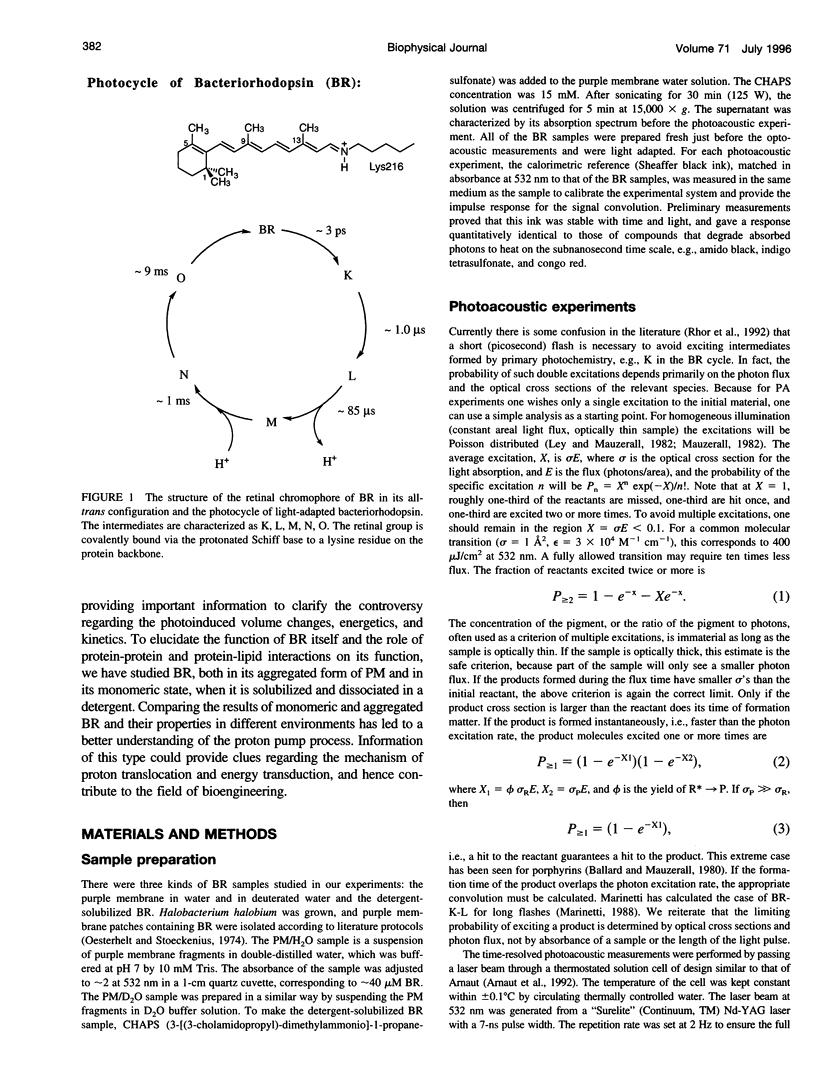
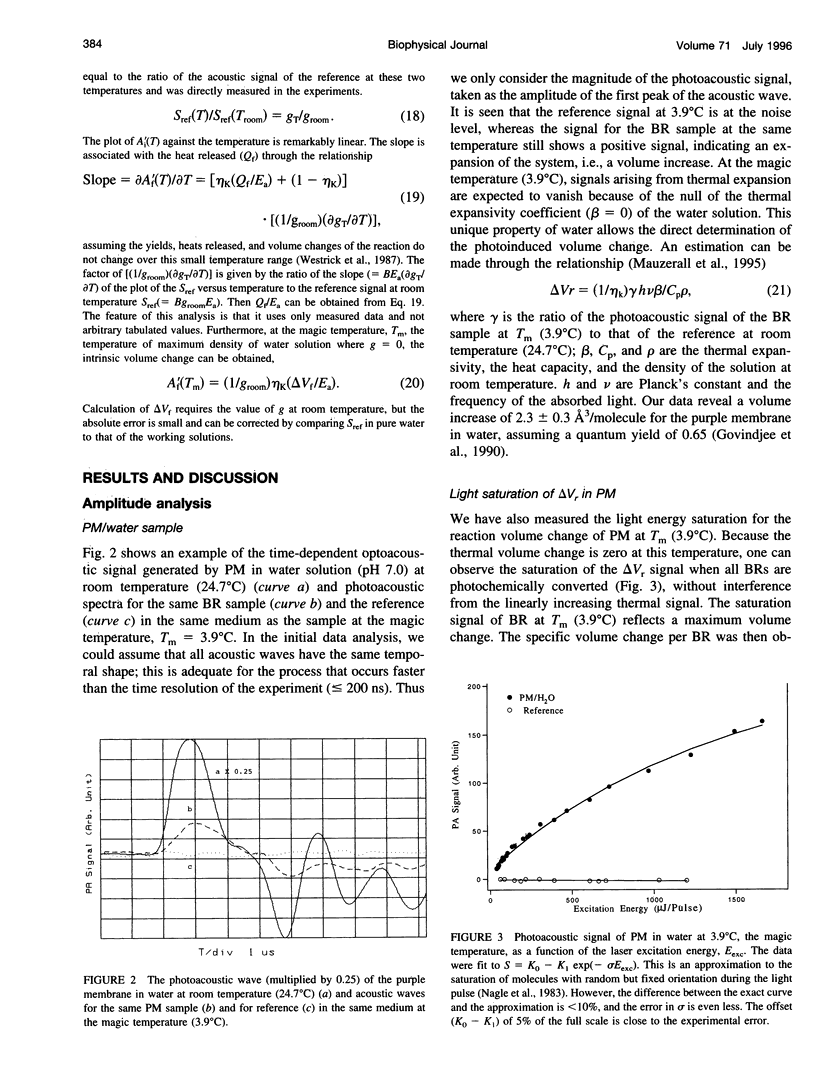
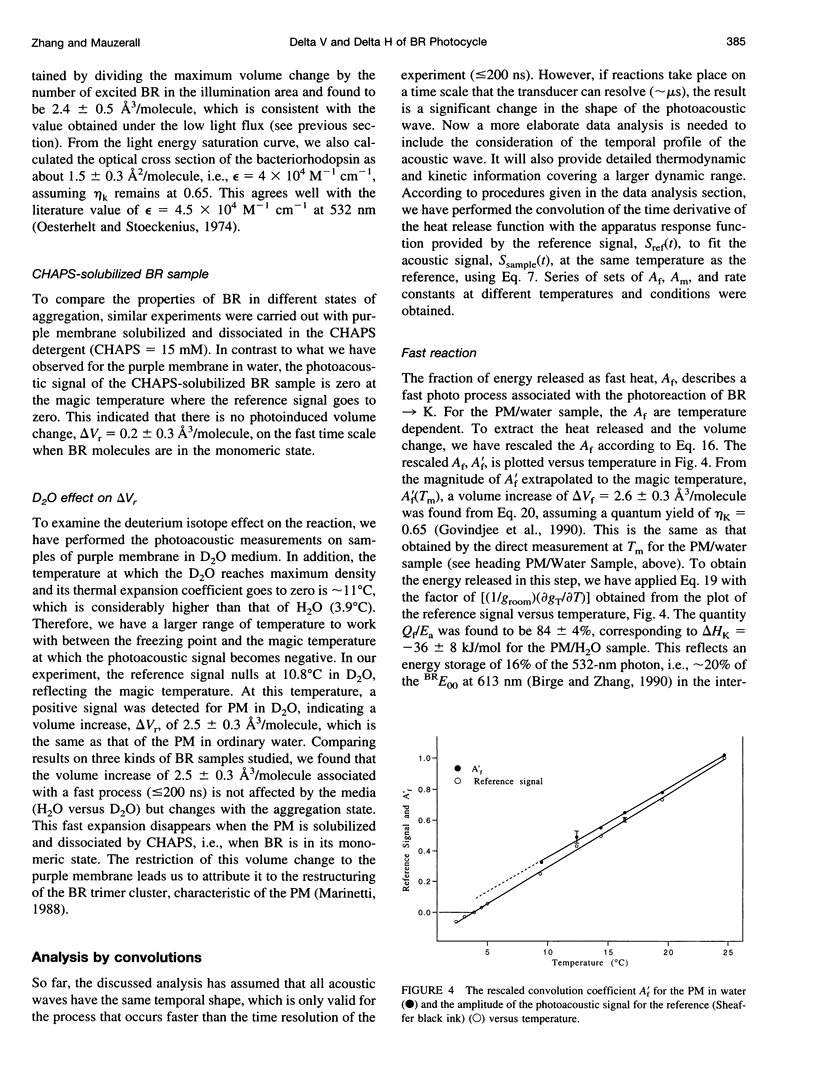
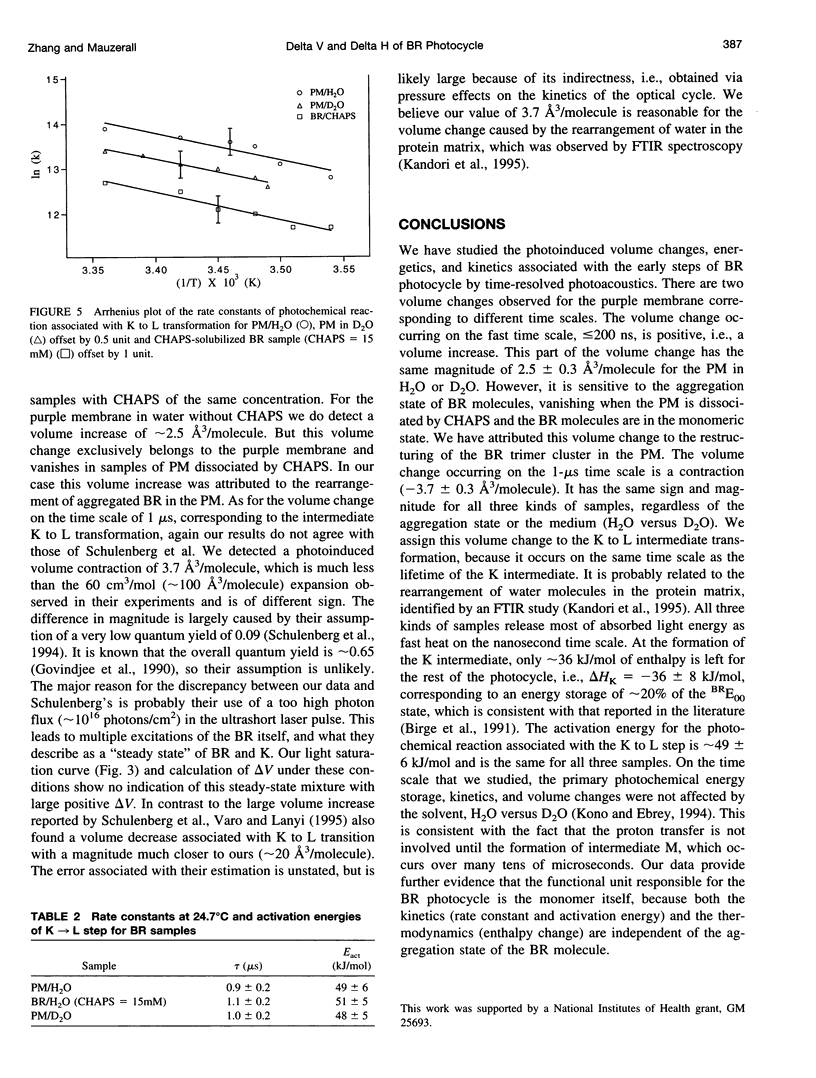
Abstract
We have studied the photoinduced volume changes, energetics, and kinetics in the early steps of the bacteriorhodopsin (BR) photocycle with pulsed, time-resolved photoacoustics. Our data show that there are two volume changes. The fast volume change ( < or = 200 ns) is an expansion (2.5 +/- 0.3 A3/molecule) and is observed exclusively in the purple membrane (PM), vanishing in the 3-[(3-cholamidopropyl)-dimethylammonio] -1-propane-sulfonate-sulfonate-solubilized BR sample; the slow change (approximately 1 micros) is a volume contraction (-3.7 +/- 0.3 A3/molecule). The fast expansion is assigned to the restructuring of the aggregated BR in the PM, and the 1-micros contraction to the change in hydrogen bonding of water at Asp 212 (Kandori et al. 1995. J. Am. Chem. Soc. 117:2118-2119). The formation of the K intermediate releases most of the absorbed energy as heat, with delta Hk = -36 +/- 8 kJ/mol. The activation energy of the K --> L step is 49 +/- 6 kJ/mol, but the enthalpy change is small, -4 +/- 10 kJ/mol. On the time scale we studied, the primary photochemical kinetics, enthalpy, and volume changes are not affected by substituting the solvent D2O for H2O. Comparing data on monomeric and aggregated BR, we conclude that the functional unit for the photocycle is the BR monomer, because both the kinetics (rate constant and activation energy) and the enthalpy changes are independent of its aggregation state.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dencher N. A., Kohl K. D., Heyn M. P. Photochemical cycle and light-dark adaptation of monomeric and aggregated bacteriorhodopsin in various lipid environments. Biochemistry. 1983 Mar 15;22(6):1323–1334. doi: 10.1021/bi00275a002. [DOI] [PubMed] [Google Scholar]
- Govindjee R., Balashov S. P., Ebrey T. G. Quantum efficiency of the photochemical cycle of bacteriorhodopsin. Biophys J. 1990 Sep;58(3):597–608. doi: 10.1016/S0006-3495(90)82403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiek S., Dencher N. A. Monomeric and aggregated bacteriorhodopsin: Single-turnover proton transport stoichiometry and photochemistry. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9509–9513. doi: 10.1073/pnas.85.24.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Kubota K., Tominaga Y., Fujime S., Otomo J., Ikegami A. Dynamic light scattering study of suspensions of purple membrane. Biophys Chem. 1985 Nov;23(1-2):15–29. doi: 10.1016/0301-4622(85)80060-0. [DOI] [PubMed] [Google Scholar]
- Marinetti T. Nonproton ion release by purple membranes exhibits cooperativity as shown by determination of the optical cross-section. Biophys J. 1988 Aug;54(2):197–204. doi: 10.1016/S0006-3495(88)82948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. C., Gunner M. R., Zhang J. W. Volume contraction on photoexcitation of the reaction center from Rhodobacter sphaeroides R-26: internal probe of dielectrics. Biophys J. 1995 Jan;68(1):275–280. doi: 10.1016/S0006-3495(95)80185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C. L., Peters K. S. A photoacoustic calorimetry study of horse carboxymyoglobin on the 10-nanosecond time scale. Biophys J. 1993 Oct;65(4):1660–1665. doi: 10.1016/S0006-3495(93)81223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Schulenberg P. J., Rohr M., Gärtner W., Braslavsky S. E. Photoinduced volume changes associated with the early transformations of bacteriorhodopsin: a laser-induced optoacoustic spectroscopy study. Biophys J. 1994 Mar;66(3 Pt 1):838–843. doi: 10.1016/s0006-3495(94)80860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert F., Mäntele W. Investigation of the primary photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):565–573. doi: 10.1111/j.1432-1033.1983.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Trissl H. W. Photoelectric measurements of purple membranes. Photochem Photobiol. 1990 Jun;51(6):793–818. [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Effects of hydrostatic pressure on the kinetics reveal a volume increase during the bacteriorhodopsin photocycle. Biochemistry. 1995 Sep 26;34(38):12161–12169. doi: 10.1021/bi00038a009. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Westrick J. A., Peters K. S., Ropp J. D., Sligar S. G. Role of the arginine-45 salt bridge in ligand dissociation from sperm whale carboxymyoglobin as probed by photoacoustic calorimetry. Biochemistry. 1990 Jul 17;29(28):6741–6746. doi: 10.1021/bi00480a026. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


