Abstract
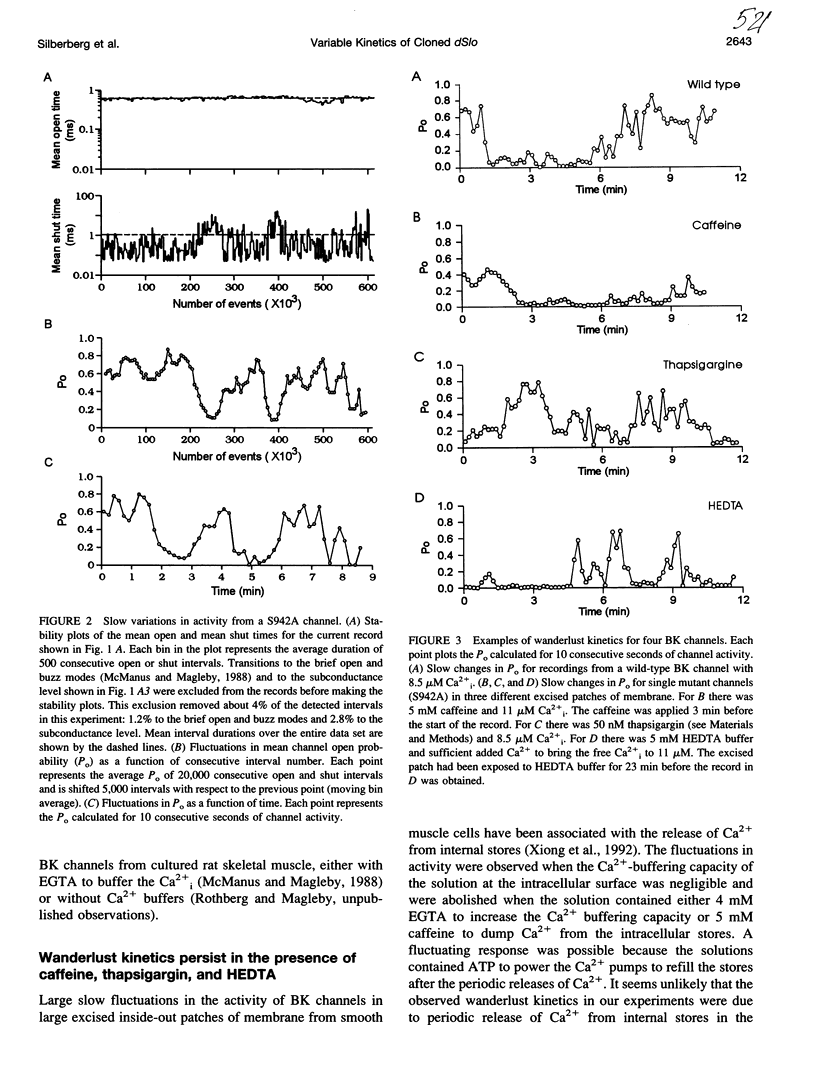
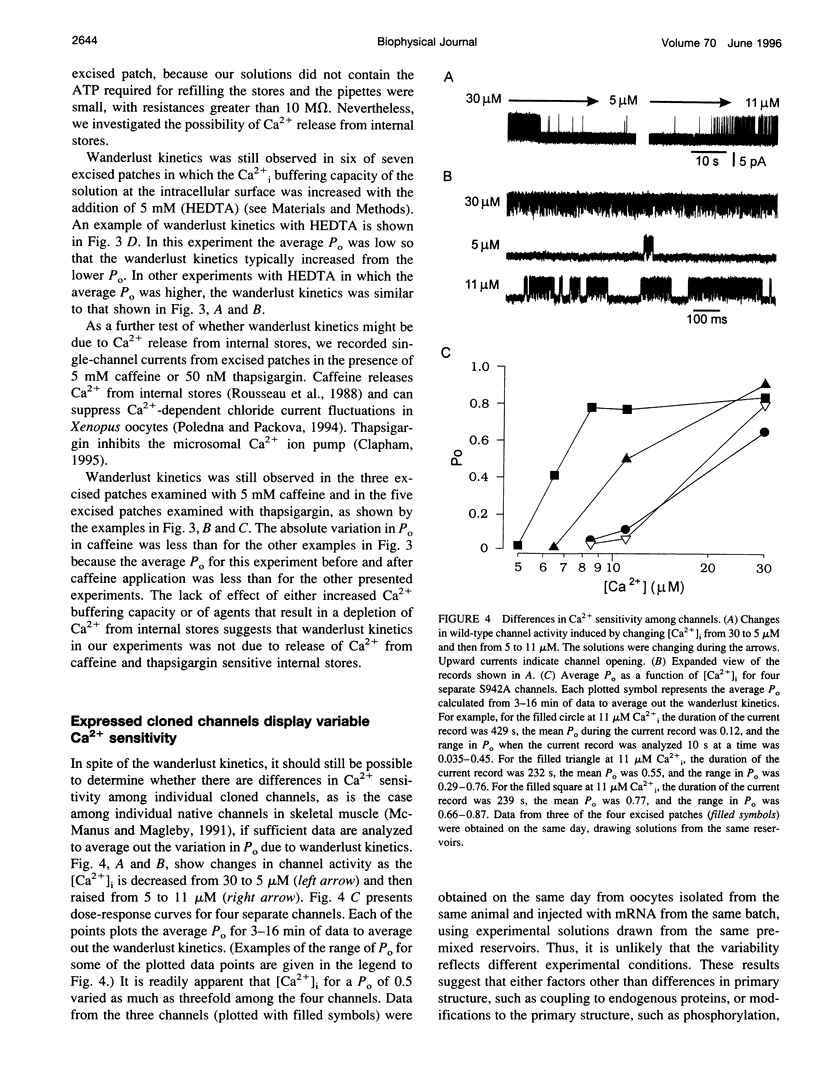
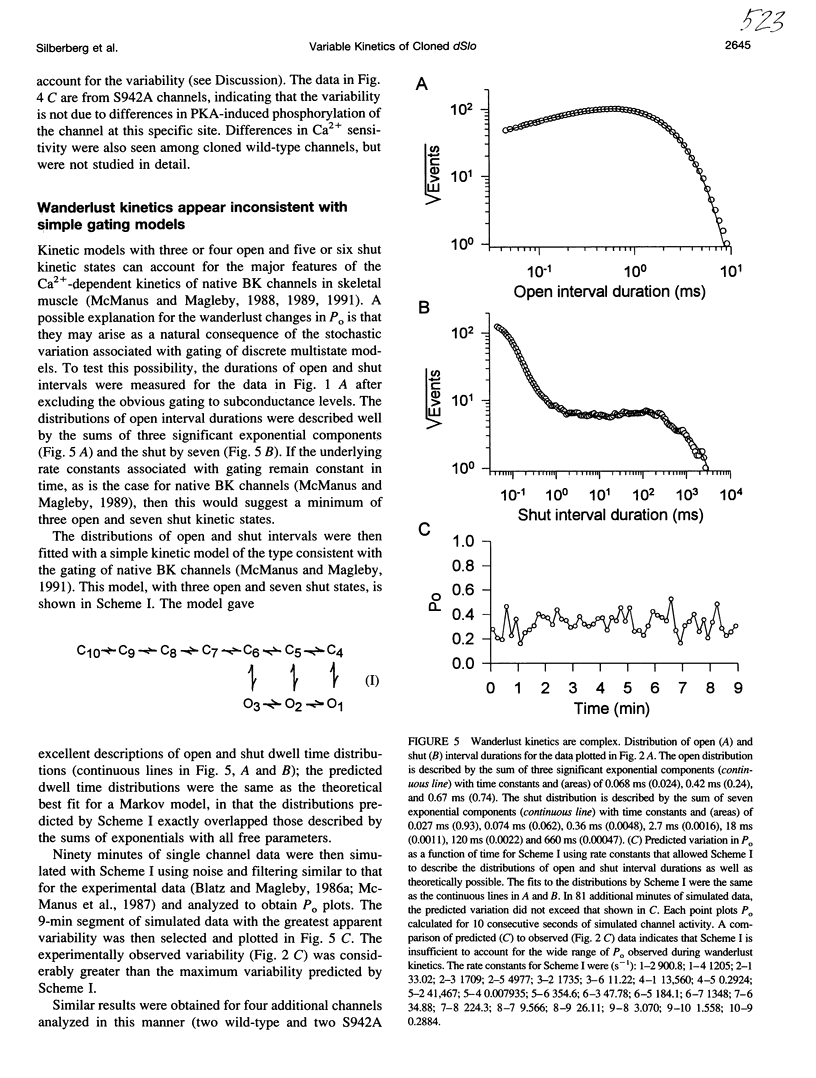
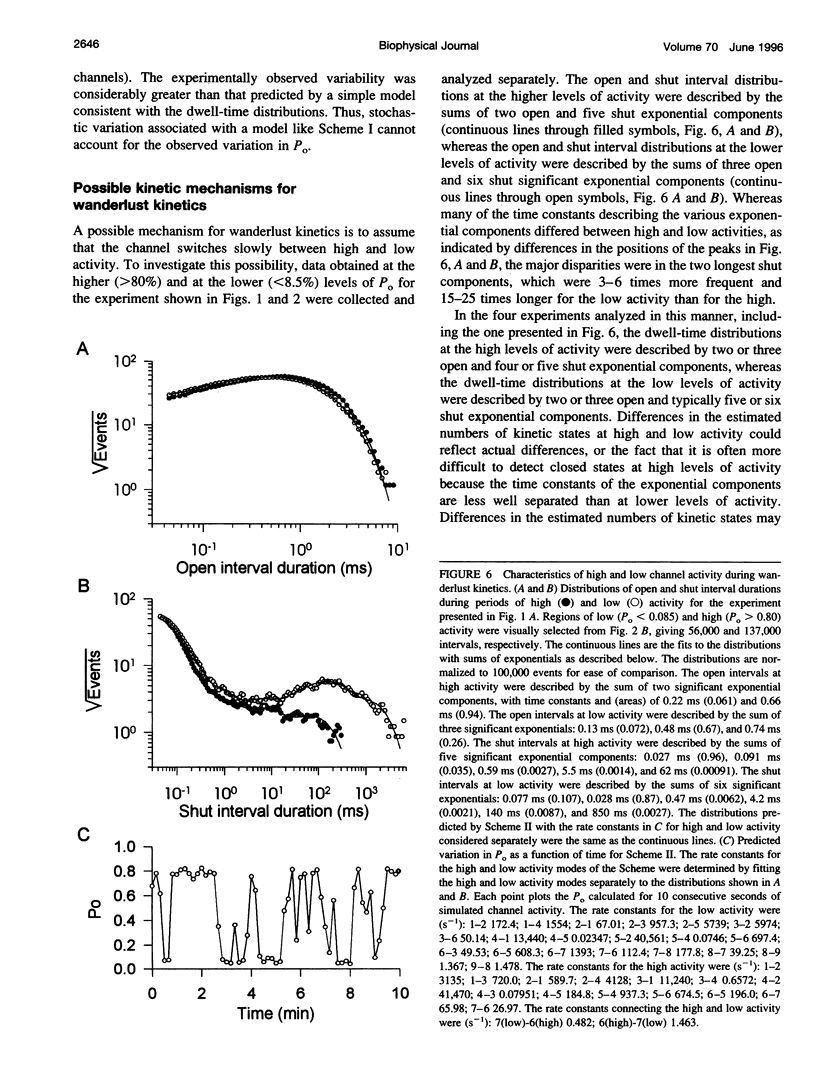
Cloned large conductance Ca2+-activated K+ channels (BK or maxi-K+ channels) from Drosophila (dSlo) were expressed in Xenopus oocytes and studied in excised membrane patches with the patch-clamp technique. Both a natural variant and a mutant that eliminated a putative cyclic AMP-dependent protein kinase phosphorylation site exhibited large, slow fluctuations in open probability with time. These fluctuations, termed "wanderlust kinetics," occurred with a time course of tens of seconds to minutes and had kinetic properties inconsistent with simple gating models. Wanderlust kinetics was still observed in the presence of 5mM caffeine or 50 nM thapsigargin, or when the Ca2+ buffering capacity of the solution was increased by the addition of 5 mM HEDTA, suggesting that the wanderlust kinetics did not arise from Ca2+ release from caffeine and thapsigargin sensitive internal stores in the excised patch. The slow changes in kinetics associated with wanderlust kinetics could be generated with a discrete-state Markov model with transitions among three or more kinetic modes with different levels of open probability. To average out the wanderlust kinetics, large amounts of data were analyzed and demonstrated up to a threefold difference in the [Ca2+]i required for an open probability of 0.5 among channels expressed from the same injected mRNA. These findings indicate that cloned dSlo channels in excised patches from Xenopus oocytes can exhibit large variability in gating properties, both within a single channel and among channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Adelman J. P., Shen K. Z., Kavanaugh M. P., Warren R. A., Wu Y. N., Lagrutta A., Bond C. T., North R. A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992 Aug;9(2):209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Atkinson N. S., Robertson G. A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991 Aug 2;253(5019):551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K., Jackson M. B. Phosphorylation and dephosphorylation modulate a Ca(2+)-activated K+ channel in rat peptidergic nerve terminals. J Physiol. 1994 Mar 1;475(2):241–254. doi: 10.1113/jphysiol.1994.sp020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina V., Omelyanenko V., Heyes B., Ryan U., Bregestovski P. Variations of membrane cholesterol alter the kinetics of Ca2(+)-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 1989 Dec;415(3):262–268. doi: 10.1007/BF00370875. [DOI] [PubMed] [Google Scholar]
- Bregestovski P. D., Bolotina V. N. Membrane fluidity and kinetics of Ca2+-dependent potassium channels. Biomed Biochim Acta. 1989;48(5-6):S382–S387. [PubMed] [Google Scholar]
- Butler A., Tsunoda S., McCobb D. P., Wei A., Salkoff L. mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channels. Science. 1993 Jul 9;261(5118):221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Stow J. L., Prat A. G., Ausiello D. A. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. 1991 Nov;261(5 Pt 1):C882–C888. doi: 10.1152/ajpcell.1991.261.5.C882. [DOI] [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Esguerra M., Wang J., Foster C. D., Adelman J. P., North R. A., Levitan I. B. Cloned Ca(2+)-dependent K+ channel modulated by a functionally associated protein kinase. Nature. 1994 Jun 16;369(6481):563–565. doi: 10.1038/369563a0. [DOI] [PubMed] [Google Scholar]
- Farley J., Rudy B. Multiple types of voltage-dependent Ca2+-activated K+ channels of large conductance in rat brain synaptosomal membranes. Biophys J. 1988 Jun;53(6):919–934. doi: 10.1016/S0006-3495(88)83173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M., Knaus H. G., McManus O. B., Giangiacomo K. M., Kaczorowski G. J., Garcia M. L. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. J Biol Chem. 1994 Jan 7;269(1):676–682. [PubMed] [Google Scholar]
- Gardiner D. M., Grey R. D. Membrane junctions in Xenopus eggs: their distribution suggests a role in calcium regulation. J Cell Biol. 1983 Apr;96(4):1159–1163. doi: 10.1083/jcb.96.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A. J., Kojima H., Carr J. A., Colquhoun D. Expression of cloned receptor subunits produces multiple receptors. Proc Biol Sci. 1990 Nov 22;242(1304):108–112. doi: 10.1098/rspb.1990.0112. [DOI] [PubMed] [Google Scholar]
- Gomez T. M., Snow D. M., Letourneau P. C. Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron. 1995 Jun;14(6):1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+. Neuron. 1993 May;10(5):797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Lagrutta A., Shen K. Z., North R. A., Adelman J. P. Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem. 1994 Aug 12;269(32):20347–20351. [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Latorre R., Vergara C., Hidalgo C. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):805–809. doi: 10.1073/pnas.79.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Helms L. M., Pallanck L., Ganetzky B., Swanson R., Leonard R. J. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995 Mar;14(3):645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
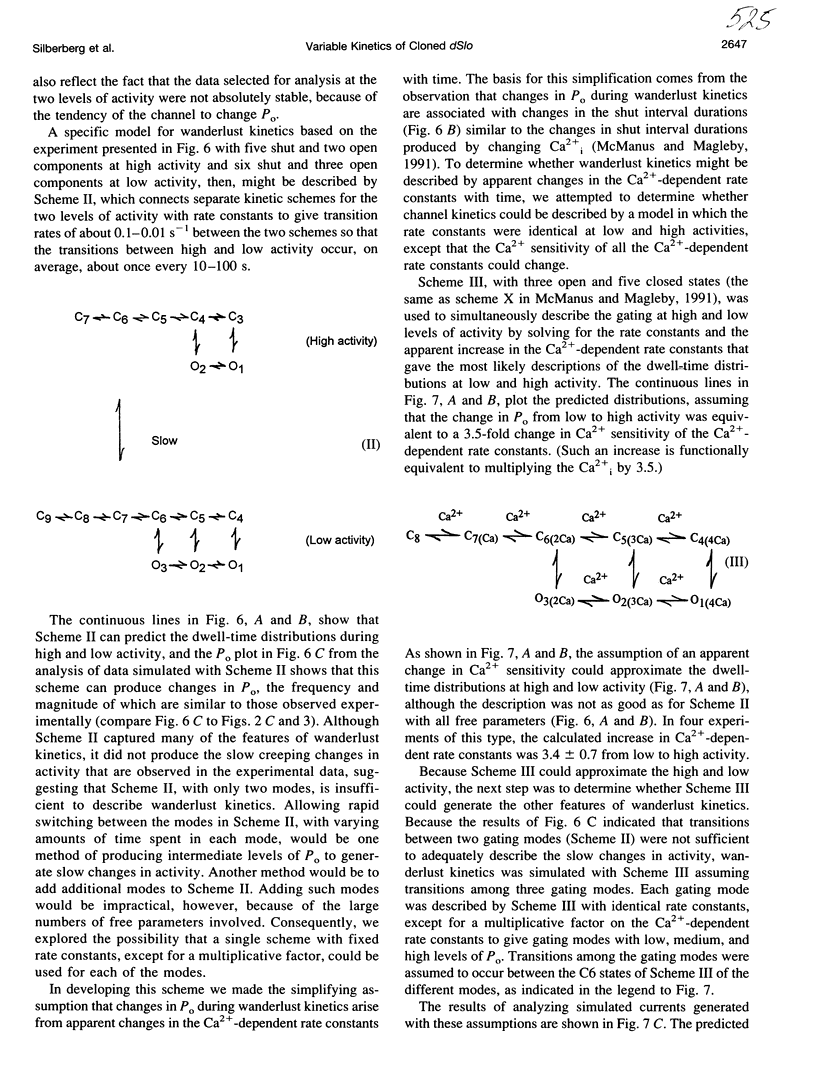
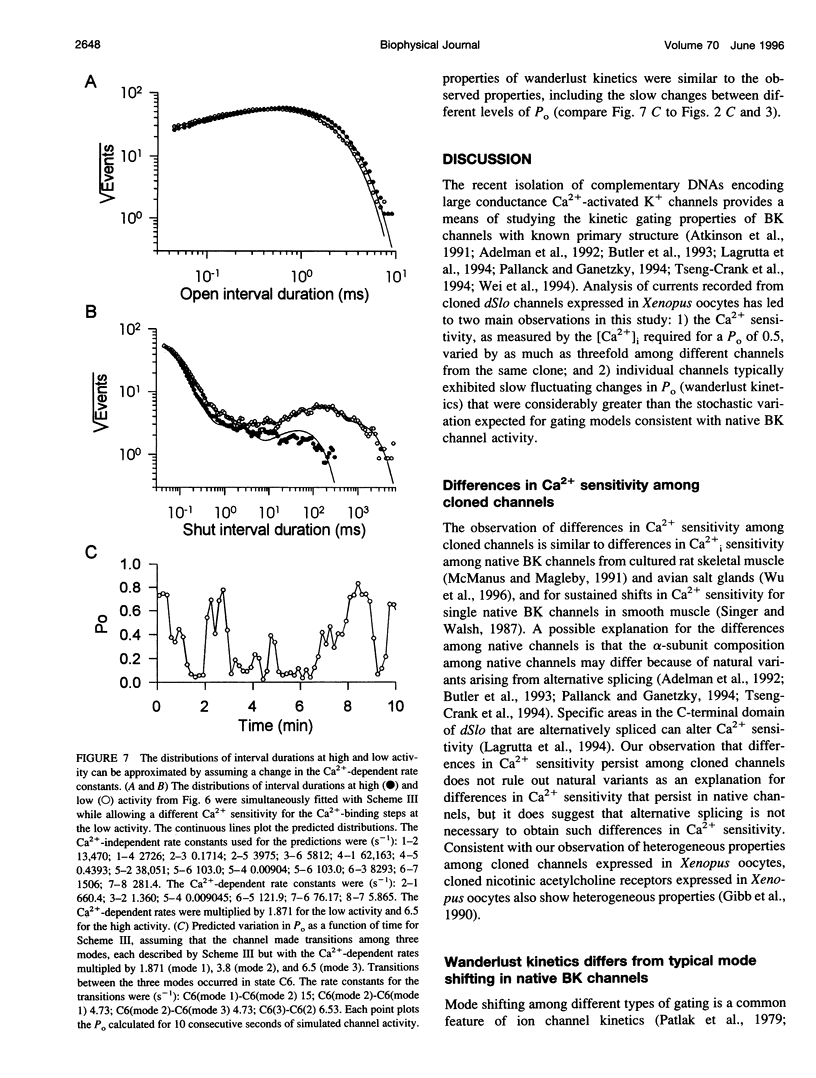
- McManus O. B., Magleby K. L. Accounting for the Ca(2+)-dependent kinetics of single large-conductance Ca(2+)-activated K+ channels in rat skeletal muscle. J Physiol. 1991 Nov;443:739–777. doi: 10.1113/jphysiol.1991.sp018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Alvarez O., Vergara C., Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo D., Brehm P. Modal shifts in acetylcholine receptor channel gating confer subunit-dependent desensitization. Science. 1993 Jun 18;260(5115):1811–1814. doi: 10.1126/science.8511590. [DOI] [PubMed] [Google Scholar]
- Pallanck L., Ganetzky B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet. 1994 Aug;3(8):1239–1243. doi: 10.1093/hmg/3.8.1239. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M., Horn R. Opentime heterogeneity during bursting of sodium channels in frog skeletal muscle. Biophys J. 1986 Mar;49(3):773–777. doi: 10.1016/S0006-3495(86)83704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poledna J., Packová V. Caffeine suppresses chloride current fluctuations in calcium-overloaded Xenopus laevis oocytes. Physiol Res. 1994;43(4):253–256. [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991 Jun;11(6):1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Shen K. Z., Lagrutta A., Davies N. W., Standen N. B., Adelman J. P., North R. A. Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: evidence for tetrameric channel formation. Pflugers Arch. 1994 Mar;426(5):440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Miyazaki K., Ikeda M., Kawaguchi Y., Sakai O. F-actin network may regulate a Cl- channel in renal proximal tubule cells. J Membr Biol. 1993 May;134(1):31–39. doi: 10.1007/BF00233473. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J., Foster C. D., Krause J. D., Mertz R., Godinot N., DiChiara T. J., Reinhart P. H. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994 Dec;13(6):1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Wei A., Solaro C., Lingle C., Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 1994 Sep;13(3):671–681. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- White R. E., Schonbrunn A., Armstrong D. L. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991 Jun 13;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Xiong Z. L., Kitamura K., Kuriyama H. Evidence for contribution of Ca2+ storage sites on unitary K+ channel currents in inside-out membrane of rabbit portal vein. Pflugers Arch. 1992 Jan;420(1):112–114. doi: 10.1007/BF00378651. [DOI] [PubMed] [Google Scholar]
- de Peyer J. E., Cachelin A. B., Levitan I. B., Reuter H. Ca2+ -activated K+ conductance in internally perfused snail neurons is enhanced by protein phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4207–4211. doi: 10.1073/pnas.79.13.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]


