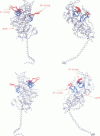
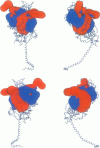
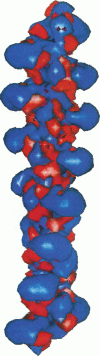
Abstract
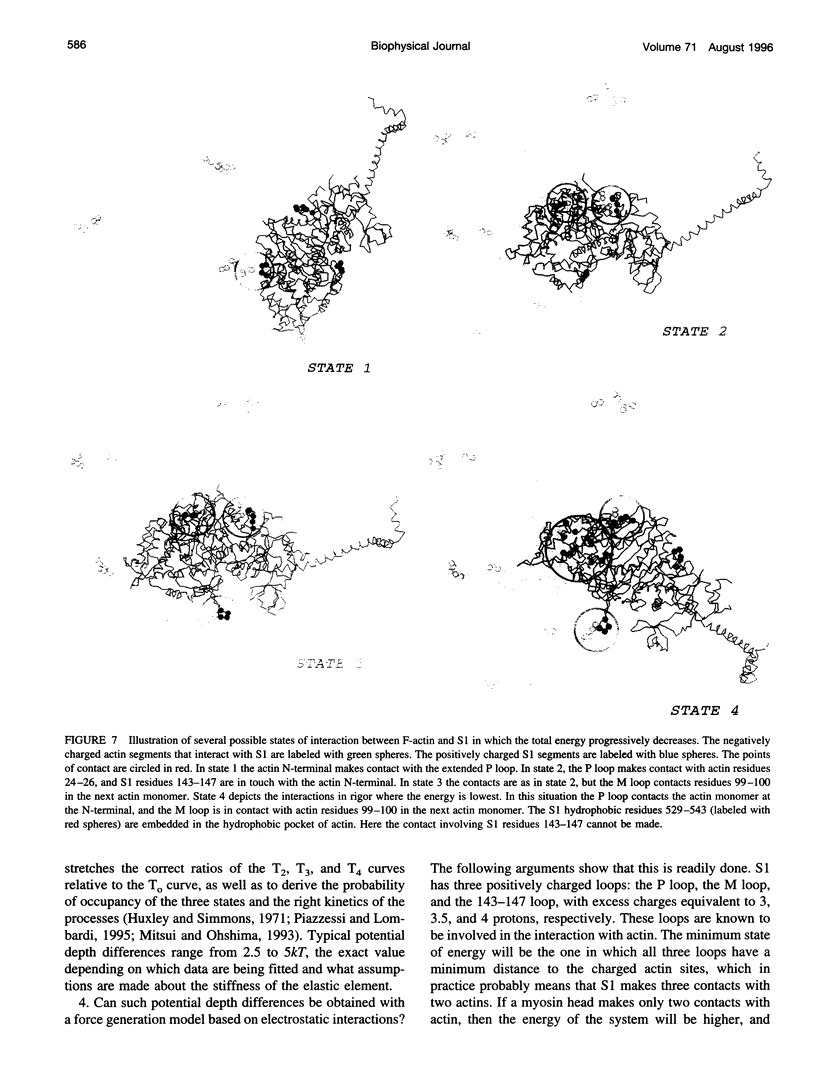
Poisson-Boltzmann calculations of the distribution of electrostatic potentials around an actin filament in physiological-strength solutions show that negative isopotential surfaces protrude into the solvent. Each protrusion follows the actin two-start helix and is located on the sites implicated in the formation of the actomyosin complex. Molecular dynamic calculations on the S1 portion of the myosin molecule indicate that in the presence of ATP the crystallographically invisible loops (comprising residues 624-649 and 564-579) remain on the surface, whereas in the absence of ATP they can move toward the actin-binding sites and experience electrostatic forces that range from 1 to 10 pN. The molecular dynamics calculations also suggest that during the ATP cycle there exist at least three states of electrostatic interactions between the loops and actin. Every time a new interaction is formed, the strain in the myosin head increases and the energy of the complex decreases by 2kT to 5kT. This can explain muscular contraction in terms of a Huxley-Simmons-type mechanism, while requiring only rearrangements of small mobile S1 segments rather than the large shape changes in the myosin molecule postulated by the conventional tilting head model.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S., Reisler E. Role of sequence 18-29 on actin in actomyosin interactions. Biochemistry. 1993 May 18;32(19):5051–5056. doi: 10.1021/bi00070a012. [DOI] [PubMed] [Google Scholar]
- Amos L. A., Huxley H. E., Holmes K. C., Goody R. S., Taylor K. A. Structural evidence that myosin heads may interact with two sites on F-actin. Nature. 1982 Sep 30;299(5882):467–469. doi: 10.1038/299467a0. [DOI] [PubMed] [Google Scholar]
- Andreev O. A., Borejdo J. The myosin head can bind two actin monomers. Biochem Biophys Res Commun. 1991 May 31;177(1):350–356. doi: 10.1016/0006-291x(91)91990-t. [DOI] [PubMed] [Google Scholar]
- Aspenström P., Karlsson R. Interference with myosin subfragment-1 binding by site-directed mutagenesis of actin. Eur J Biochem. 1991 Aug 15;200(1):35–41. doi: 10.1111/j.1432-1033.1991.tb21045.x. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Chaussepied P., Audemard E., Kassab R. Functional characterization of skeletal F-actin labeled on the NH2-terminal segment of residues 1-28. Eur J Biochem. 1989 May 15;181(3):747–754. doi: 10.1111/j.1432-1033.1989.tb14787.x. [DOI] [PubMed] [Google Scholar]
- Bonafé N., Chaussepied P. A single myosin head can be cross-linked to the N termini of two adjacent actin monomers. Biophys J. 1995 Apr;68(4 Suppl):35S–43S. [PMC free article] [PubMed] [Google Scholar]
- Bordas J., Diakun G. P., Diaz F. G., Harries J. E., Lewis R. A., Lowy J., Mant G. R., Martin-Fernandez M. L., Towns-Andrews E. Two-dimensional time-resolved X-ray diffraction studies of live isometrically contracting frog sartorius muscle. J Muscle Res Cell Motil. 1993 Jun;14(3):311–324. doi: 10.1007/BF00123096. [DOI] [PubMed] [Google Scholar]
- Chaussepied P. Interaction between stretch of residues 633-642 (actin binding site) and nucleotide binding site on skeletal myosin subfragment 1 heavy chain. Biochemistry. 1989 Nov 14;28(23):9123–9128. doi: 10.1021/bi00449a025. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Morales M. F. Modifying preselected sites on proteins: the stretch of residues 633-642 of the myosin heavy chain is part of the actin-binding site. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7471–7475. doi: 10.1073/pnas.85.20.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. K., Blake W. T., Rubenstein P. A. Removal of the amino-terminal acidic residues of yeast actin. Studies in vitro and in vivo. J Biol Chem. 1992 May 5;267(13):9430–9436. [PubMed] [Google Scholar]
- Cook R. K., Root D., Miller C., Reisler E., Rubenstein P. A. Enhanced stimulation of myosin subfragment 1 ATPase activity by addition of negatively charged residues to the yeast actin NH2 terminus. J Biol Chem. 1993 Feb 5;268(4):2410–2415. [PubMed] [Google Scholar]
- Dan-Goor M., Muhlrad A. Antibody directed against the 142-148 sequence of the myosin heavy chain interferes with myosin-actin interaction. Biochemistry. 1991 Jan 15;30(2):400–405. doi: 10.1021/bi00216a014. [DOI] [PubMed] [Google Scholar]
- DasGupta G., Reisler E. Antibody against the amino terminus of alpha-actin inhibits actomyosin interactions in the presence of ATP. J Mol Biol. 1989 Jun 20;207(4):833–836. doi: 10.1016/0022-2836(89)90249-0. [DOI] [PubMed] [Google Scholar]
- DasGupta G., Reisler E. Nucleotide-induced changes in the interaction of myosin subfragment 1 with actin: detection by antibodies against the N-terminal segment of actin. Biochemistry. 1991 Oct 15;30(41):9961–9966. doi: 10.1021/bi00105a021. [DOI] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Gerstein M., Schulz G., Chothia C. Domain closure in adenylate kinase. Joints on either side of two helices close like neighboring fingers. J Mol Biol. 1993 Jan 20;229(2):494–501. doi: 10.1006/jmbi.1993.1048. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Calculation of electrostatic potentials in an enzyme active site. Nature. 1987 Nov 5;330(6143):84–86. doi: 10.1038/330084a0. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Straatsma T. P., McCammon J. A., Ripoll D. R., Faerman C. H., Axelsen P. H., Silman I., Sussman J. L. Open "back door" in a molecular dynamics simulation of acetylcholinesterase. Science. 1994 Mar 4;263(5151):1276–1278. doi: 10.1126/science.8122110. [DOI] [PubMed] [Google Scholar]
- Hirose K., Franzini-Armstrong C., Goldman Y. E., Murray J. M. Structural changes in muscle crossbridges accompanying force generation. J Cell Biol. 1994 Nov;127(3):763–778. doi: 10.1083/jcb.127.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Lenart T. D., Murray J. M., Franzini-Armstrong C., Goldman Y. E. Flash and smash: rapid freezing of muscle fibers activated by photolysis of caged ATP. Biophys J. 1993 Jul;65(1):397–408. doi: 10.1016/S0006-3495(93)81061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock-DeGregori S. E., Varnell T. A. Tropomyosin has discrete actin-binding sites with sevenfold and fourteenfold periodicities. J Mol Biol. 1990 Aug 20;214(4):885–896. doi: 10.1016/0022-2836(90)90343-K. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Honig B., Nicholls A. Classical electrostatics in biology and chemistry. Science. 1995 May 26;268(5214):1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Hyde C. C., Ahmed S. A., Padlan E. A., Miles E. W., Davies D. R. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988 Nov 25;263(33):17857–17871. [PubMed] [Google Scholar]
- Ishijima A., Harada Y., Kojima H., Funatsu T., Higuchi H., Yanagida T. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem Biophys Res Commun. 1994 Mar 15;199(2):1057–1063. doi: 10.1006/bbrc.1994.1336. [DOI] [PubMed] [Google Scholar]
- Johara M., Toyoshima Y. Y., Ishijima A., Kojima H., Yanagida T., Sutoh K. Charge-reversion mutagenesis of Dictyostelium actin to map the surface recognized by myosin during ATP-driven sliding motion. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2127–2131. doi: 10.1073/pnas.90.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Kögler H., Moir A. J., Trayer I. P., Rüegg J. C. Peptide competition of actin activation of myosin-subfragment 1 ATPase by an amino terminal actin fragment. FEBS Lett. 1991 Dec 2;294(1-2):31–34. doi: 10.1016/0014-5793(91)81336-7. [DOI] [PubMed] [Google Scholar]
- Lehman W., Craig R., Vibert P. Ca(2+)-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994 Mar 3;368(6466):65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- Lorenz M., Popp D., Holmes K. C. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993 Dec 5;234(3):826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- Maita T., Hayashida M., Tanioka Y., Komine Y., Matsuda G. The primary structure of the myosin head. Proc Natl Acad Sci U S A. 1987 Jan;84(2):416–420. doi: 10.1073/pnas.84.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M. L., Bordas J., Diakun G., Harries J., Lowy J., Mant G. R., Svensson A., Towns-Andrews E. Time-resolved X-ray diffraction studies of myosin head movements in live frog sartorius muscle during isometric and isotonic contractions. J Muscle Res Cell Motil. 1994 Jun;15(3):319–348. doi: 10.1007/BF00123484. [DOI] [PubMed] [Google Scholar]
- Mejean C., Boyer M., Labbé J. P., Marlier L., Benyamin Y., Roustan C. Anti-actin antibodies. An immunological approach to the myosin-actin and the tropomyosin-actin interfaces. Biochem J. 1987 Jun 15;244(3):571–577. doi: 10.1042/bj2440571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Kalnoski M., Yunossi Z., Bulinski J. C., Reisler E. Antibodies directed against N-terminal residues on actin do not block acto-myosin binding. Biochemistry. 1987 Sep 22;26(19):6064–6070. doi: 10.1021/bi00393a018. [DOI] [PubMed] [Google Scholar]
- Müller C. W., Schulz G. E. Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5A refined at 1.9 A resolution. A model for a catalytic transition state. J Mol Biol. 1992 Mar 5;224(1):159–177. doi: 10.1016/0022-2836(92)90582-5. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Krengel U., Petsko G. A., Goody R. S., Kabsch W., Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990 Aug;9(8):2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G., Lombardi V. A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys J. 1995 May;68(5):1966–1979. doi: 10.1016/S0006-3495(95)80374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Rogers N. K., Moore G. R., Sternberg M. J. Electrostatic interactions in globular proteins: calculation of the pH dependence of the redox potential of cytochrome c551. J Mol Biol. 1985 Apr 20;182(4):613–616. doi: 10.1016/0022-2836(85)90248-7. [DOI] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. How molecular motors work. Nature. 1994 Dec 8;372(6506):515–518. doi: 10.1038/372515a0. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Hayes F. R., Russell A. J., Thomas P. G., Fersht A. R. Prediction of electrostatic effects of engineering of protein charges. Nature. 1987 Nov 5;330(6143):86–88. doi: 10.1038/330086a0. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Ando M., Sutoh K., Toyoshima Y. Y. Site-directed mutations of Dictyostelium actin: disruption of a negative charge cluster at the N terminus. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7711–7714. doi: 10.1073/pnas.88.17.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L. A., de Vos A. M., Milburn M. V., Kim S. H. Crystal structures at 2.2 A resolution of the catalytic domains of normal ras protein and an oncogenic mutant complexed with GDP. J Mol Biol. 1991 Feb 5;217(3):503–516. doi: 10.1016/0022-2836(91)90753-s. [DOI] [PubMed] [Google Scholar]
- Van Eyk J. E., Hodges R. S. A synthetic peptide of the N-terminus of actin interacts with myosin. Biochemistry. 1991 Dec 17;30(50):11676–11682. doi: 10.1021/bi00114a010. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. Binding manner of actin to the lysine-rich sequence of myosin subfragment 1 in the presence and absence of ATP. Biochemistry. 1989 Jun 27;28(13):5573–5577. doi: 10.1021/bi00439a035. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Lawson D., Rayment I. Is myosin a "back door" enzyme? Biophys J. 1995 Apr;68(4 Suppl):44S–49S. [PMC free article] [PubMed] [Google Scholar]