosolic photoreceptor: unusual fold, active site, and chromophore. Biochemistry. 1995 May 16;34(19):6278–6287. doi: 10.1021/bi00019a004. [DOI] [PubMed] [Google Scholar]
- Chen B. L., Baase W. A., Schellman J. A. Low-temperature unfolding of a mutant of phage T4 lysozyme. 2. Kinetic investigations. Biochemistry. 1989 Jan 24;28(2):691–699. doi: 10.1021/bi00428a042. [DOI] [PubMed] [Google Scholar]
- Chen B. L., Schellman J. A. Low-temperature unfolding of a mutant of phage T4 lysozyme. 1. Equilibrium studies. Biochemistry. 1989 Jan 24;28(2):685–691. doi: 10.1021/bi00428a041. [DOI] [PubMed] [Google Scholar]
- Devault D. Quantum mechanical tunnelling in biological systems. Q Rev Biophys. 1980 Nov;13(4):387–564. doi: 10.1017/s003358350000175x. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh Z. T., Hideg K., Hubbell W. L. Photoactivated conformational changes in rhodopsin: a time-resolved spin label study. Science. 1993 Nov 26;262(5138):1416–1419. doi: 10.1126/science.8248781. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hoff W. D., Düx P., Hård K., Devreese B., Nugteren-Roodzant I. M., Crielaard W., Boelens R., Kaptein R., van Beeumen J., Hellingwerf K. J. Thiol ester-linked p-coumaric acid as a new photoactive prosthetic group in a protein with rhodopsin-like photochemistry. Biochemistry. 1994 Nov 29;33(47):13959–13962. doi: 10.1021/bi00251a001. [DOI] [PubMed] [Google Scholar]
- Hoff W. D., Sprenger W. W., Postma P. W., Meyer T. E., Veenhuis M., Leguijt T., Hellingwerf K. J. The photoactive yellow protein from Ectothiorhodospira halophila as studied with a highly specific polyclonal antiserum: (intra)cellular localization, regulation of expression, and taxonomic distribution of cross-reacting proteins. J Bacteriol. 1994 Jul;176(13):3920–3927. doi: 10.1128/jb.176.13.3920-3927.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff W. D., van Stokkum I. H., van Ramesdonk H. J., van Brederode M. E., Brouwer A. M., Fitch J. C., Meyer T. E., van Grondelle R., Hellingwerf K. J. Measurement and global analysis of the absorbance changes in the photocycle of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys J. 1994 Oct;67(4):1691–1705. doi: 10.1016/S0006-3495(94)80643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. E., Fersht A. R. Folding of chymotrypsin inhibitor 2. 2. Influence of proline isomerization on the folding kinetics and thermodynamic characterization of the transition state of folding. Biochemistry. 1991 Oct 29;30(43):10436–10443. doi: 10.1021/bi00107a011. [DOI] [PubMed] [Google Scholar]
- Makhatadze G. I., Privalov P. L. Heat capacity of proteins. I. Partial molar heat capacity of individual amino acid residues in aqueous solution: hydration effect. J Mol Biol. 1990 May 20;213(2):375–384. doi: 10.1016/S0022-2836(05)80197-4. [DOI] [PubMed] [Google Scholar]
- McRee D. E., Tainer J. A., Meyer T. E., Van Beeumen J., Cusanovich M. A., Getzoff E. D. Crystallographic structure of a photoreceptor protein at 2.4 A resolution. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6533–6537. doi: 10.1073/pnas.86.17.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Fitch J. C., Bartsch R. G., Tollin G., Cusanovich M. A. Soluble cytochromes and a photoactive yellow protein isolated from the moderately halophilic purple phototrophic bacterium, Rhodospirillum salexigens. Biochim Biophys Acta. 1990 Apr 26;1016(3):364–370. doi: 10.1016/0005-2728(90)90170-9. [DOI] [PubMed] [Google Scholar]
- Meyer T. E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim Biophys Acta. 1985 Jan 23;806(1):175–183. doi: 10.1016/0005-2728(85)90094-5. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Tollin G., Causgrove T. P., Cheng P., Blankenship R. E. Picosecond decay kinetics and quantum yield of fluorescence of the photoactive yellow protein from the halophilic purple phototrophic bacterium, Ectothiorhodospira halophila. Biophys J. 1991 May;59(5):988–991. doi: 10.1016/S0006-3495(91)82313-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Tollin G., Hazzard J. H., Cusanovich M. A. Photoactive yellow protein from the purple phototrophic bacterium, Ectothiorhodospira halophila. Quantum yield of photobleaching and effects of temperature, alcohols, glycerol, and sucrose on kinetics of photobleaching and recovery. Biophys J. 1989 Sep;56(3):559–564. doi: 10.1016/S0006-3495(89)82703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Yakali E., Cusanovich M. A., Tollin G. Properties of a water-soluble, yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry. 1987 Jan 27;26(2):418–423. doi: 10.1021/bi00376a012. [DOI] [PubMed] [Google Scholar]
- Mizutani Y., Tokutomi S., Kaminaka S., Kitagawa T. Ultraviolet resonance Raman spectra of pea intact, large, and small phytochromes: differences in molecular topography of the red- and far-red-absorbing forms. Biochemistry. 1993 Jul 13;32(27):6916–6922. doi: 10.1021/bi00078a015. [DOI] [PubMed] [Google Scholar]
- Ng K., Getzoff E. D., Moffat K. Optical studies of a bacterial photoreceptor protein, photoactive yellow protein, in single crystals. Biochemistry. 1995 Jan 24;34(3):879–890. doi: 10.1021/bi00003a022. [DOI] [PubMed] [Google Scholar]
- Pace C. N. Conformational stability of globular proteins. Trends Biochem Sci. 1990 Jan;15(1):14–17. doi: 10.1016/0968-0004(90)90124-t. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Gill S. J. Stability of protein structure and hydrophobic interaction. Adv Protein Chem. 1988;39:191–234. doi: 10.1016/s0065-3233(08)60377-0. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Makhatadze G. I. Contribution of hydration and non-covalent interactions to the heat capacity effect on protein unfolding. J Mol Biol. 1992 Apr 5;224(3):715–723. doi: 10.1016/0022-2836(92)90555-x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Potekhin S. A. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Rath P., DeCaluwé L. L., Bovee-Geurts P. H., DeGrip W. J., Rothschild K. J. Fourier transform infrared difference spectroscopy of rhodopsin mutants: light activation of rhodopsin causes hydrogen-bonding change in residue aspartic acid-83 during meta II formation. Biochemistry. 1993 Oct 5;32(39):10277–10282. doi: 10.1021/bi00090a001. [DOI] [PubMed] [Google Scholar]
- Resek J. F., Farahbakhsh Z. T., Hubbell W. L., Khorana H. G. Formation of the meta II photointermediate is accompanied by conformational changes in the cytoplasmic surface of rhodopsin. Biochemistry. 1993 Nov 16;32(45):12025–12032. doi: 10.1021/bi00096a012. [DOI] [PubMed] [Google Scholar]
- Salamon Z., Meyer T. E., Tollin G. Photobleaching of the photoactive yellow protein from Ectothiorhodospira halophila promotes binding to lipid bilayers: evidence from surface plasmon resonance spectroscopy. Biophys J. 1995 Feb;68(2):648–654. doi: 10.1016/S0006-3495(95)80225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa S., Sugihara M. Characterization of the transition state of lysozyme unfolding. I. Effect of protein-solvent interactions on the transition state. Biopolymers. 1984 Nov;23(11 Pt 2):2473–2488. doi: 10.1002/bip.360231122. [DOI] [PubMed] [Google Scholar]
- Sprenger W. W., Hoff W. D., Armitage J. P., Hellingwerf K. J. The eubacterium Ectothiorhodospira halophila is negatively phototactic, with a wavelength dependence that fits the absorption spectrum of the photoactive yellow protein. J Bacteriol. 1993 May;175(10):3096–3104. doi: 10.1128/jb.175.10.3096-3104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beeumen J. J., Devreese B. V., Van Bun S. M., Hoff W. D., Hellingwerf K. J., Meyer T. E., McRee D. E., Cusanovich M. A. Primary structure of a photoactive yellow protein from the phototrophic bacterium Ectothiorhodospira halophila, with evidence for the mass and the binding site of the chromophore. Protein Sci. 1993 Jul;2(7):1114–1125. doi: 10.1002/pro.5560020706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Wells T. A., Nakazawa M., Manabe K., Song P. S. A conformational change associated with the phototransformation of Pisum phytochrome A as probed by fluorescence quenching. Biochemistry. 1994 Jan 25;33(3):708–712. doi: 10.1021/bi00169a012. [DOI] [PubMed] [Google Scholar]
- Xie D., Bhakuni V., Freire E. Calorimetric determination of the energetics of the molten globule intermediate in protein folding: apo-alpha-lactalbumin. Biochemistry. 1991 Nov 5;30(44):10673–10678. doi: 10.1021/bi00108a010. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. T. Photoreversible change in the conformation of phytochrome as probed with a covalently bound fluorescent sulfhydryl reagent, N-(9-acridinyl)maleimide. Biochim Biophys Acta. 1993 Jun 4;1163(3):227–233. doi: 10.1016/0167-4838(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Yan B., Nakanishi K., Spudich J. L. Mechanism of activation of sensory rhodopsin I: evidence for a steric trigger. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9412–9416. doi: 10.1073/pnas.88.21.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Takahashi T., Johnson R., Spudich J. L. Identification of signaling states of a sensory receptor by modulation of lifetimes of stimulus-induced conformations: the case of sensory rhodopsin II. Biochemistry. 1991 Nov 5;30(44):10686–10692. doi: 10.1021/bi00108a012. [DOI] [PubMed] [Google Scholar]
- van Brederode M. E., Gensch T., Hoff W. D., Hellingwerf K. J., Braslavsky S. E. Photoinduced volume change and energy storage associated with the early transformations of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys J. 1995 Mar;68(3):1101–1109. doi: 10.1016/S0006-3495(95)80284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


