Abstract
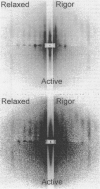
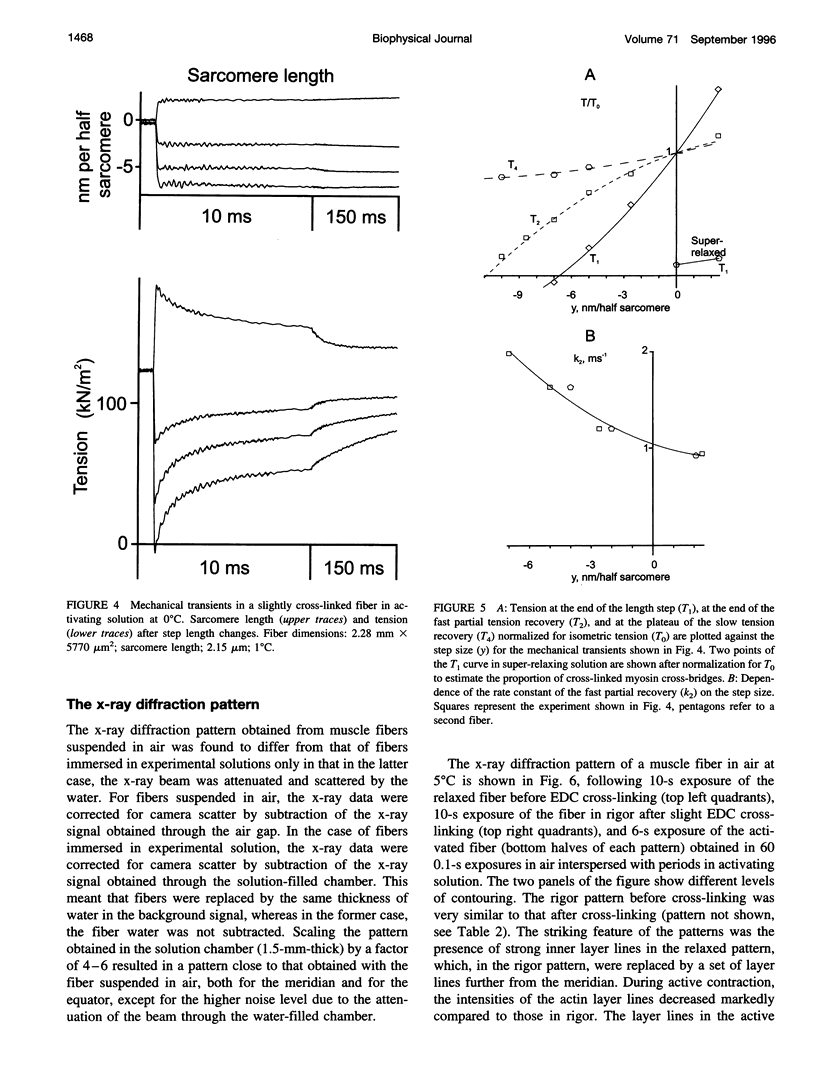
We show prolonged contraction of permeabilized muscle fibers of the frog during which structural order, as judged from low-angle x-ray diffraction, was preserved by means of partial cross-linking of the fibers using the zero-length cross-linker 1-ethyl-3-[3-dimethylamino)propyl]carbodiimide. Ten to twenty percent of the myosin cross-bridges were cross-linked, allowing the remaining 80-90% to cycle and generate force. These fibers displayed a well-preserved sarcomeric order and mechanical characteristics similar to those of intact muscle fibers. The intensity of the brightest meridional reflection at 14.5 nm, resulting from the projection of cross-bridges evenly spaced along the myofilament length, decreased by 60% as a relaxed fiber was deprived of ATP and entered the rigor state. Upon activation of a rigorized fiber by the addition of ATP, the intensity of this reflection returned to 97% of the relaxed value, suggesting that the overall orientation of cross-bridges in the active muscle was more perpendicular to the filament axis than in rigor. Following a small-amplitude length step applied to the active fibers, the reflection intensity decreased for both releases and stretches. In rigor, however, a small stretch increased the amplitude of the reflection by 35%. These findings show the close link between cross-bridge orientation and tension changes.
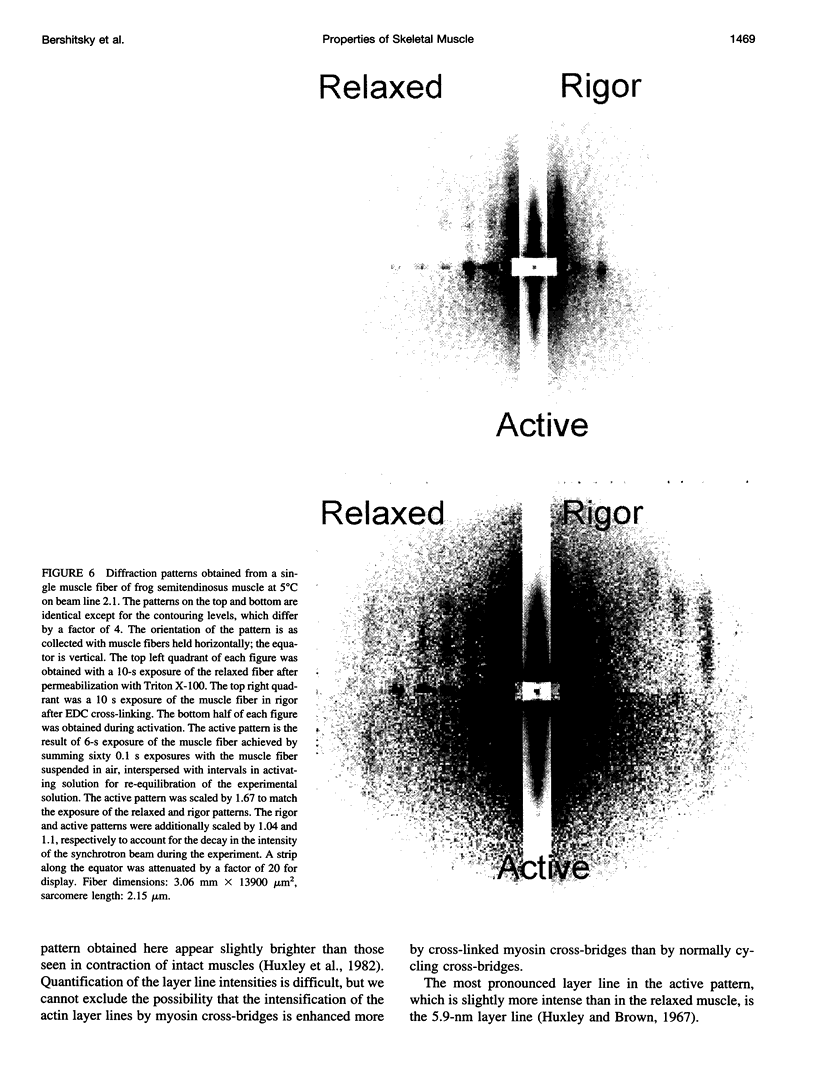
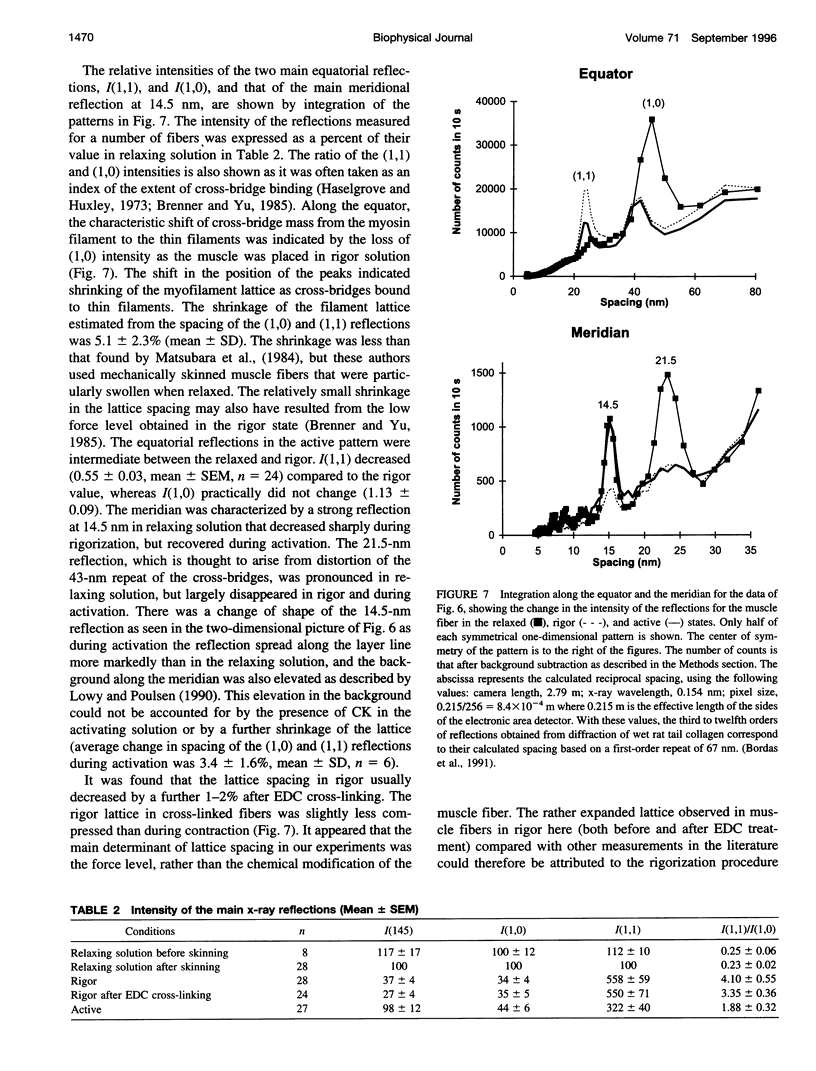
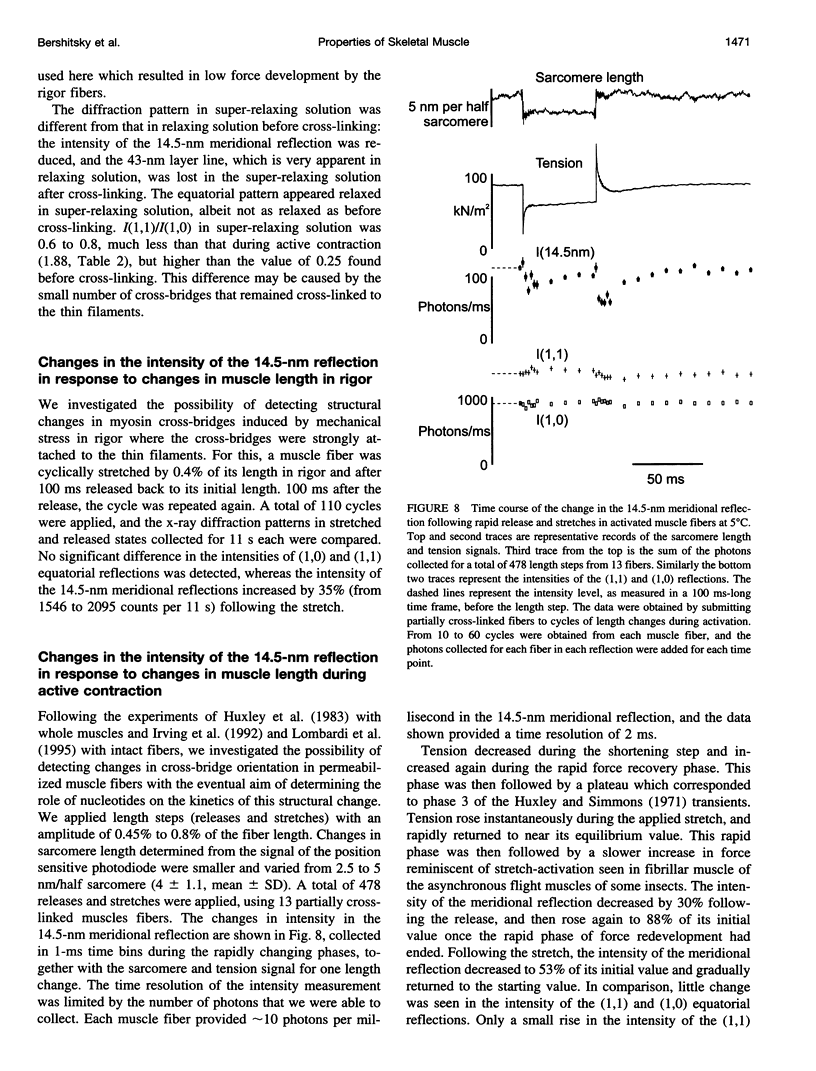
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bershitsky SYu, Tsaturyan A. K. Effect of joule temperature jump on tension and stiffness of skinned rabbit muscle fibers. Biophys J. 1989 Nov;56(5):809–816. doi: 10.1016/S0006-3495(89)82727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershitsky S. Y., Tsaturyan A. K. Force generation and work production by covalently cross-linked actin-myosin cross-bridges in rabbit muscle fibers. Biophys J. 1995 Sep;69(3):1011–1021. doi: 10.1016/S0006-3495(95)79976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershitsky S. Y., Tsaturyan A. K. Tension responses to joule temperature jump in skinned rabbit muscle fibres. J Physiol. 1992 Feb;447:425–448. doi: 10.1113/jphysiol.1992.sp019010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordas J., Diakun G. P., Diaz F. G., Harries J. E., Lewis R. A., Lowy J., Mant G. R., Martin-Fernandez M. L., Towns-Andrews E. Two-dimensional time-resolved X-ray diffraction studies of live isometrically contracting frog sartorius muscle. J Muscle Res Cell Motil. 1993 Jun;14(3):311–324. doi: 10.1007/BF00123096. [DOI] [PubMed] [Google Scholar]
- Bordas J., Diakun G. P., Harries J. E., Lewis R. A., Mant G. R., Martin-Fernandez M. L., Towns-Andrews E. Two-dimensional time resolved X-ray diffraction of muscle: recent results. Adv Biophys. 1991;27:15–33. doi: 10.1016/0065-227x(91)90005-x. [DOI] [PubMed] [Google Scholar]
- Brenner B., Yu L. C. Equatorial x-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys J. 1985 Nov;48(5):829–834. doi: 10.1016/S0006-3495(85)83841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C. Structural changes in the actomyosin cross-bridges associated with force generation. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5252–5256. doi: 10.1073/pnas.90.11.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984 Jul;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. The stiffness of frog skinned muscle fibres at altered lateral filament spacing. J Physiol. 1986 Sep;378:175–194. doi: 10.1113/jphysiol.1986.sp016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarek Z., Gergely J. Zero-length crosslinking procedure with the use of active esters. Anal Biochem. 1990 Feb 15;185(1):131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. A personal view of muscle and motility mechanisms. Annu Rev Physiol. 1996;58:1–19. doi: 10.1146/annurev.ph.58.030196.000245. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. J Mol Biol. 1983 Sep 15;169(2):469–506. doi: 10.1016/s0022-2836(83)80062-x. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Stewart A., Sosa H., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994 Dec;67(6):2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Irving M., Lombardi V., Piazzesi G., Ferenczi M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature. 1992 May 14;357(6374):156–158. doi: 10.1038/357156a0. [DOI] [PubMed] [Google Scholar]
- Irving M., St Claire Allen T., Sabido-David C., Craik J. S., Brandmeier B., Kendrick-Jones J., Corrie J. E., Trentham D. R., Goldman Y. E. Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature. 1995 Jun 22;375(6533):688–691. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- Iwamoto H., Podolsky R. J. Crossbridge rotation in EDC-crosslinked striated muscle fibers. Adv Exp Med Biol. 1993;332:393–407. doi: 10.1007/978-1-4615-2872-2_37. [DOI] [PubMed] [Google Scholar]
- Leszyk J., Grabarek Z., Gergely J., Collins J. H. Characterization of zero-length cross-links between rabbit skeletal muscle troponin C and troponin I: evidence for direct interaction between the inhibitory region of troponin I and the NH2-terminal, regulatory domain of troponin C. Biochemistry. 1990 Jan 9;29(1):299–304. doi: 10.1021/bi00453a041. [DOI] [PubMed] [Google Scholar]
- Lombardi V., Piazzesi G., Ferenczi M. A., Thirlwell H., Dobbie I., Irving M. Elastic distortion of myosin heads and repriming of the working stroke in muscle. Nature. 1995 Apr 6;374(6522):553–555. doi: 10.1038/374553a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R. Studies of the diffuse x-ray scattering from contracting frog skeletal muscles. Biophys J. 1990 May;57(5):977–985. doi: 10.1016/S0006-3495(90)82617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinchik S., Yu L. C. Analysis of equatorial x-ray diffraction patterns from muscle fibers: factors that affect the intensities. Biophys J. 1995 May;68(5):2023–2031. doi: 10.1016/S0006-3495(95)80379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Nishiye E., Somlyo A. V., Török K., Somlyo A. P. The effects of MgADP on cross-bridge kinetics: a laser flash photolysis study of guinea-pig smooth muscle. J Physiol. 1993 Jan;460:247–271. doi: 10.1113/jphysiol.1993.sp019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G., Lombardi V., Ferenczi M. A., Thirlwell H., Dobbie I., Irving M. Changes in the x-ray diffraction pattern from single, intact muscle fibers produced by rapid shortening and stretch. Biophys J. 1995 Apr;68(4 Suppl):92S–98S. [PMC free article] [PubMed] [Google Scholar]
- Poole K. J., Rapp G., Maéda Y., Goody R. S. The time course of changes in the equatorial diffraction patterns from different muscle types on photolysis of caged-ATP. Adv Exp Med Biol. 1988;226:391–404. [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Squire J., Harford J. Time-resolved studies of crossbridge movement: why use X-rays? Why use fish muscle? Adv Exp Med Biol. 1993;332:435–450. doi: 10.1007/978-1-4615-2872-2_40. [DOI] [PubMed] [Google Scholar]
- Tawada K., Kimura M. Stiffness of carbodiimide-crosslinked glycerinated muscle fibres in rigor and relaxing solutions at high salt concentrations. J Muscle Res Cell Motil. 1986 Aug;7(4):339–350. doi: 10.1007/BF01753655. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]