Abstract
Complex formation between horse heart cytochrome c (cyt c) and bovine cytochrome c oxidase (cco) incorporated into a supported planar egg phosphatidylcholine membrane containing varying amounts of cardiolipin (CL) (0-20 mol%) has been studied under low (10 mM) and medium (160 mM) ionic strength conditions by surface plasmon resonance (SPR) spectroscopy. Both specific and nonspecific modes of cyt c binding are observed. The dissociation constant of the specific interaction between cyt c and cco increases from approximately 6.5 microM at low ionic strength to 18 microM at medium ionic strength, whereas the final saturation level of bound protein is independent of salt concentration and corresponds to approximately 53% of the total cco molecules present in the membrane. This suggests a 1:1 binding stoichiometry between the two proteins. The nonspecific binding component is governed by electrostatic interactions between cyt c and the membrane lipids and results in a partially ionic strength-reversible protein-membrane association. Thus, hydrophobic interactions between cyt c and the membrane, which are the predominant mode of binding in the absence of cco, are greatly suppressed. Both the amount of nonspecifically bound protein and the binding affinity can be varied over a broad range by changing the ionic strength and the extent of CL incorporation into the membrane. Under conditions approximating the physiological state in the mitochondrion (i.e., 20 mol% CL and medium ionic strength), 1-1.5 cyt c molecules are bound to the lipid phase per molecule of cco, with a dissociation constant of 0.1 microM. The possible physiological significance of these observations is discussed.
Full text
PDF

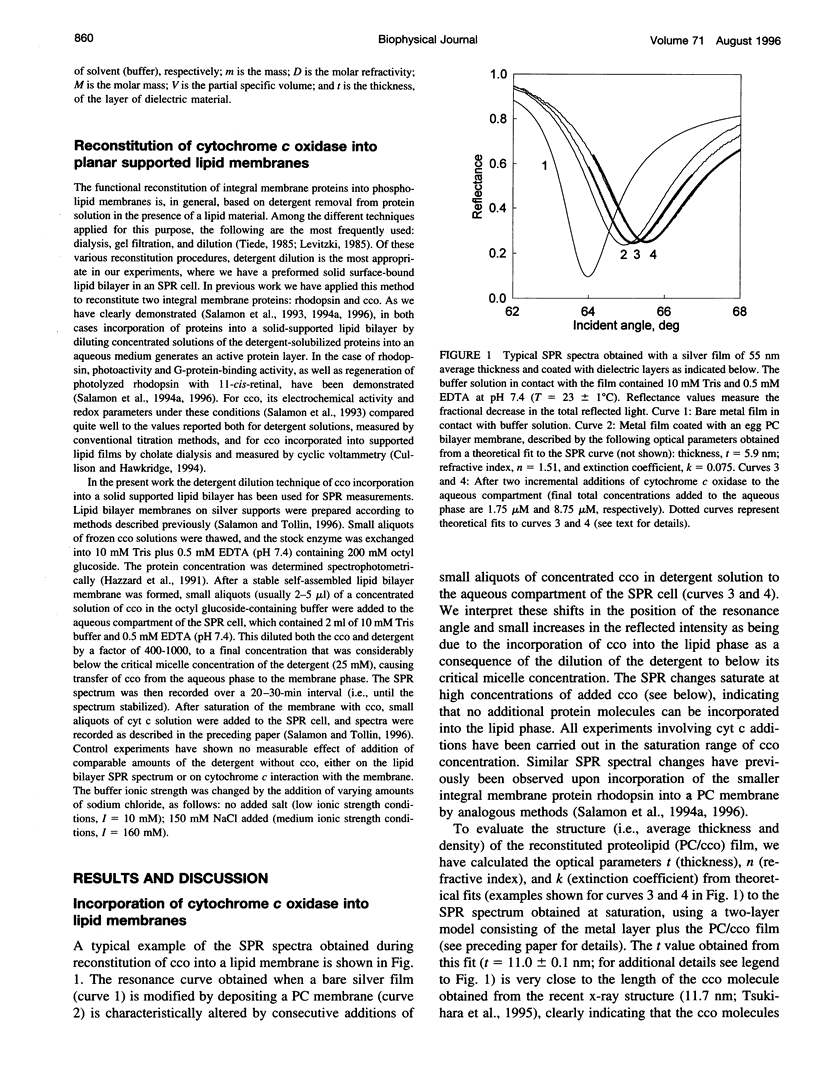
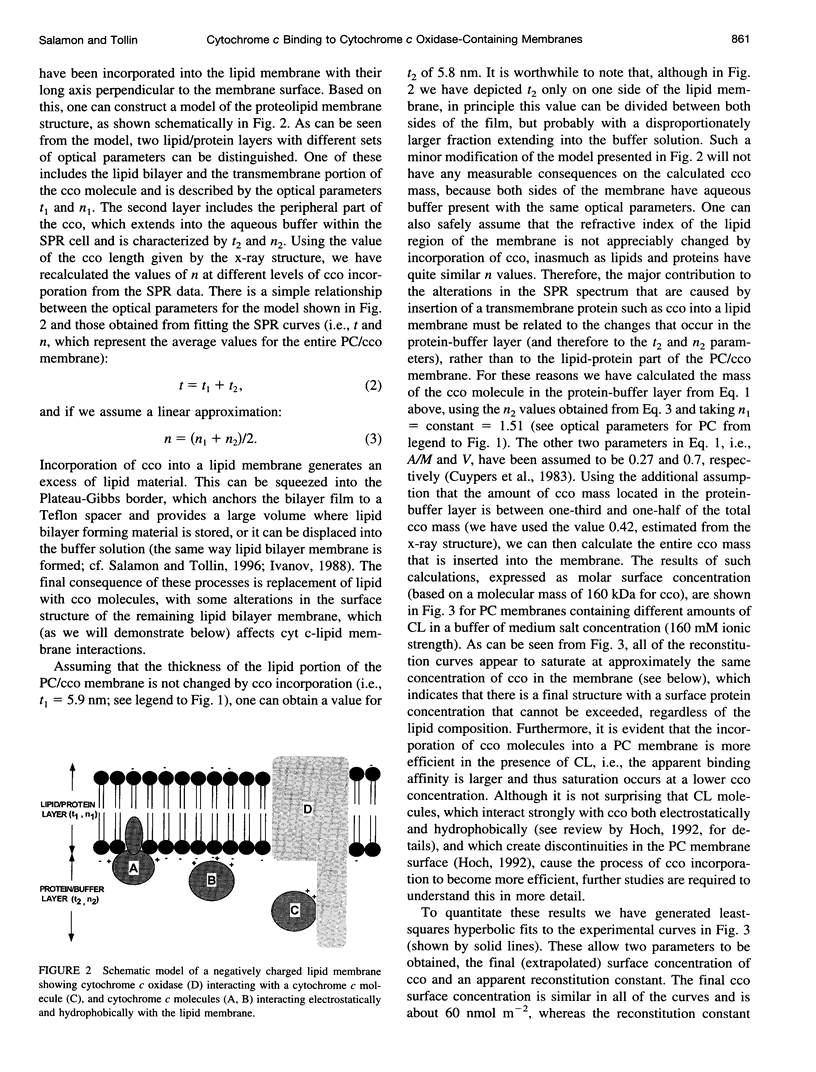
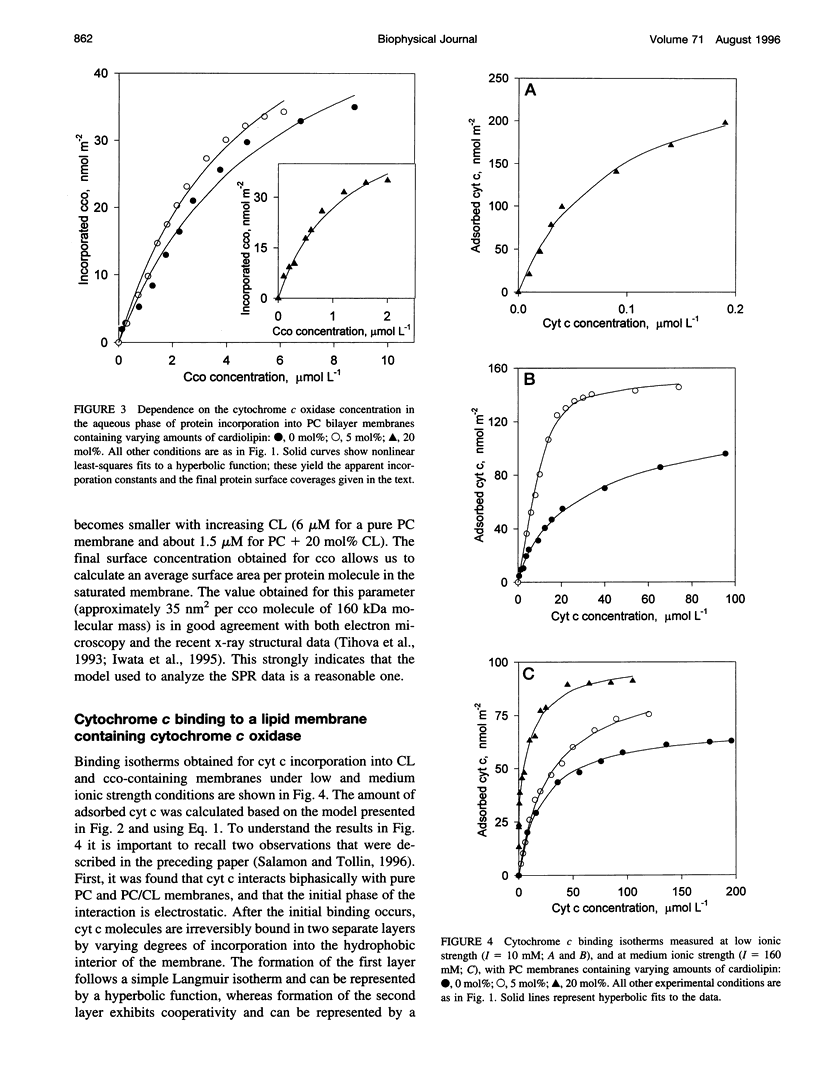


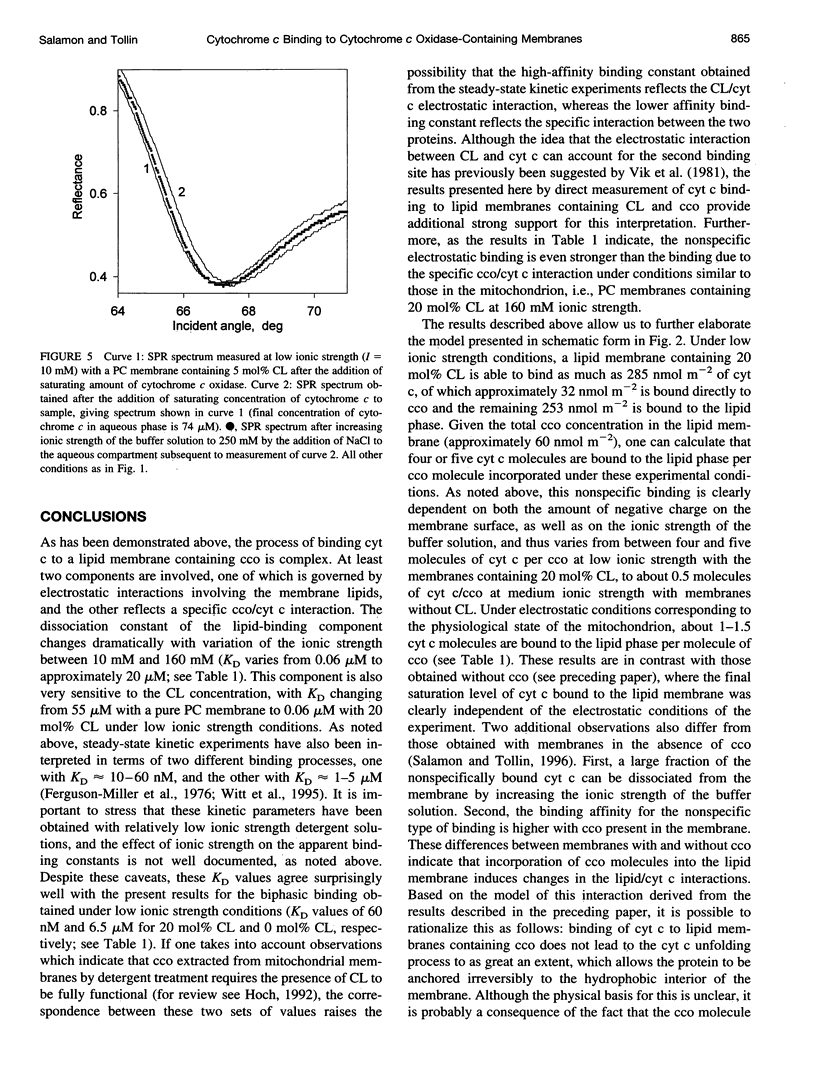


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antalis T. M., Palmer G. Kinetic characterization of the interaction between cytochrome oxidase and cytochrome c. J Biol Chem. 1982 Jun 10;257(11):6194–6206. [PubMed] [Google Scholar]
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Bolgiano B., Smith L., Davies H. C. Kinetics of the interaction of the cytochrome c oxidase of Paracoccus denitrificans with its own and bovine cytochrome c. Biochim Biophys Acta. 1988 Apr 22;933(2):341–350. doi: 10.1016/0005-2728(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Bolli R., Nałecz K. A., Azzi A. The aggregation state of bovine heart cytochrome c oxidase and its kinetics in monomeric and dimeric form. Arch Biochem Biophys. 1985 Jul;240(1):102–116. doi: 10.1016/0003-9861(85)90012-8. [DOI] [PubMed] [Google Scholar]
- Bondeson K., Frostell-Karlsson A., Fägerstam L., Magnusson G. Lactose repressor-operator DNA interactions: kinetic analysis by a surface plasmon resonance biosensor. Anal Biochem. 1993 Oct;214(1):245–251. doi: 10.1006/abio.1993.1484. [DOI] [PubMed] [Google Scholar]
- Brzezinski P., Malmström B. G. The mechanism of electron gating in proton pumping cytochrome c oxidase: the effect of pH and temperature on internal electron transfer. Biochim Biophys Acta. 1987 Oct 29;894(1):29–38. doi: 10.1016/0005-2728(87)90209-x. [DOI] [PubMed] [Google Scholar]
- Calhoun M. W., Thomas J. W., Gennis R. B. The cytochrome oxidase superfamily of redox-driven proton pumps. Trends Biochem Sci. 1994 Aug;19(8):325–330. doi: 10.1016/0968-0004(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A. Structure and function of cytochrome c oxidase. Annu Rev Biochem. 1990;59:569–596. doi: 10.1146/annurev.bi.59.070190.003033. [DOI] [PubMed] [Google Scholar]
- Cooper C. E. The steady-state kinetics of cytochrome c oxidation by cytochrome oxidase. Biochim Biophys Acta. 1990 Jun 26;1017(3):187–203. doi: 10.1016/0005-2728(90)90184-6. [DOI] [PubMed] [Google Scholar]
- Copeland R. A., Chan S. I. Proton translocation in proteins. Annu Rev Phys Chem. 1989;40:671–698. doi: 10.1146/annurev.pc.40.100189.003323. [DOI] [PubMed] [Google Scholar]
- Cuypers P. A., Corsel J. W., Janssen M. P., Kop J. M., Hermens W. T., Hemker H. C. The adsorption of prothrombin to phosphatidylserine multilayers quantitated by ellipsometry. J Biol Chem. 1983 Feb 25;258(4):2426–2431. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Garber E. A., Margoliash E. Interaction of cytochrome c with cytochrome c oxidase: an understanding of the high- to low-affinity transition. Biochim Biophys Acta. 1990 Feb 2;1015(2):279–287. doi: 10.1016/0005-2728(90)90032-y. [DOI] [PubMed] [Google Scholar]
- Gennis R., Ferguson-Miller S. Structure of cytochrome c oxidase, energy generator of aerobic life. Science. 1995 Aug 25;269(5227):1063–1064. doi: 10.1126/science.7652553. [DOI] [PubMed] [Google Scholar]
- Gupta S., Morgan T. R., Gordan G. S. Calcitonin gene-related peptide in hepatorenal syndrome. A possible mediator of peripheral vasodilation? J Clin Gastroenterol. 1992 Mar;14(2):122–126. doi: 10.1097/00004836-199203000-00010. [DOI] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R., Chazotte B., Gupte S. S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986 Oct;18(5):331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Hazzard J. T., Rong S. Y., Tollin G. Ionic strength dependence of the kinetics of electron transfer from bovine mitochondrial cytochrome c to bovine cytochrome c oxidase. Biochemistry. 1991 Jan 8;30(1):213–222. doi: 10.1021/bi00215a031. [DOI] [PubMed] [Google Scholar]
- Hoch F. L. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992 Mar 26;1113(1):71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Iwata S., Ostermeier C., Ludwig B., Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995 Aug 24;376(6542):660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Reconstitution of membrane receptor systems. Biochim Biophys Acta. 1985 Jun 12;822(1):127–153. doi: 10.1016/0304-4157(85)90005-x. [DOI] [PubMed] [Google Scholar]
- MUELLER P., RUDIN D. O., TIEN H. T., WESCOTT W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962 Jun 9;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- Malatesta F., Antonini G., Sarti P., Brunori M. Structure and function of a molecular machine: cytochrome c oxidase. Biophys Chem. 1995 Mar;54(1):1–33. doi: 10.1016/0301-4622(94)00117-3. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome oxidase: some unsolved problems and controversial issues. Arch Biochem Biophys. 1990 Aug 1;280(2):233–241. doi: 10.1016/0003-9861(90)90325-s. [DOI] [PubMed] [Google Scholar]
- Michel B., Bosshard H. R. Oxidation of cytochrome c by cytochrome c oxidase: spectroscopic binding studies and steady-state kinetics support a conformational transition mechanism. Biochemistry. 1989 Jan 10;28(1):244–252. doi: 10.1021/bi00427a034. [DOI] [PubMed] [Google Scholar]
- Michel B., Bosshard H. R. Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase. J Biol Chem. 1984 Aug 25;259(16):10085–10091. [PubMed] [Google Scholar]
- Morgan J. E., Wikström M. Steady-state redox behavior of cytochrome c, cytochrome a, and CuA of cytochrome c oxidase in intact rat liver mitochondria. Biochemistry. 1991 Jan 29;30(4):948–958. doi: 10.1021/bi00218a010. [DOI] [PubMed] [Google Scholar]
- Nałecz K. A., Bolli R., Azzi A. Preparation of monomeric cytochrome C oxidase: its kinetics differ from those of the dimeric enzyme. Biochem Biophys Res Commun. 1983 Jul 29;114(2):822–828. doi: 10.1016/0006-291x(83)90855-0. [DOI] [PubMed] [Google Scholar]
- Nicholls P. The steady state behaviour of cytochrome c oxidase in proteoliposomes. FEBS Lett. 1993 Jul 26;327(2):194–198. doi: 10.1016/0014-5793(93)80168-t. [DOI] [PubMed] [Google Scholar]
- Ortega-Lopez J., Robinson N. C. Cytochrome c oxidase: biphasic kinetics result from incomplete reduction of cytochrome a by cytochrome c bound to the high-affinity site. Biochemistry. 1995 Aug 8;34(31):10000–10008. doi: 10.1021/bi00031a023. [DOI] [PubMed] [Google Scholar]
- Panda M., Robinson N. C. Kinetics and mechanism for the binding of HCN to cytochrome c oxidase. Biochemistry. 1995 Aug 8;34(31):10009–10018. doi: 10.1021/bi00031a024. [DOI] [PubMed] [Google Scholar]
- Plant A. L., Brigham-Burke M., Petrella E. C., O'Shannessy D. J. Phospholipid/alkanethiol bilayers for cell-surface receptor studies by surface plasmon resonance. Anal Biochem. 1995 Apr 10;226(2):342–348. doi: 10.1006/abio.1995.1234. [DOI] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. The cytochrome c oxidase binding site on cytochrome c. Differential chemical modification of lysine residues in free and oxidase-bound cytochrome c. J Biol Chem. 1978 Sep 10;253(17):6045–6053. [PubMed] [Google Scholar]
- Salamon Z., Meyer T. E., Tollin G. Photobleaching of the photoactive yellow protein from Ectothiorhodospira halophila promotes binding to lipid bilayers: evidence from surface plasmon resonance spectroscopy. Biophys J. 1995 Feb;68(2):648–654. doi: 10.1016/S0006-3495(95)80225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon Z., Wang Y., Brown M. F., Macleod H. A., Tollin G. Conformational changes in rhodopsin probed by surface plasmon resonance spectroscopy. Biochemistry. 1994 Nov 22;33(46):13706–13711. doi: 10.1021/bi00250a022. [DOI] [PubMed] [Google Scholar]
- Salamon Z., Wang Y., Soulages J. L., Brown M. F., Tollin G. Surface plasmon resonance spectroscopy studies of membrane proteins: transducin binding and activation by rhodopsin monitored in thin membrane films. Biophys J. 1996 Jul;71(1):283–294. doi: 10.1016/S0006-3495(96)79224-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon Z., Wang Y., Tollin G., Macleod H. A. Assembly and molecular organization of self-assembled lipid bilayers on solid substrates monitored by surface plasmon resonance spectroscopy. Biochim Biophys Acta. 1994 Nov 2;1195(2):267–275. doi: 10.1016/0005-2736(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Schuster S. C., Swanson R. V., Alex L. A., Bourret R. B., Simon M. I. Assembly and function of a quaternary signal transduction complex monitored by surface plasmon resonance. Nature. 1993 Sep 23;365(6444):343–347. doi: 10.1038/365343a0. [DOI] [PubMed] [Google Scholar]
- Soulages J. L., Salamon Z., Wells M. A., Tollin G. Low concentrations of diacylglycerol promote the binding of apolipophorin III to a phospholipid bilayer: a surface plasmon resonance spectroscopy study. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5650–5654. doi: 10.1073/pnas.92.12.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Dye D., Margoliash E. Single catalytic site model for the oxidation of ferrocytochrome c by mitochondrial cytochrome c oxidase. Proc Natl Acad Sci U S A. 1984 Jan;81(2):347–351. doi: 10.1073/pnas.81.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R. V., Schuster S. C., Simon M. I. Expression of CheA fragments which define domains encoding kinase, phosphotransfer, and CheY binding activities. Biochemistry. 1993 Aug 3;32(30):7623–7629. doi: 10.1021/bi00081a004. [DOI] [PubMed] [Google Scholar]
- Tiede D. M. Incorporation of membrane proteins into interfacial films: model membranes for electrical and structural characterization. Biochim Biophys Acta. 1985 Dec;811(4):357–379. doi: 10.1016/0304-4173(85)90007-2. [DOI] [PubMed] [Google Scholar]
- Tihova M., Tattrie B., Nicholls P. Electron microscopy of cytochrome c oxidase-containing proteoliposomes: imaging analysis of protein orientation and monomer-dimer behaviour. Biochem J. 1993 Jun 15;292(Pt 3):933–946. doi: 10.1042/bj2920933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpower B. L., Gennis R. B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995 Aug 25;269(5227):1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H., Zickermann V., Ludwig B. Site-directed mutagenesis of cytochrome c oxidase reveals two acidic residues involved in the binding of cytochrome c. Biochim Biophys Acta. 1995 Jun 1;1230(1-2):74–76. doi: 10.1016/0005-2728(95)00050-s. [DOI] [PubMed] [Google Scholar]



