Abstract
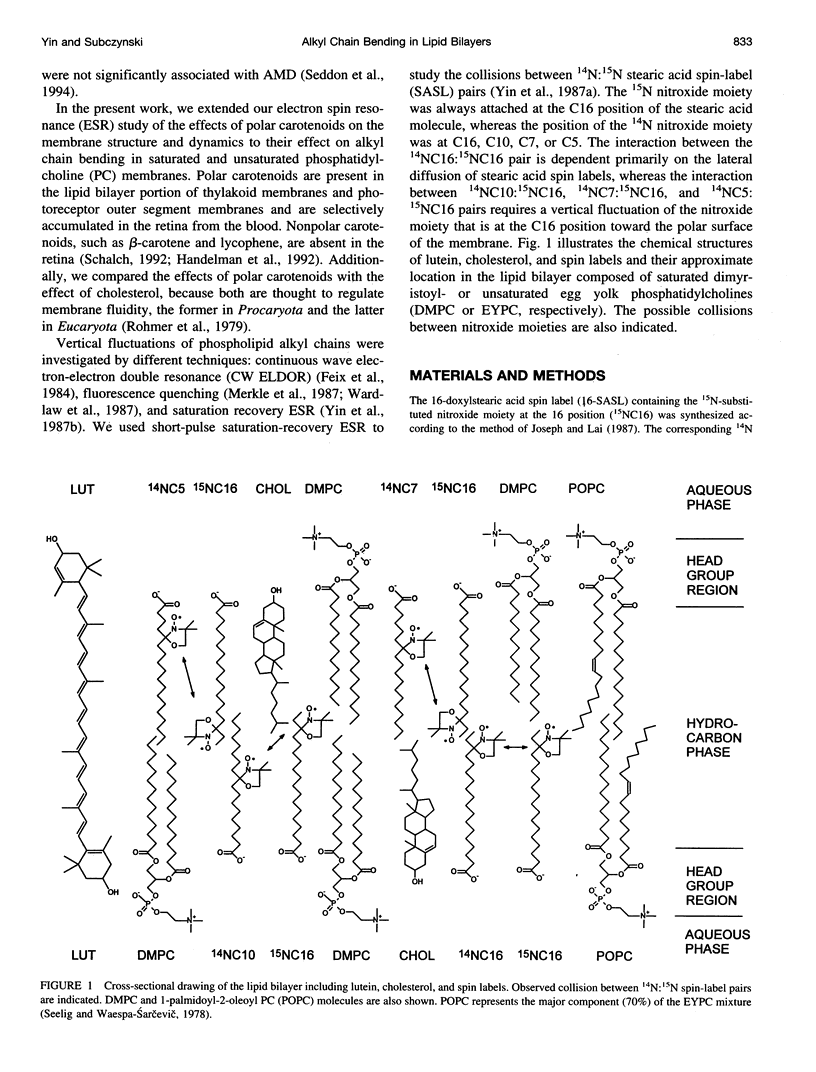
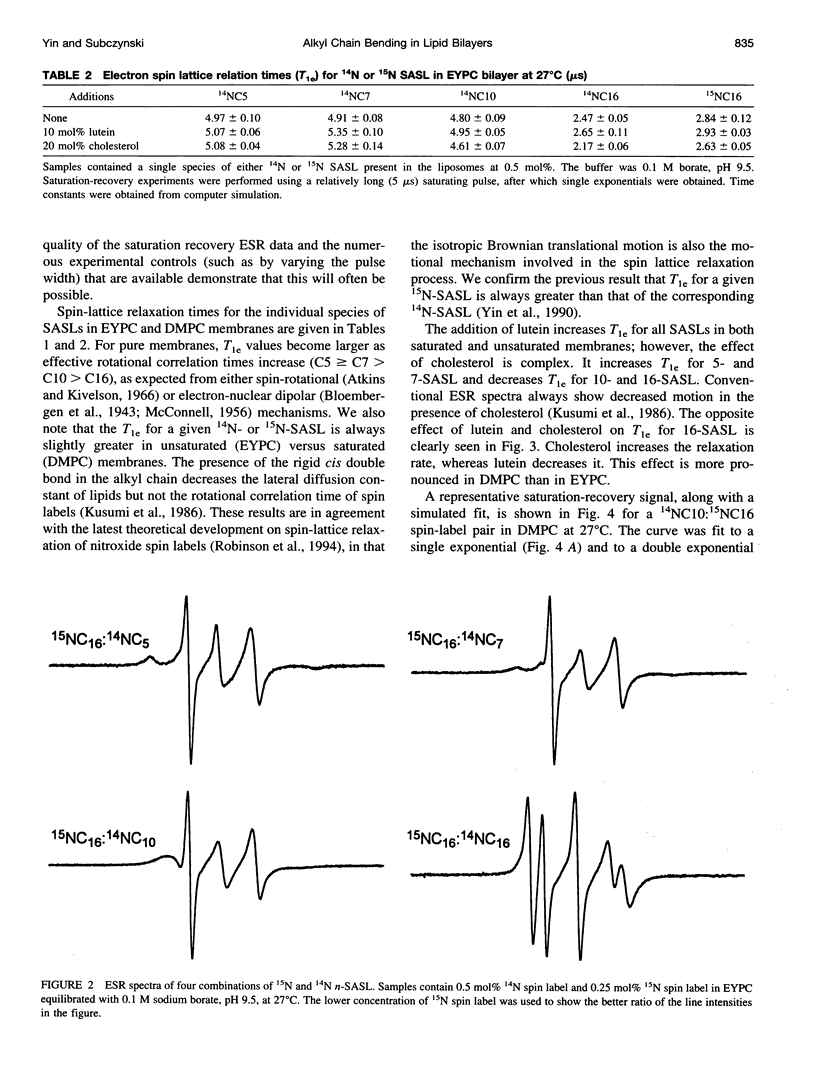
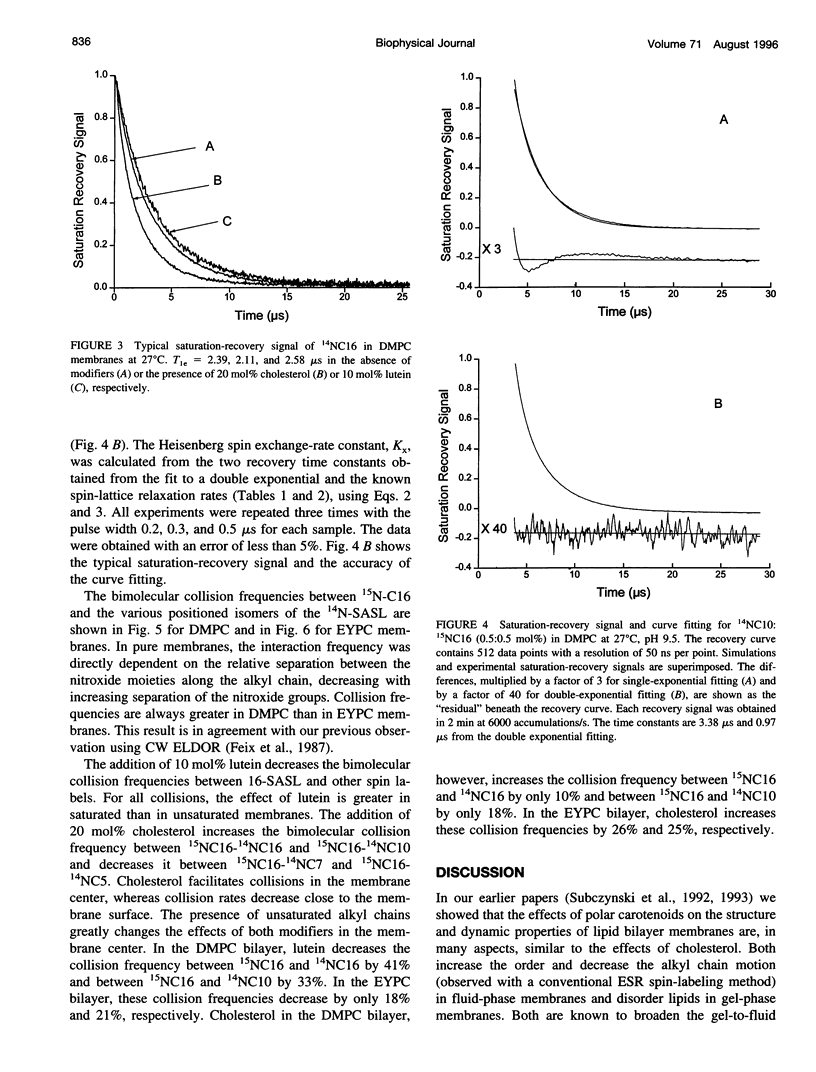
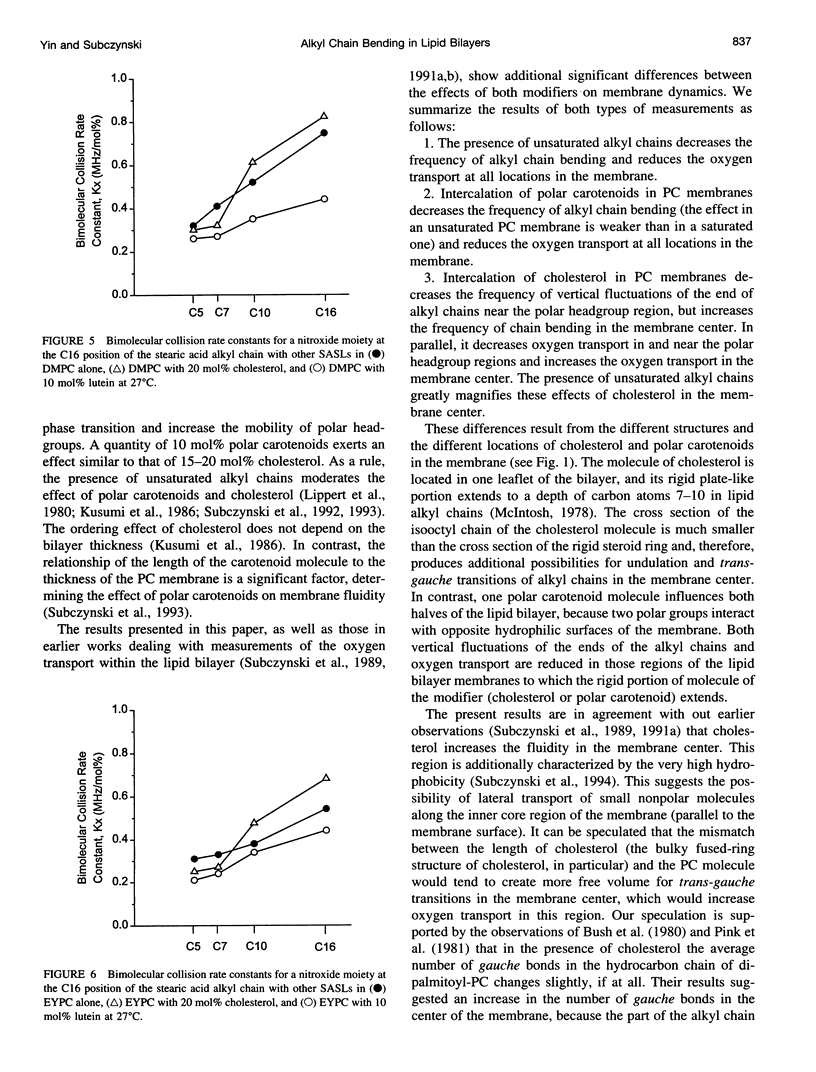
A short pulse saturation recovery electron spin resonance technique has been used to study the effects of polar carotenoid-lutein and cholesterol on interactions of 14N:15N stearic acid spin-label pairs in fluid-phase phosphatidylcholine (PC) membranes. Bimolecular collisions for pairs consisting of various combinations of [14N]-16-, [14N]-10-, [14N]-7-, or [14N]-5-doxylstearate and [15N]-16-doxylstearate in dimyristoyl-PC (DMPC) or egg yolk PC (EYPC) membranes were measured at 27 degrees C. In the absence and presence of lutein or cholesterol for both lipid systems, the collision rates were ordered as 16:5 < 16:7 < 16:10 < 16:16. For all spin-label pairs studied, interaction frequencies were greater in DMPC than in EYPC. Polar carotenoid-lutein reduces the collision frequency for all spin-label pairs, whereas cholesterol reduces the collision frequency for 16:5 and 16:7 pairs and increases the collision frequency in the membrane center for 16:10 and 16:16 pairs. The presence of unsaturated alkyl chains greatly reduces the effect of lutein but magnifies the effect of cholesterol in the membrane center. The observed differences in the effects of these modifiers on alkyl chain bending result from differences in the structure of cholesterol and polar carotenoid and from their different localization within the lipid bilayer membrane. These studies further confirm the occurrence of vertical fluctuations of alkyl chain ends toward the bilayer surface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendich A., Olson J. A. Biological actions of carotenoids. FASEB J. 1989 Jun;3(8):1927–1932. [PubMed] [Google Scholar]
- Bone R. A., Landrum J. T., Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Res. 1992 Jan;32(1):105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- Bone R. A., Landrum J. T., Hime G. W., Cains A., Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993 May;34(6):2033–2040. [PubMed] [Google Scholar]
- Bone R. A., Landrum J. T. Macular pigment in Henle fiber membranes: a model for Haidinger's brushes. Vision Res. 1984;24(2):103–108. doi: 10.1016/0042-6989(84)90094-4. [DOI] [PubMed] [Google Scholar]
- Bone R. A., Landrum J. T., Tarsis S. L. Preliminary identification of the human macular pigment. Vision Res. 1985;25(11):1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- Bramley P. M., Mackenzie A. Regulation of carotenoid biosynthesis. Curr Top Cell Regul. 1988;29:291–343. doi: 10.1016/b978-0-12-152829-4.50009-4. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Bush S. F., Adams R. G., Levin I. W. Structural reorganizations in lipid bilayer systems: effect of hydration and sterol addition on Raman spectra of dipalmitoylphosphatidylcholine multilayers. Biochemistry. 1980 Sep 16;19(19):4429–4436. doi: 10.1021/bi00560a008. [DOI] [PubMed] [Google Scholar]
- Cohen Y., Yalovsky S., Nechushtai R. Integration and assembly of photosynthetic protein complexes in chloroplast thylakoid membranes. Biochim Biophys Acta. 1995 May 8;1241(1):1–30. doi: 10.1016/0304-4157(94)00012-3. [DOI] [PubMed] [Google Scholar]
- Conn P. F., Schalch W., Truscott T. G. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B. 1991 Oct;11(1):41–47. doi: 10.1016/1011-1344(91)80266-k. [DOI] [PubMed] [Google Scholar]
- Feix J. B., Popp C. A., Venkataramu S. D., Beth A. H., Park J. H., Hyde J. S. An electron-electron double-resonance study of interactions between [14N]- and [15N]stearic acid spin-label pairs: lateral diffusion and vertical fluctuations in dimyristoylphosphatidylcholine. Biochemistry. 1984 May 8;23(10):2293–2299. doi: 10.1021/bi00305a032. [DOI] [PubMed] [Google Scholar]
- Feix J. B., Yin J. J., Hyde J. S. Interactions of 14N:15N stearic acid spin-label pairs: effects of host lipid alkyl chain length and unsaturation. Biochemistry. 1987 Jun 30;26(13):3850–3855. doi: 10.1021/bi00387a017. [DOI] [PubMed] [Google Scholar]
- Foote C. S., Chang Y. C., Denny R. W. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J Am Chem Soc. 1970 Aug 26;92(17):5216–5218. doi: 10.1021/ja00720a036. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M., SISTROM W. R., COHENBAZIRE G., STANIER R. Y., CALVIN M. Function of carotenoids in photosynthesis. Nature. 1955 Dec 24;176(4495):1211–1215. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- Handelman G. J., Dratz E. A., Reay C. C., van Kuijk J. G. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988 Jun;29(6):850–855. [PubMed] [Google Scholar]
- Handelman G. J., Snodderly D. M., Adler A. J., Russett M. D., Dratz E. A. Measurement of carotenoids in human and monkey retinas. Methods Enzymol. 1992;213:220–230. doi: 10.1016/0076-6879(92)13123-f. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Actions of carotenoids in biological systems. Annu Rev Nutr. 1993;13:561–587. doi: 10.1146/annurev.nu.13.070193.003021. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7(6):617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Kusumi A., Subczynski W. K., Pasenkiewicz-Gierula M., Hyde J. S., Merkle H. Spin-label studies on phosphatidylcholine-cholesterol membranes: effects of alkyl chain length and unsaturation in the fluid phase. Biochim Biophys Acta. 1986 Jan 29;854(2):307–317. doi: 10.1016/0005-2736(86)90124-0. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Laiken S. L., Printz M. P. Kinetic class analysis of hydrogen-exchange data. Biochemistry. 1970 Mar 31;9(7):1547–1553. doi: 10.1021/bi00809a011. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Lindsay R. M., Schultz R. Laser-Raman investigation of lysozyme-phospholipid interactions. Biochim Biophys Acta. 1980 Jun 20;599(1):32–41. doi: 10.1016/0005-2736(80)90054-1. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Merkle H., Subczynski W. K., Kusumi A. Dynamic fluorescence quenching studies on lipid mobilities in phosphatidylcholine-cholesterol membranes. Biochim Biophys Acta. 1987 Feb 26;897(2):238–248. doi: 10.1016/0005-2736(87)90420-2. [DOI] [PubMed] [Google Scholar]
- Olson J. A. Provitamin A function of carotenoids: the conversion of beta-carotene into vitamin A. J Nutr. 1989 Jan;119(1):105–108. doi: 10.1093/jn/119.1.105. [DOI] [PubMed] [Google Scholar]
- Pink D. A., Green T. J., Chapman D. Raman scattering in bilayers of saturated phosphatidylcholines and cholesterol. Experiment and theory. Biochemistry. 1981 Nov 10;20(23):6692–6698. doi: 10.1021/bi00526a026. [DOI] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Haas D. A., Mailer C. Molecular dynamics in liquids: spin-lattice relaxation of nitroxide spin labels. Science. 1994 Jan 28;263(5146):490–493. doi: 10.1126/science.8290958. [DOI] [PubMed] [Google Scholar]
- Rohmer M., Bouvier P., Ourisson G. Molecular evolution of biomembranes: structural equivalents and phylogenetic precursors of sterols. Proc Natl Acad Sci U S A. 1979 Feb;76(2):847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Hidalgo E., Olmedilla B. Carotenoids. Int J Vitam Nutr Res. 1993;63(4):265–269. [PubMed] [Google Scholar]
- Schalch W. Carotenoids in the retina--a review of their possible role in preventing or limiting damage caused by light and oxygen. EXS. 1992;62:280–298. doi: 10.1007/978-3-0348-7460-1_29. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Ajani U. A., Sperduto R. D., Hiller R., Blair N., Burton T. C., Farber M. D., Gragoudas E. S., Haller J., Miller D. T. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994 Nov 9;272(18):1413–1420. [PubMed] [Google Scholar]
- Seddon J. M., Hennekens C. H. Vitamins, minerals, and macular degeneration. Promising but unproven hypotheses. Arch Ophthalmol. 1994 Feb;112(2):176–179. doi: 10.1001/archopht.1994.01090140052021. [DOI] [PubMed] [Google Scholar]
- Seelig J., Waespe-Sarcevic N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry. 1978 Aug 8;17(16):3310–3315. doi: 10.1021/bi00609a021. [DOI] [PubMed] [Google Scholar]
- Shin Y. K., Freed J. H. Dynamic imaging of lateral diffusion by electron spin resonance and study of rotational dynamics in model membranes. Effect of cholesterol. Biophys J. 1989 Mar;55(3):537–550. doi: 10.1016/S0006-3495(89)82847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Stahl W., Sundquist A. R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992 Sep 30;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Smith I. C. A deuterium nuclear magnetic resonance study of the condensing effect of cholesterol on egg phosphatidylcholine bilayer membranes. I. Perdeuterated fatty acid probes. Chem Phys Lipids. 1976 Oct;17(2-3):251–263. doi: 10.1016/0009-3084(76)90070-0. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S., Kusumi A. Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: a pulse ESR spin labeling study. Biochemistry. 1991 Sep 3;30(35):8578–8590. doi: 10.1021/bi00099a013. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S., Kusumi A. Oxygen permeability of phosphatidylcholine--cholesterol membranes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4474–4478. doi: 10.1073/pnas.86.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski W. K., Markowska E., Gruszecki W. I., Sielewiesiuk J. Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: a spin-label study. Biochim Biophys Acta. 1992 Mar 23;1105(1):97–108. doi: 10.1016/0005-2736(92)90167-k. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Markowska E., Sielewiesiuk J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim Biophys Acta. 1991 Sep 10;1068(1):68–72. doi: 10.1016/0005-2736(91)90061-c. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Markowska E., Sielewiesiuk J. Spin-label studies on phosphatidylcholine-polar carotenoid membranes: effects of alkyl-chain length and unsaturation. Biochim Biophys Acta. 1993 Aug 15;1150(2):173–181. doi: 10.1016/0005-2736(93)90087-g. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Wisniewska A., Yin J. J., Hyde J. S., Kusumi A. Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry. 1994 Jun 21;33(24):7670–7681. doi: 10.1021/bi00190a022. [DOI] [PubMed] [Google Scholar]
- Wardlaw J. R., Sawyer W. H., Ghiggino K. P. Vertical fluctuations of phospholipid acyl chains in bilayers. FEBS Lett. 1987 Oct 19;223(1):20–24. doi: 10.1016/0014-5793(87)80502-1. [DOI] [PubMed] [Google Scholar]
- Yin J. J., Feix J. B., Hyde J. S. Mapping of collision frequencies for stearic acid spin labels by saturation-recovery electron paramagnetic resonance. Biophys J. 1990 Sep;58(3):713–720. doi: 10.1016/S0006-3495(90)82414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. J., Feix J. B., Hyde J. S. The effects of cholesterol on lateral diffusion and vertical fluctuations in lipid bilayers. An electron-electron double resonance (ELDOR) study. Biophys J. 1987 Dec;52(6):1031–1038. doi: 10.1016/S0006-3495(87)83296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. J., Pasenkiewicz-Gierula M., Hyde J. S. Lateral diffusion of lipids in membranes by pulse saturation recovery electron spin resonance. Proc Natl Acad Sci U S A. 1987 Feb;84(4):964–968. doi: 10.1073/pnas.84.4.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988 Jan-Feb;32(4):252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]


