Abstract
Fluorescence microphotolysis was combined with confocal laser-scanning microscopy to yield a method, herein referred to as line-scanning microphotolysis (LINESCAMP), for the measurement of molecular transport at a lateral resolution of approximately 0.34 microns and a temporal resolution of approximately 0.5 ms. A confocal microscope was operated in the line scan mode, while the laser beam power could be switched during scanning between low monitoring and high photolysing levels in less then a microsecond. The number and location of line segments to be photolysed could be freely determined. The length of the photolysed segments could be also chosen and was only limited by diffraction. Together with instrumentation a new, completely general, theoretical framework for the evaluation of diffusion measurements was developed. Based on the numerical simulation of diffusion processes employing a modified Crank-Nicholson scheme, the theory could be applied to any photobleaching geometry and profile as the initial condition and took into account the convolution with the microscope point spread function. With small diffraction-limited areas, the method yielded accurate values for diffusion coefficients in the range between approximately 10(-4) and 1 micron2 s-1. A first application of the method to the diffusion of a fluorescently labeled tracer inside the cell nucleus showed the potential of the method for the study of complex biological systems.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Georgiou G. N., Morrison I. E., Stevenson G. V., Cherry R. J. Tracking of cell surface receptors by fluorescence digital imaging microscopy using a charge-coupled device camera. Low-density lipoprotein and influenza virus receptor mobility at 4 degrees C. J Cell Sci. 1992 Feb;101(Pt 2):415–425. doi: 10.1242/jcs.101.2.415. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Webb W. W. Fluorescent low density lipoprotein for observation of dynamics of individual receptor complexes on cultured human fibroblasts. J Cell Biol. 1981 Sep;90(3):595–604. doi: 10.1083/jcb.90.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland K. M., So P. T., Gratton E. Two-photon fluorescence correlation spectroscopy: method and application to the intracellular environment. Biophys J. 1995 Feb;68(2):694–701. doi: 10.1016/S0006-3495(95)80230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
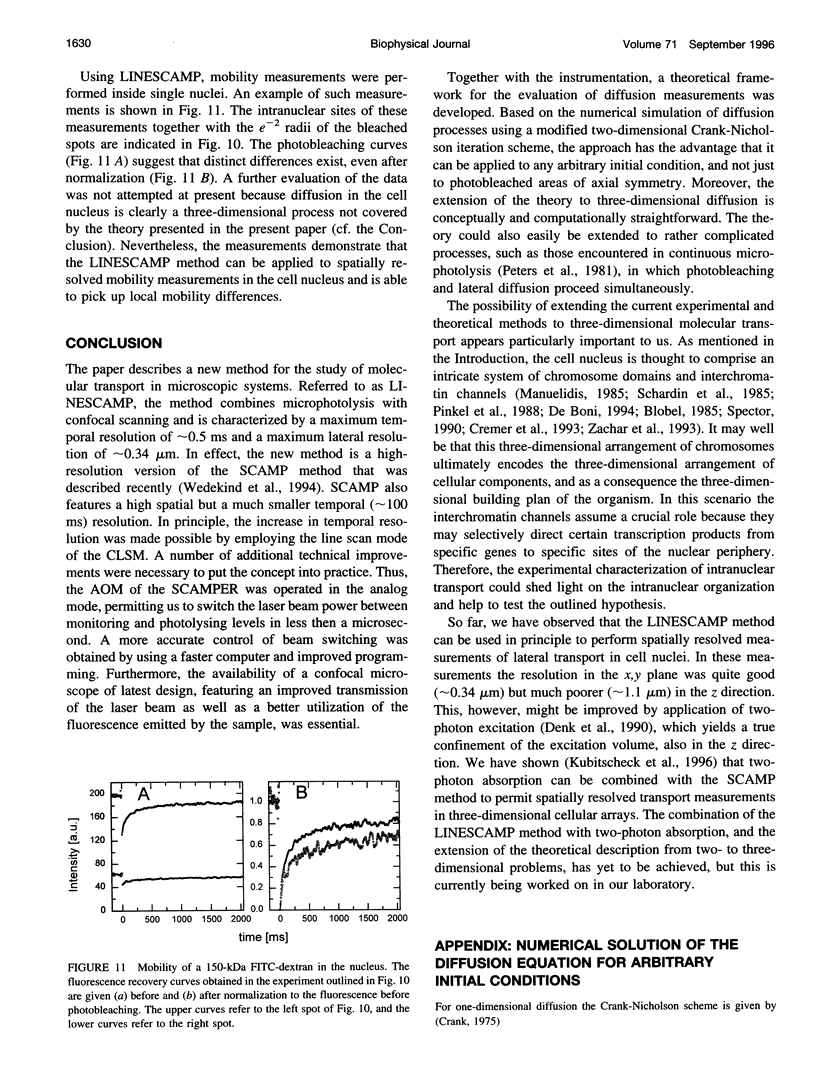
- Betzig E., Trautman J. K., Harris T. D., Weiner J. S., Kostelak R. L. Breaking the diffraction barrier: optical microscopy on a nanometric scale. Science. 1991 Mar 22;251(5000):1468–1470. doi: 10.1126/science.251.5000.1468. [DOI] [PubMed] [Google Scholar]
- Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T., Kurz A., Zirbel R., Dietzel S., Rinke B., Schröck E., Speicher M. R., Mathieu U., Jauch A., Emmerich P. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol. 1993;58:777–792. doi: 10.1101/sqb.1993.058.01.085. [DOI] [PubMed] [Google Scholar]
- De Boni U. The interphase nucleus as a dynamic structure. Int Rev Cytol. 1994;150:149–171. doi: 10.1016/s0074-7696(08)61541-7. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Moeremans M., De Mey J. Probing microtubule-dependent intracellular motility with nanometre particle video ultramicroscopy (nanovid ultramicroscopy). Cytobios. 1985;43(174S):273–283. [PubMed] [Google Scholar]
- Edidin M. Patches, posts and fences: proteins and plasma membrane domains. Trends Cell Biol. 1992 Dec;2(12):376–380. doi: 10.1016/0962-8924(92)90050-w. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zúiga M. C., Sheetz M. P. Truncation mutants define and locate cytoplasmic barriers to lateral mobility of membrane glycoproteins. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3378–3382. doi: 10.1073/pnas.91.8.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficner R., Huber R. Refined crystal structure of phycoerythrin from Porphyridium cruentum at 0.23-nm resolution and localization of the gamma subunit. Eur J Biochem. 1993 Nov 15;218(1):103–106. doi: 10.1111/j.1432-1033.1993.tb18356.x. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Jacobson K. A closer look at how membrane proteins move. Biophys J. 1993 Nov;65(5):1754–1755. doi: 10.1016/S0006-3495(93)81231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Kerr R. A. Who will win the el nino sweepstakes this time? Science. 1990 Apr 27;248(4954):445–445. doi: 10.1126/science.248.4954.445. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitscheck U., Wedekind P., Peters R. Lateral diffusion measurement at high spatial resolution by scanning microphotolysis in a confocal microscope. Biophys J. 1994 Sep;67(3):948–956. doi: 10.1016/S0006-3495(94)80596-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A., Sako Y., Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993 Nov;65(5):2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I., Scholz M., Peters R. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J Cell Biol. 1986 Apr;102(4):1183–1190. doi: 10.1083/jcb.102.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K. Physical properties of cytoplasm. Curr Opin Cell Biol. 1994 Feb;6(1):3–9. doi: 10.1016/0955-0674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Individual interphase chromosome domains revealed by in situ hybridization. Hum Genet. 1985;71(4):288–293. doi: 10.1007/BF00388453. [DOI] [PubMed] [Google Scholar]
- Peters R., Brünger A., Schulten K. Continuous fluorescence microphotolysis: A sensitive method for study of diffusion processes in single cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):962–966. doi: 10.1073/pnas.78.2.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardin M., Cremer T., Hager H. D., Lang M. Specific staining of human chromosomes in Chinese hamster x man hybrid cell lines demonstrates interphase chromosome territories. Hum Genet. 1985;71(4):281–287. doi: 10.1007/BF00388452. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Tschödrich-Rotter M., Peters R., Krohne G. Properties of fluorescently labeled Xenopus lamin A in vivo. Eur J Cell Biol. 1994 Oct;65(1):70–81. [PubMed] [Google Scholar]
- Soumpasis D. M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 Jan;41(1):95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Higher order nuclear organization: three-dimensional distribution of small nuclear ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1990 Jan;87(1):147–151. doi: 10.1073/pnas.87.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschödrich-Rotter M., Kubitscheck U., Ugochukwu G., Buckley J. T., Peters R. Optical single-channel analysis of the aerolysin pore in erythrocyte membranes. Biophys J. 1996 Feb;70(2):723–732. doi: 10.1016/S0006-3495(96)79612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind P., Kubitscheck U., Peters R. Scanning microphotolysis: a new photobleaching technique based on fast intensity modulation of a scanned laser beam and confocal imaging. J Microsc. 1994 Oct;176(Pt 1):23–33. doi: 10.1111/j.1365-2818.1994.tb03496.x. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Kramer J., Mims I. P., Bingham P. M. Evidence for channeled diffusion of pre-mRNAs during nuclear RNA transport in metazoans. J Cell Biol. 1993 May;121(4):729–742. doi: 10.1083/jcb.121.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]