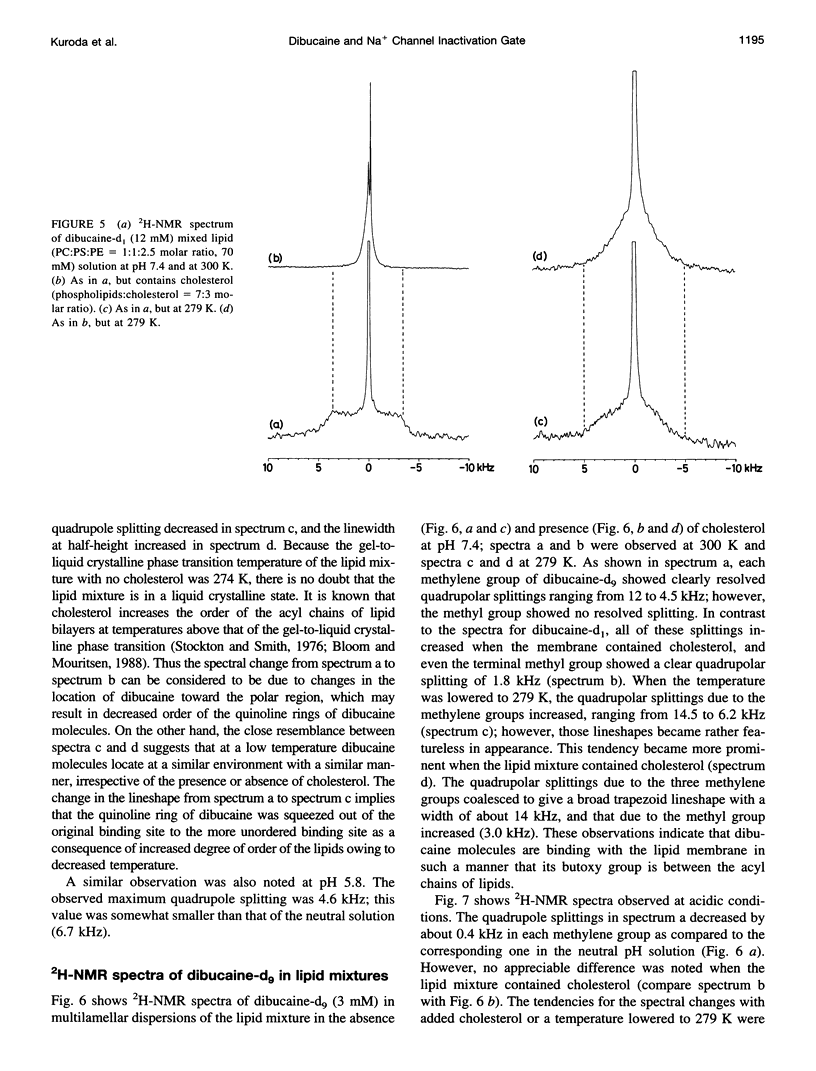
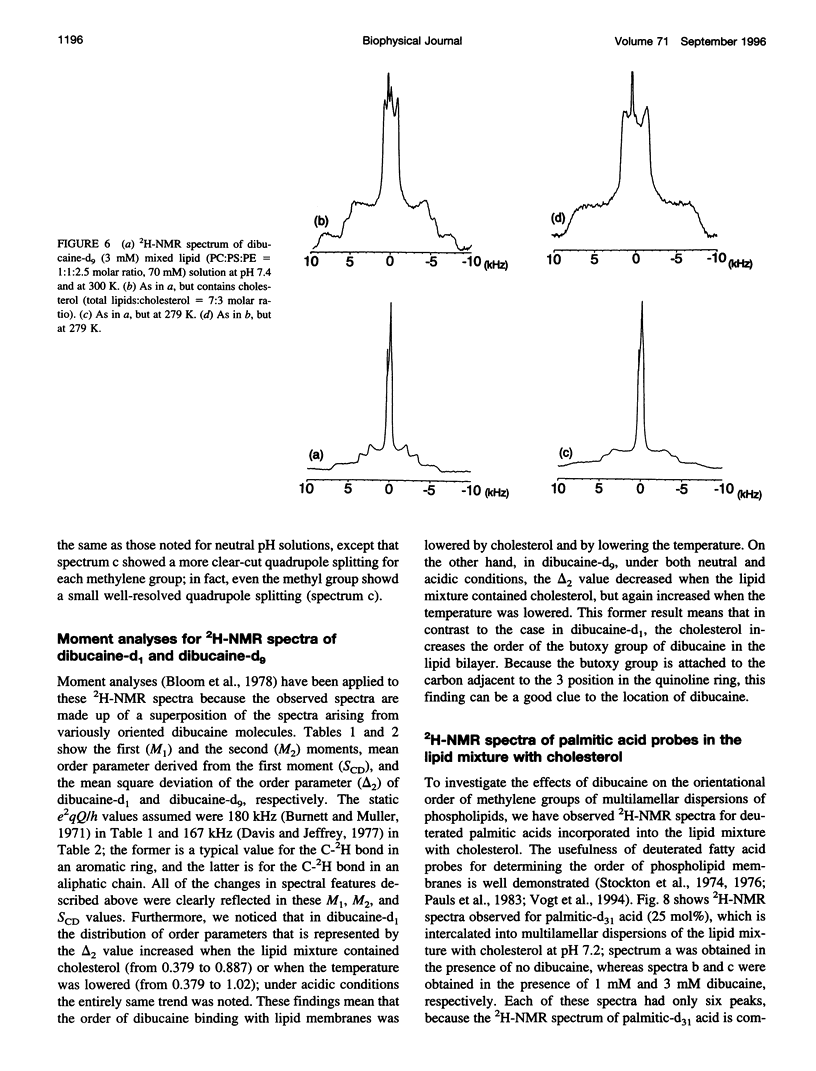
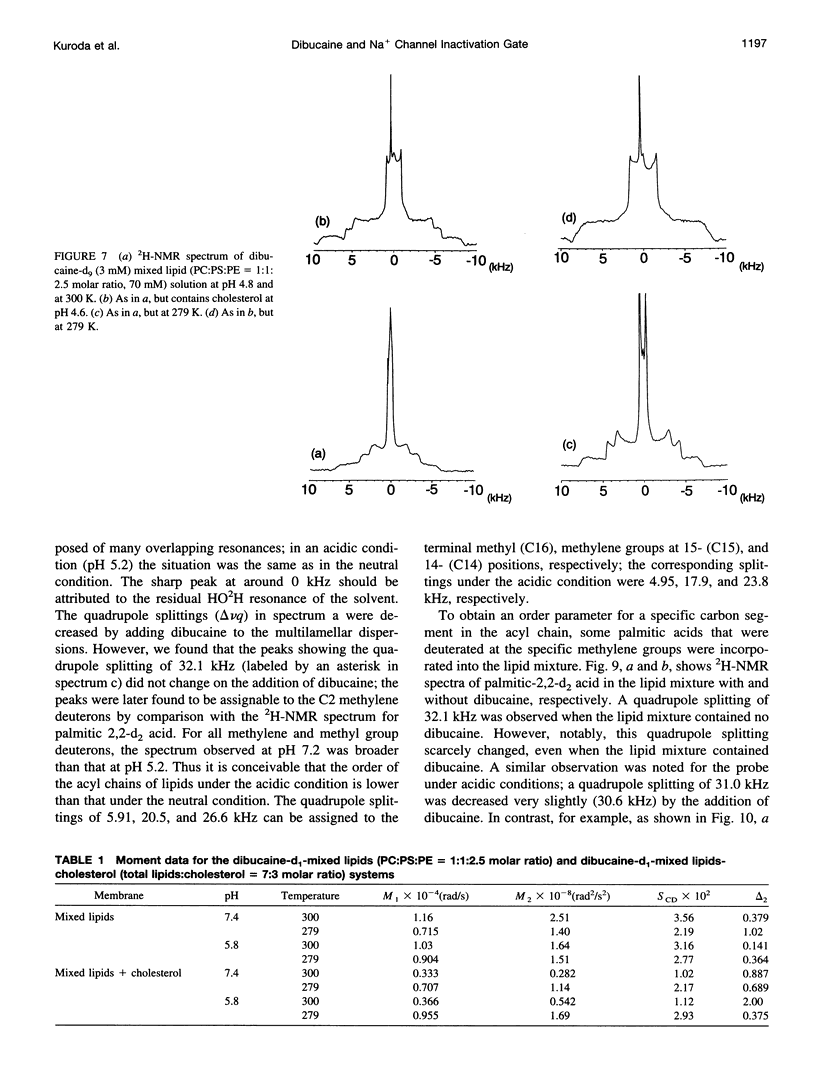
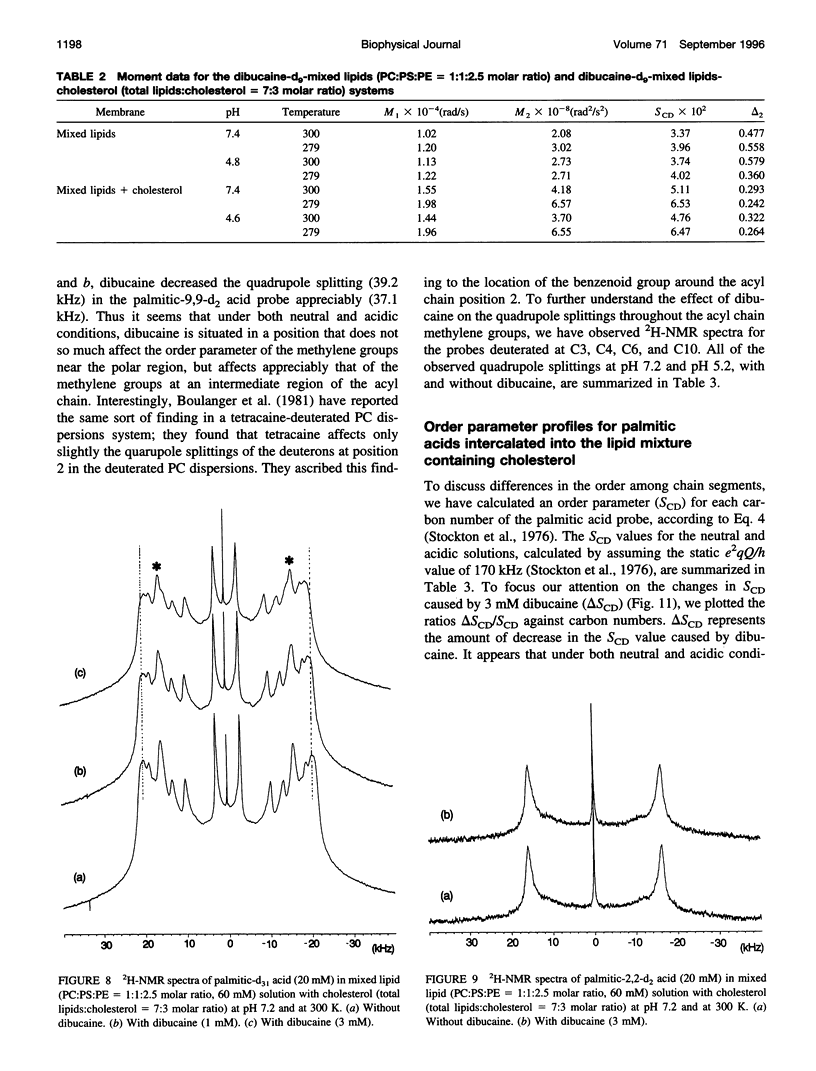
Abstract
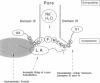
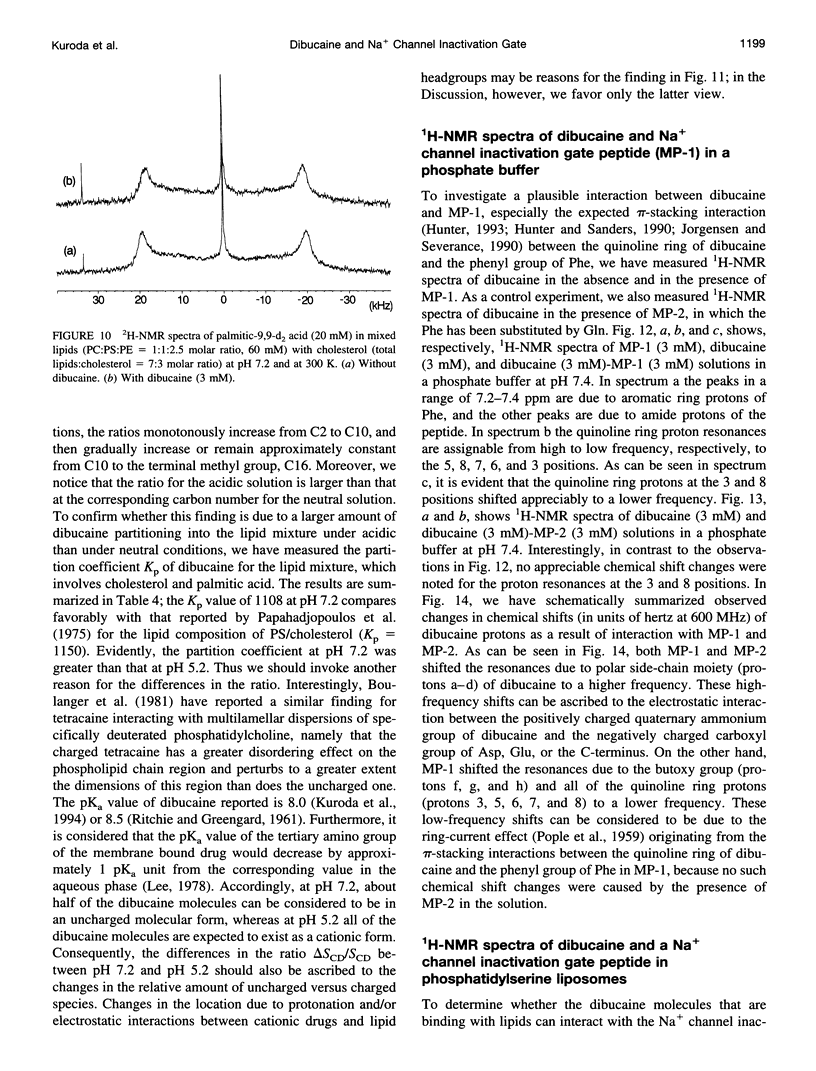
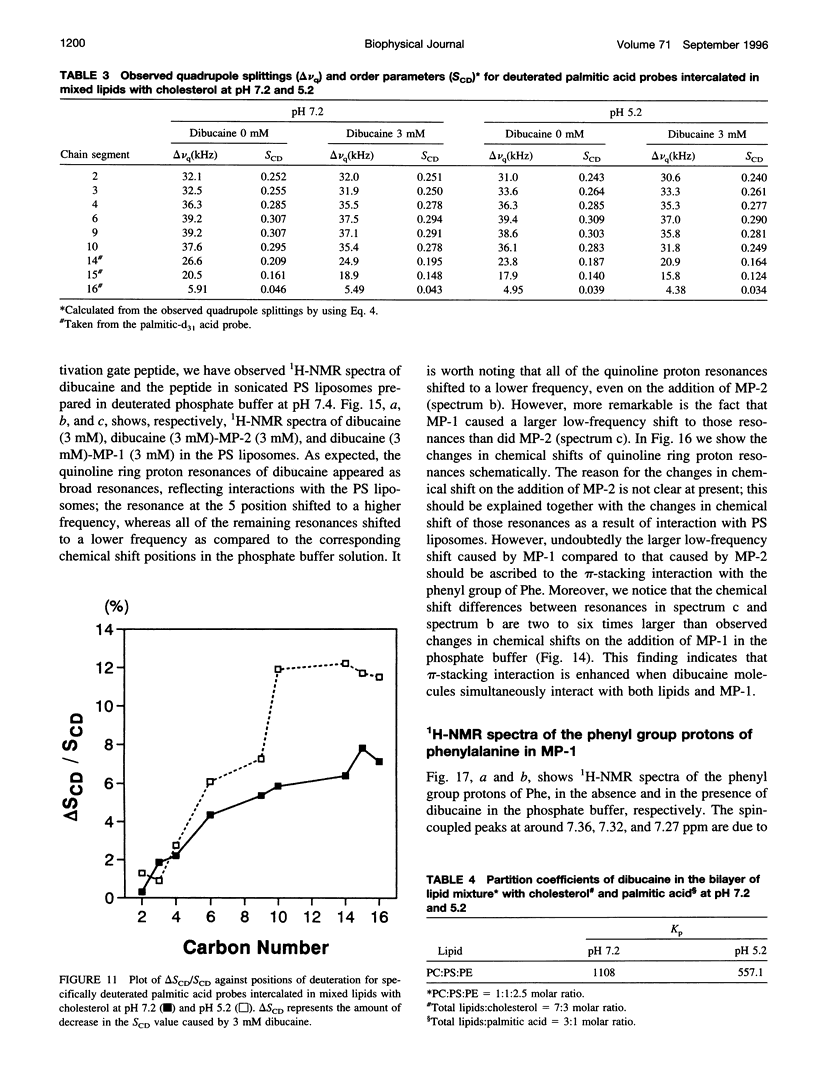
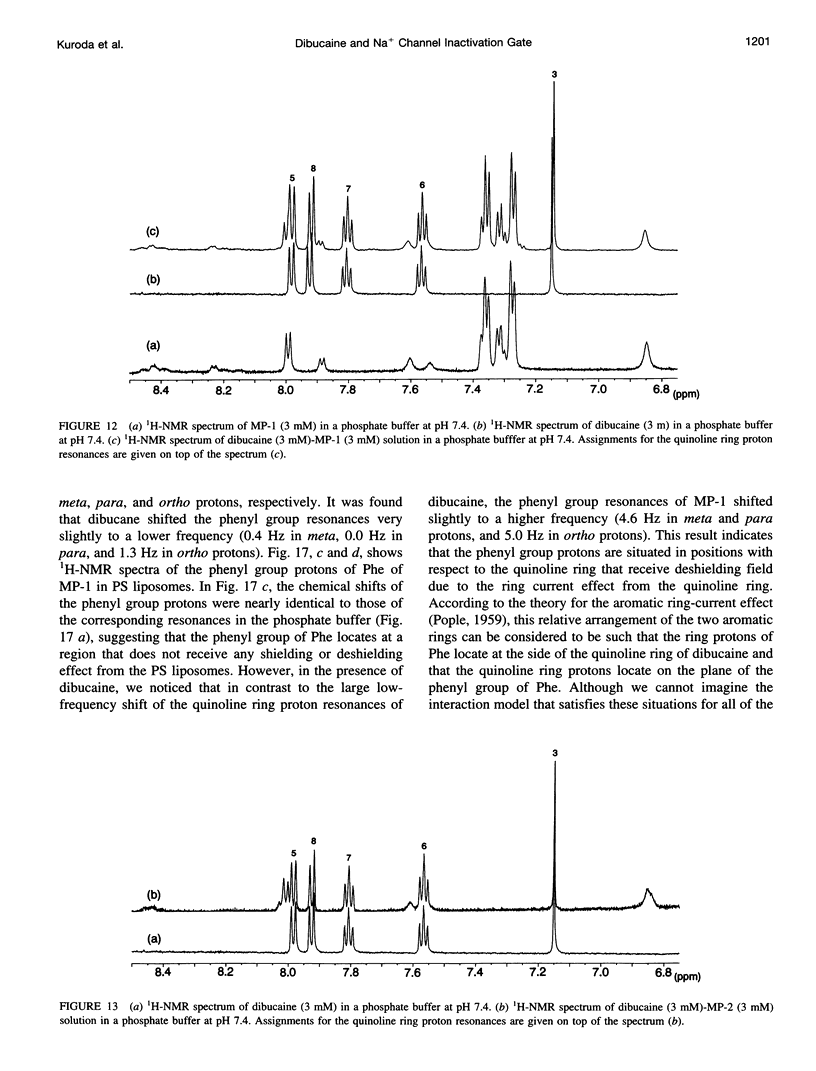
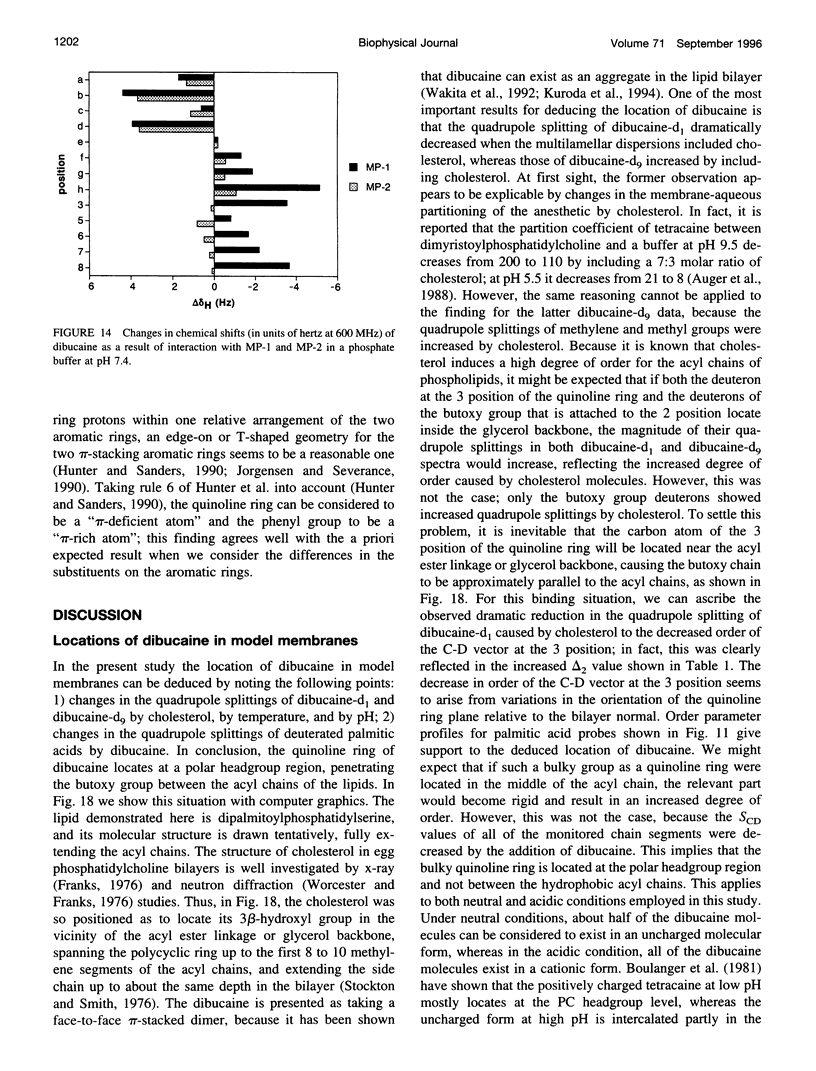
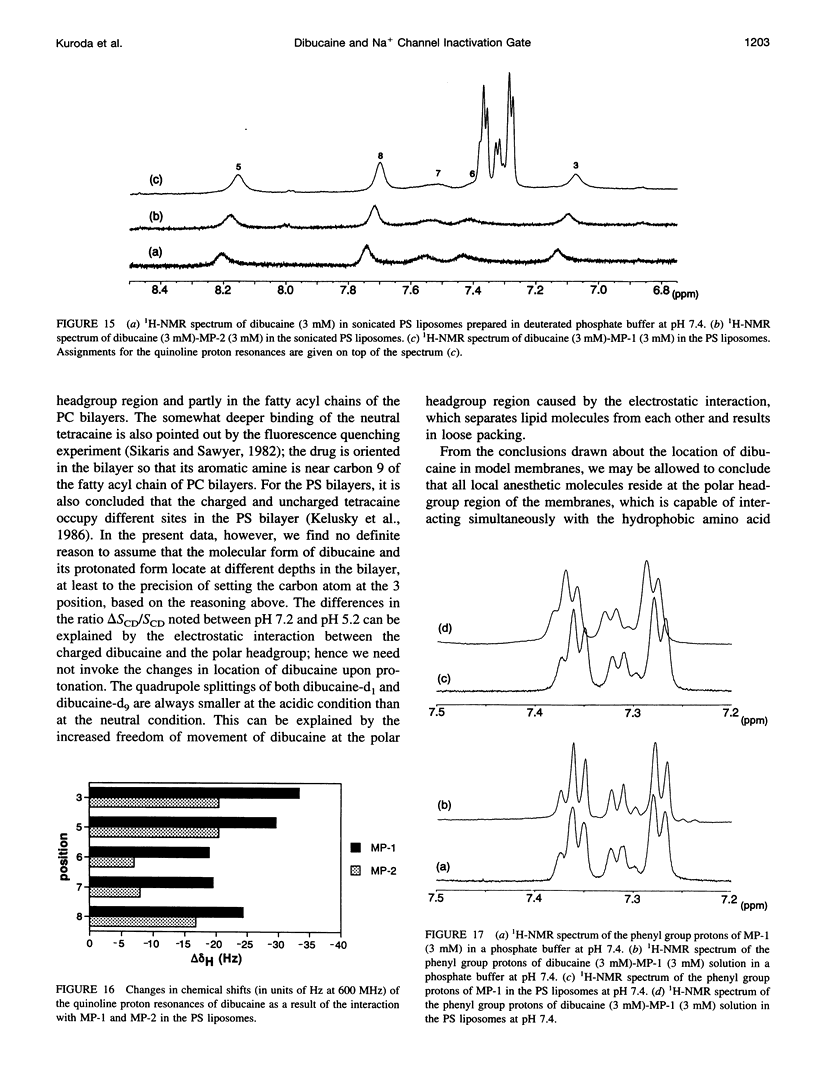
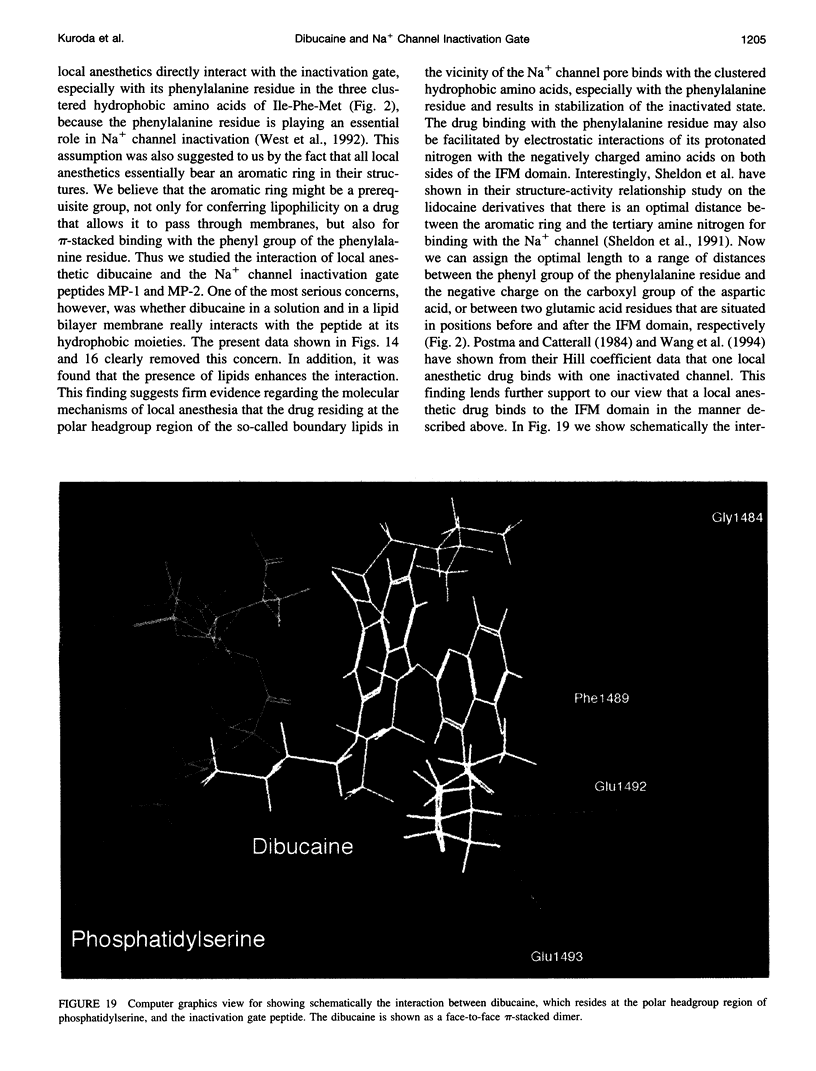
To study the molecular mechanisms of local anesthesia, locations of local anesthetic dibucaine in model membranes and the interactions of dibucaine with a Na+ channel inactivation gate peptide have been studied by 2H- and 1H-NMR spectroscopies. The 2H-NMR spectra of dibucaine-d9 and dibucaine-d1, which are deuterated at the butoxy group and at the 3 position in its quinoline ring, respectively, have been observed in multilamellar dispersions of the lipid mixture composed of phosphatidylcholine, phosphatidylserine, and phosphatidylethanolamine. 2H-NMR spectra of deuterated palmitic acids incorporated, as a probe, into the lipid mixture containing cholesterol have also been observed. An order parameter, SCD, for each carbon segment was calculated from the observed quadrupole splittings. Combining these results, we concluded that first, the butoxy group of dibucaine is penetrating between the acyl chains of lipids in the model membranes, and second, the quinoline ring of dibucaine is located at the polar region of lipids but not at the hydrophobic acyl chain moiety. These results mean that dibucaine is situated in a favorable position that permits it to interact with a cluster of hydrophobic amino acids (Ile-Phe-Met) within the intracellular linker between domains III and IV of Na+ channel protein, which functions as an inactivation gate. To confirm whether the dibucaine molecule at the surface region of lipids can really interact with the hydrophobic amino acids, we synthesized a model peptide that includes the hydrophobic amino acids (Ac-GGQDIFMTEEQK-OH, MP-1), the amino acid sequence of which corresponds to the linker part of rat brain type IIA Na+ channel, and the one in which Phe has been substituted by Gln (MP-2), and measured 1H-NMR spectra in both phosphate buffer and phosphatidylserine liposomes. It was found that the quinoline ring of dibucaine can interact with the aromatic ring of Phe by stacking of the rings; moreover, the interaction can be reinforced by the presence of lipids. In conclusion, we wish to propose that local anesthesia originates from the pi-stacking interaction between aromatic rings of an anesthetic molecule located at the polar headgroup region of the so-called boundary lipids and of the Phe in the intracellular linker between domains III and IV of the Na+ channel protein, prolonging the inactivated state and consequently making it impossible to proceed to the resting state.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger M., Jarrell H. C., Smith I. C. Interactions of the local anesthetic tetracaine with membranes containing phosphatidylcholine and cholesterol: a 2H NMR study. Biochemistry. 1988 Jun 28;27(13):4660–4667. doi: 10.1021/bi00413a012. [DOI] [PubMed] [Google Scholar]
- Bennett P. B., Valenzuela C., Chen L. Q., Kallen R. G. On the molecular nature of the lidocaine receptor of cardiac Na+ channels. Modification of block by alterations in the alpha-subunit III-IV interdomain. Circ Res. 1995 Sep;77(3):584–592. doi: 10.1161/01.res.77.3.584. [DOI] [PubMed] [Google Scholar]
- Boulanger Y., Schreier S., Smith I. C. Molecular details of anesthetic--lipid interaction as seen by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1981 Nov 24;20(24):6824–6830. doi: 10.1021/bi00527a013. [DOI] [PubMed] [Google Scholar]
- Chacko G. K., Villegas G. M., Barnola F. V., Villegas R., Goldman D. E. The polypeptide and the phospholipid components of axon plasma membranes. Biochim Biophys Acta. 1976 Aug 4;443(1):19–32. doi: 10.1016/0005-2736(76)90488-0. [DOI] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Davis J. H., Maraviglia B., Weeks G., Godin D. V. Bilayer rigidity of the erythrocyte membrane2H-NMR of a perdeuterated palmitic acid probe. Biochim Biophys Acta. 1979 Jan 19;550(2):362–366. doi: 10.1016/0005-2736(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Dibble A. R., Feigenson G. W. Detection of coexisting fluid phospholipid phases by equilibrium Ca2+ binding: peptide-poor L alpha and peptide-rich HII phase coexistence in gramicidin A'/phospholipid dispersions. Biochemistry. 1994 Nov 8;33(44):12945–12953. doi: 10.1021/bi00248a002. [DOI] [PubMed] [Google Scholar]
- Franks N. P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. I. X-ray diffraction. J Mol Biol. 1976 Jan 25;100(3):345–358. doi: 10.1016/s0022-2836(76)80067-8. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelusky E. C., Boulanger Y., Schreier S., Smith I. C. A 2H-NMR study on the interactions of the local anesthetic tetracaine with membranes containing phosphatidylserine. Biochim Biophys Acta. 1986 Mar 27;856(1):85–90. doi: 10.1016/0005-2736(86)90013-1. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Wakita M., Nakagawa T. Interaction between dibucaine and pig erythrocyte membranes as studied by NOESY experiments in 1H-NMR spectroscopy. Which form of dibucaine interacts more strongly, cationic or uncharged? Chem Pharm Bull (Tokyo) 1994 Dec;42(12):2418–2425. doi: 10.1248/cpb.42.2418. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Effects of charged drugs on the phase transition temperatures of phospholipid bilayers. Biochim Biophys Acta. 1978 Dec 4;514(1):95–104. doi: 10.1016/0005-2736(78)90079-2. [DOI] [PubMed] [Google Scholar]
- Maraviglia B., Davis J. H., Bloom M., Westerman J., Wirtz K. W. Human erythrocyte membranes are fluid down to -5 degrees C. Biochim Biophys Acta. 1982 Mar 23;686(1):137–140. doi: 10.1016/0005-2736(82)90160-2. [DOI] [PubMed] [Google Scholar]
- McPhee J. C., Ragsdale D. S., Scheuer T., Catterall W. A. A critical role for transmembrane segment IVS6 of the sodium channel alpha subunit in fast inactivation. J Biol Chem. 1995 May 19;270(20):12025–12034. doi: 10.1074/jbc.270.20.12025. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Frazier T., Yamada M. The site of action and active form of local anesthetics. I. Theory and pH experiments with tertiary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):32–44. [PubMed] [Google Scholar]
- Nichol C. P., Davis J. H., Weeks G., Bloom M. Quantitative study of the fluidity of Escherichia coli membranes using deuterium magnetic resonance. Biochemistry. 1980 Feb 5;19(3):451–457. doi: 10.1021/bi00544a008. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Meadows M., Rice D., Jacobs R. Spectroscopic studies of specifically deuterium labeled membrane systems. Nuclear magnetic resonance investigation of the effects of cholesterol in model systems. Biochemistry. 1978 Jul 11;17(14):2727–2740. doi: 10.1021/bi00607a006. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Poste G., Shepherd G. Effects of local anesthetics on membrane properties. I. Changes in the fluidity of phospholipid bilayers. Biochim Biophys Acta. 1975 Jul 18;394(4):504–519. doi: 10.1016/0005-2736(75)90137-6. [DOI] [PubMed] [Google Scholar]
- Patton D. E., West J. W., Catterall W. A., Goldin A. L. Amino acid residues required for fast Na(+)-channel inactivation: charge neutralizations and deletions in the III-IV linker. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10905–10909. doi: 10.1073/pnas.89.22.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma S. W., Catterall W. A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by local anesthetics. Mol Pharmacol. 1984 Mar;25(2):219–227. [PubMed] [Google Scholar]
- RITCHIE J. M., GREENGARD P. On the active structure of local anesthetics. J Pharmacol Exp Ther. 1961 Aug;133:241–245. [PubMed] [Google Scholar]
- Ragsdale D. S., McPhee J. C., Scheuer T., Catterall W. A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994 Sep 16;265(5179):1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Sheldon R. S., Hill R. J., Taouis M., Wilson L. M. Aminoalkyl structural requirements for interaction of lidocaine with the class I antiarrhythmic drug receptor on rat cardiac myocytes. Mol Pharmacol. 1991 May;39(5):609–614. [PubMed] [Google Scholar]
- Sikaris K. A., Sawyer W. H. The interaction of local anaesthetics with synthetic phospholipid bilayers. Biochem Pharmacol. 1982 Aug 15;31(16):2625–2631. doi: 10.1016/0006-2952(82)90709-2. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Polnaszek C. F., Leitch L. C., Tulloch A. P., Smith I. C. A study of mobility and order in model membranes using 2H NMR relaxation rates and quadrupole splittings of specifically deuterated lipids. Biochem Biophys Res Commun. 1974 Sep 23;60(2):844–850. doi: 10.1016/0006-291x(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Polnaszek C. F., Tulloch A. P., Hasan F., Smith I. C. Molecular motion and order in single-bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures. A deuterium nuclear magnetic resonance study of specifically labeled lipids. Biochemistry. 1976 Mar 9;15(5):954–966. doi: 10.1021/bi00650a003. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Smith I. C. A deuterium nuclear magnetic resonance study of the condensing effect of cholesterol on egg phosphatidylcholine bilayer membranes. I. Perdeuterated fatty acid probes. Chem Phys Lipids. 1976 Oct;17(2-3):251–263. doi: 10.1016/0009-3084(76)90070-0. [DOI] [PubMed] [Google Scholar]
- Tournois H., Leunissen-Bijvelt J., Haest C. W., de Gier J., de Kruijff B. Gramicidin-induced hexagonal HII phase formation in erythrocyte membranes. Biochemistry. 1987 Oct 20;26(21):6613–6621. doi: 10.1021/bi00395a008. [DOI] [PubMed] [Google Scholar]
- Trudell J. R. A unitary theory of anesthesia based on lateral phase separations in nerve membranes. Anesthesiology. 1977 Jan;46(1):5–10. doi: 10.1097/00000542-197701000-00003. [DOI] [PubMed] [Google Scholar]
- Vogt T. C., Killian J. A., De Kruijff B. Structure and dynamics of the acyl chain of a transmembrane polypeptide. Biochemistry. 1994 Mar 1;33(8):2063–2070. doi: 10.1021/bi00174a012. [DOI] [PubMed] [Google Scholar]
- Wakita M., Kuroda Y., Fujiwara Y., Nakagawa T. Conformations of dibucaine and tetracaine in small unilamellar phosphatidylcholine vesicles as studied by nuclear Overhauser effects in 1H nuclear magnetic resonance spectroscopy. Chem Phys Lipids. 1992 Jul;62(1):45–54. doi: 10.1016/0009-3084(92)90053-r. [DOI] [PubMed] [Google Scholar]
- Wang G. K., Mok W. M., Wang S. Y. Charged tetracaine as an inactivation enhancer in batrachotoxin-modified Na+ channels. Biophys J. 1994 Nov;67(5):1851–1860. doi: 10.1016/S0006-3495(94)80666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Simon R., Wang S. Y. Quaternary ammonium compounds as structural probes of single batrachotoxin-activated Na+ channels. J Gen Physiol. 1991 Nov;98(5):1005–1024. doi: 10.1085/jgp.98.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman J., Boulanger Y., Ehrenberg A., Smith I. C. Charge and pH dependent drug binding to model membranes. A 2H-NMR and light absorption study. Biochim Biophys Acta. 1982 Mar 8;685(3):315–328. doi: 10.1016/0005-2736(82)90073-6. [DOI] [PubMed] [Google Scholar]
- Worcester D. L., Franks N. P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. II. Neutrol diffraction. J Mol Biol. 1976 Jan 25;100(3):359–378. doi: 10.1016/s0022-2836(76)80068-x. [DOI] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Amine blockers of the cytoplasmic mouth of sodium channels: a small structural change can abolish voltage dependence. Biophys J. 1994 Sep;67(3):1015–1027. doi: 10.1016/S0006-3495(94)80567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]