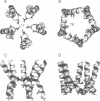
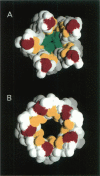
Abstract
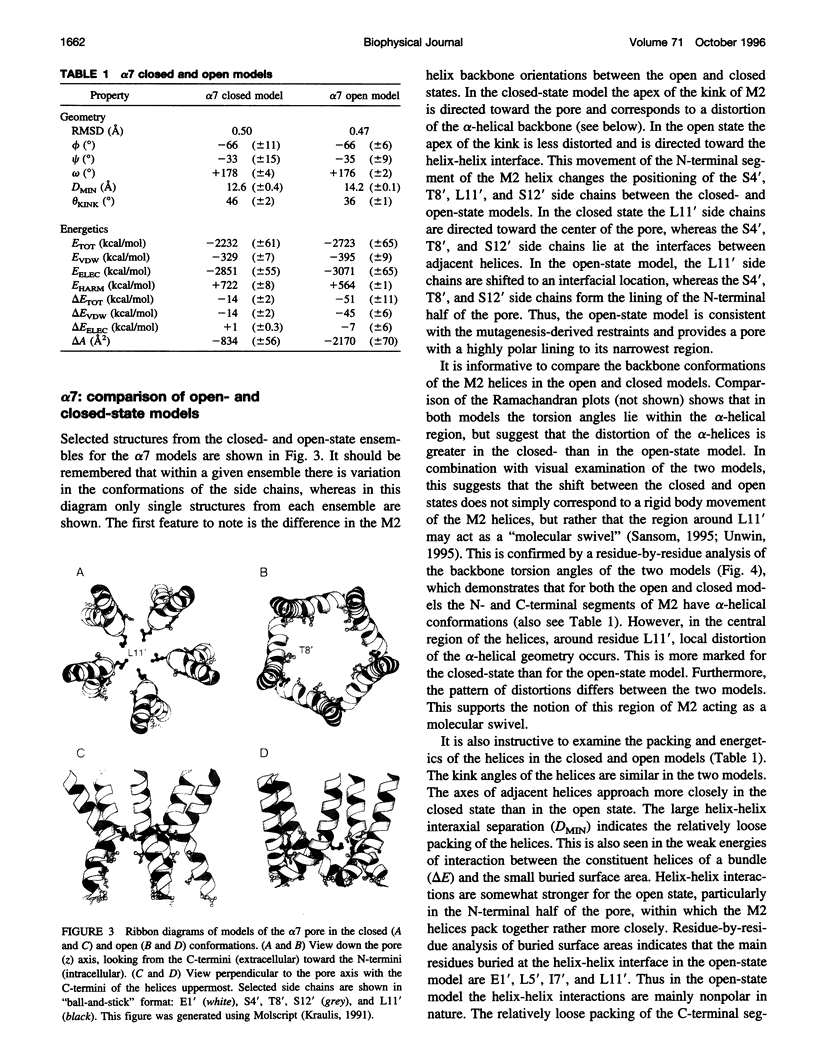
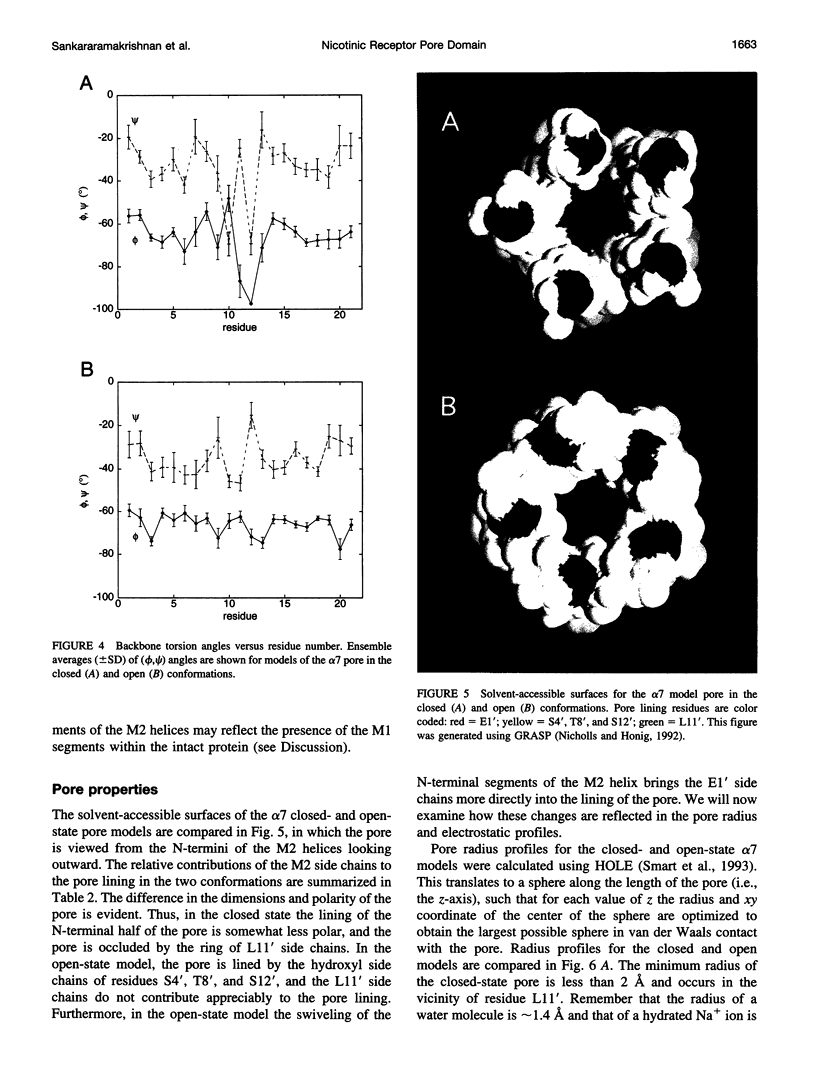
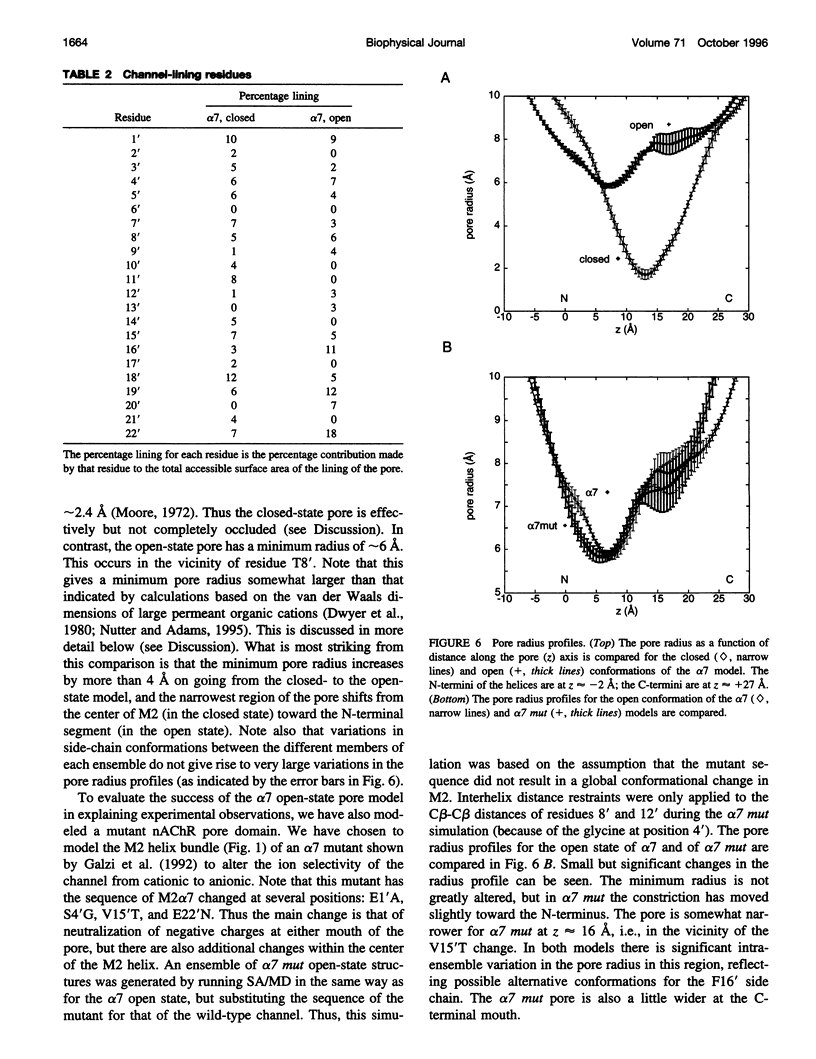
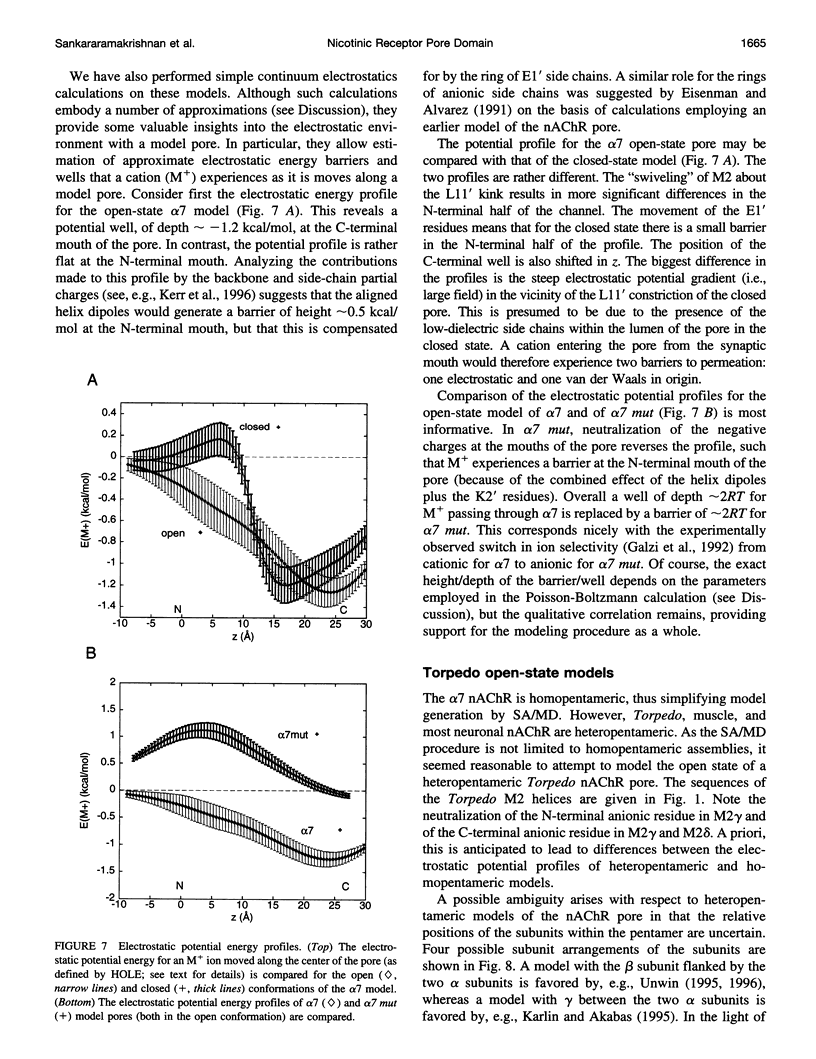
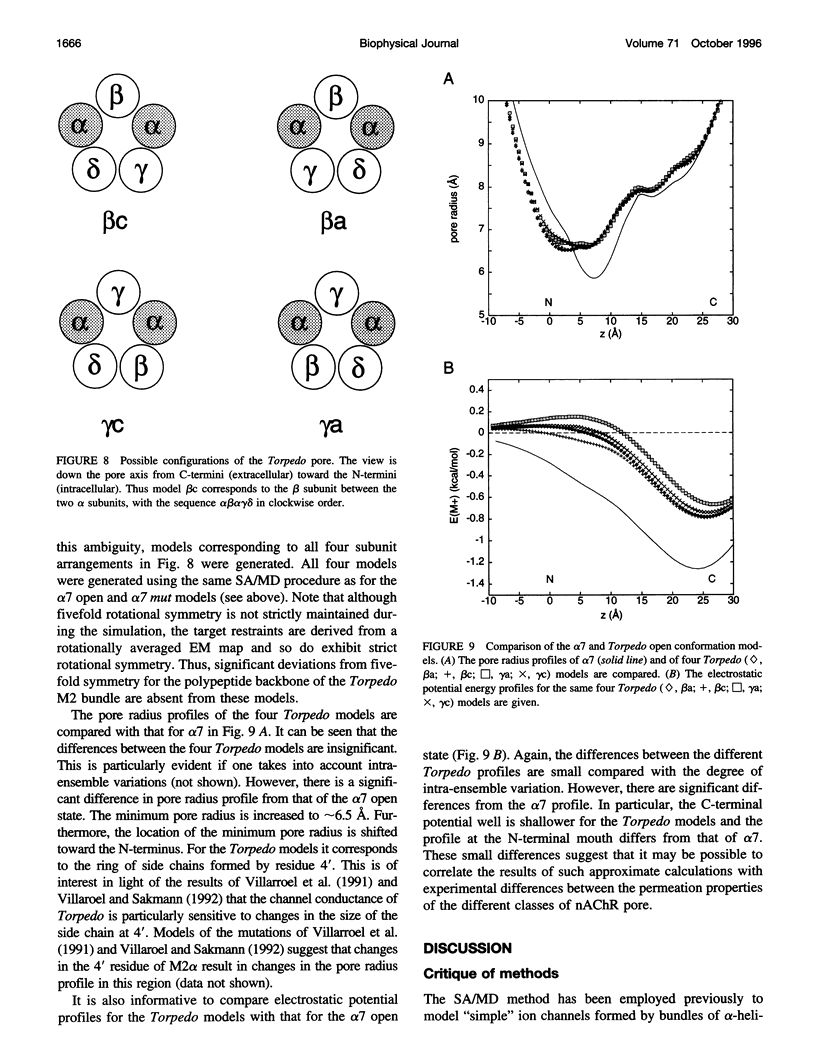
The pore domain of the nicotinic acetylcholine receptor has been modeled as a bundle of five kinked M2 helices. Models were generated via molecular dynamics simulations incorporating restraints derived from 9-A resolution cryoelectron microscopy data (Unwin, 1993; 1995), and from mutagenesis data that identify channel-lining side chains. Thus, these models conform to current experimental data but will require revision as higher resolution data become available. Models of the open and closed states of a homopentameric alpha 7 pore are compared. The minimum radius of the closed-state model is less than 2 A; the minimum radius of the open-state models is approximately 6 A. It is suggested that the presence of "bound" water molecules within the pore may reduce the effective minimum radii below these values by up to approximately 3 A. Poisson-Boltzmann calculations are used to obtain a first approximation to the potential energy of a monovalent cation as it moves along the pore axis. The differences in electrostatic potential energy profiles between the open-state models of alpha 7 and of a mutant of alpha 7 are consistent with the experimentally observed change in ion selectivity from cationic to anionic. Models of the open state of the heteropentameric Torpedo nicotinic acetylcholine receptor pore domain are also described. Relatively small differences in pore radius and electrostatic potential energy profiles are seen when the Torpedo and alpha 7 models are compared.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Kaufmann C., Archdeacon P., Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron. 1994 Oct;13(4):919–927. doi: 10.1016/0896-6273(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Akabas M. H., Stauffer D. A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992 Oct 9;258(5080):307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Bechinger B., Kim Y., Chirlian L. E., Gesell J., Neumann J. M., Montal M., Tomich J., Zasloff M., Opella S. J. Orientations of amphipathic helical peptides in membrane bilayers determined by solid-state NMR spectroscopy. J Biomol NMR. 1991 Jul;1(2):167–173. doi: 10.1007/BF01877228. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Galzi J. L., Devillers-Thiéry A., Bertrand S., Changeux J. P. Stratification of the channel domain in neurotransmitter receptors. Curr Opin Cell Biol. 1993 Aug;5(4):688–693. doi: 10.1016/0955-0674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- Breed J., Kerr I. D., Sankararamakrishnan R., Sansom M. S. Packing interactions of Aib-containing helices: molecular modeling of parallel dimers of simple hydrophobic helices and of alamethicin. Biopolymers. 1995 Jun;35(6):639–655. doi: 10.1002/bip.360350610. [DOI] [PubMed] [Google Scholar]
- Breed J., Sankararamakrishnan R., Kerr I. D., Sansom M. S. Molecular dynamics simulations of water within models of ion channels. Biophys J. 1996 Apr;70(4):1643–1661. doi: 10.1016/S0006-3495(96)79727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Galzi J. L., Devillers-Thiéry A., Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labelling and site-directed mutagenesis. Q Rev Biophys. 1992 Nov;25(4):395–432. doi: 10.1017/s0033583500004352. [DOI] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Chiu S. W., Jakobsson E., Subramaniam S., McCammon J. A. Time-correlation analysis of simulated water motion in flexible and rigid gramicidin channels. Biophys J. 1991 Jul;60(1):273–285. doi: 10.1016/S0006-3495(91)82049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. N., Labarca C., Czyzyk L., Davidson N., Lester H. A. Tris+/Na+ permeability ratios of nicotinic acetylcholine receptors are reduced by mutations near the intracellular end of the M2 region. J Gen Physiol. 1992 Apr;99(4):545–572. doi: 10.1085/jgp.99.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier S., Bertrand D., Matter J. M., Hernandez M. C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990 Dec;5(6):847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Dorman V., Partenskii M. B., Jordan P. C. A semi-microscopic Monte Carlo study of permeation energetics in a gramicidin-like channel: the origin of cation selectivity. Biophys J. 1996 Jan;70(1):121–134. doi: 10.1016/S0006-3495(96)79554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Alvarez O. Structure and function of channels and channelogs as studied by computational chemistry. J Membr Biol. 1991 Jan;119(2):109–132. doi: 10.1007/BF01871411. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Dani J. A. An introduction to molecular architecture and permeability of ion channels. Annu Rev Biophys Biophys Chem. 1987;16:205–226. doi: 10.1146/annurev.bb.16.060187.001225. [DOI] [PubMed] [Google Scholar]
- Filatov G. N., White M. M. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol. 1995 Sep;48(3):379–384. [PubMed] [Google Scholar]
- Furois-Corbin S., Pullman A. A possible model for the inner wall of the acetylcholine receptor channel. Biochim Biophys Acta. 1989 Sep 18;984(3):339–350. doi: 10.1016/0005-2736(89)90301-5. [DOI] [PubMed] [Google Scholar]
- Furois-Corbin S., Pullman A. Energy profiles in the acetylcholine receptor (AChR) channel. The MII-helix model and the role of the remaining helices. FEBS Lett. 1989 Jul 31;252(1-2):63–68. doi: 10.1016/0014-5793(89)80890-7. [DOI] [PubMed] [Google Scholar]
- Furois-Corbin S., Pullman A. The effect of point mutations on energy profiles in a model of the nicotinic acetylcholine receptor (AChR) channel. Biophys Chem. 1991 Feb;39(2):153–159. doi: 10.1016/0301-4622(91)85017-k. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Devillers-Thiéry A., Hussy N., Bertrand S., Changeux J. P., Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992 Oct 8;359(6395):500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Green M. E., Lewis J. Monte Carlo simulation of the water in a channel with charges. Biophys J. 1991 Feb;59(2):419–426. doi: 10.1016/S0006-3495(91)82235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman M., Tsfadia Y., Masad A., Nachliel E. Quantitation of physical-chemical properties of the aqueous phase inside the phoE ionic channel. Biochim Biophys Acta. 1992 Aug 24;1109(2):141–148. doi: 10.1016/0005-2736(92)90077-y. [DOI] [PubMed] [Google Scholar]
- Harvey S. C. Treatment of electrostatic effects in macromolecular modeling. Proteins. 1989;5(1):78–92. doi: 10.1002/prot.340050109. [DOI] [PubMed] [Google Scholar]
- Hucho F., Görne-Tschelnokow U., Strecker A. Beta-structure in the membrane-spanning part of the nicotinic acetylcholine receptor (or how helical are transmembrane helices?). Trends Biochem Sci. 1994 Sep;19(9):383–387. doi: 10.1016/0968-0004(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Hucho F., Hilgenfeld R. The selectivity filter of a ligand-gated ion channel. The helix-M2 model of the ion channel of the nicotinic acetylcholine receptor. FEBS Lett. 1989 Oct 23;257(1):17–23. doi: 10.1016/0014-5793(89)81775-2. [DOI] [PubMed] [Google Scholar]
- Hucho F., Oberthür W., Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986 Sep 1;205(1):137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Karlin A., Akabas M. H. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995 Dec;15(6):1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Karshikoff A., Spassov V., Cowan S. W., Ladenstein R., Schirmer T. Electrostatic properties of two porin channels from Escherichia coli. J Mol Biol. 1994 Jul 22;240(4):372–384. doi: 10.1006/jmbi.1994.1451. [DOI] [PubMed] [Google Scholar]
- Kerr I. D., Doak D. G., Sankararamakrishnan R., Breed J., Sansom M. S. Molecular modelling of Staphylococcal delta-toxin ion channels by restrained molecular dynamics. Protein Eng. 1996 Feb;9(2):161–171. doi: 10.1093/protein/9.2.161. [DOI] [PubMed] [Google Scholar]
- Kerr I. D., Sankararamakrishnan R., Smart O. S., Sansom M. S. Parallel helix bundles and ion channels: molecular modeling via simulated annealing and restrained molecular dynamics. Biophys J. 1994 Oct;67(4):1501–1515. doi: 10.1016/S0006-3495(94)80624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. D., Sansom M. S. Hydrophilic surface maps of channel-forming peptides: analysis of amphipathic helices. Eur Biophys J. 1993;22(4):269–277. doi: 10.1007/BF00180261. [DOI] [PubMed] [Google Scholar]
- Kienker P., Tomaselli G., Jurman M., Yellen G. Conductance mutations of the nicotinic acetylcholine receptor do not act by a simple electrostatic mechanism. Biophys J. 1994 Feb;66(2 Pt 1):325–334. doi: 10.1016/s0006-3495(94)80781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusch A., Schulz G. E. Refined structure of the porin from Rhodopseudomonas blastica. Comparison with the porin from Rhodobacter capsulatus. J Mol Biol. 1994 Nov 11;243(5):891–905. doi: 10.1006/jmbi.1994.1690. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Lester H. A. The permeation pathway of neurotransmitter-gated ion channels. Annu Rev Biophys Biomol Struct. 1992;21:267–292. doi: 10.1146/annurev.bb.21.060192.001411. [DOI] [PubMed] [Google Scholar]
- Montal M. Design of molecular function: channels of communication. Annu Rev Biophys Biomol Struct. 1995;24:31–57. doi: 10.1146/annurev.bb.24.060195.000335. [DOI] [PubMed] [Google Scholar]
- Nilges M., Brünger A. T. Automated modeling of coiled coils: application to the GCN4 dimerization region. Protein Eng. 1991 Aug;4(6):649–659. doi: 10.1093/protein/4.6.649. [DOI] [PubMed] [Google Scholar]
- Nilges M., Brünger A. T. Successful prediction of the coiled coil geometry of the GCN4 leucine zipper domain by simulated annealing: comparison to the X-ray structure. Proteins. 1993 Feb;15(2):133–146. doi: 10.1002/prot.340150205. [DOI] [PubMed] [Google Scholar]
- Nutter T. J., Adams D. J. Monovalent and divalent cation permeability and block of neuronal nicotinic receptor channels in rat parasympathetic ganglia. J Gen Physiol. 1995 Jun;105(6):701–723. doi: 10.1085/jgp.105.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiki S., Danho W., Madison V., Montal M. M2 delta, a candidate for the structure lining the ionic channel of the nicotinic cholinergic receptor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8703–8707. doi: 10.1073/pnas.85.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiki S., Madison V., Montal M. Bundles of amphipathic transmembrane alpha-helices as a structural motif for ion-conducting channel proteins: studies on sodium channels and acetylcholine receptors. Proteins. 1990;8(3):226–236. doi: 10.1002/prot.340080305. [DOI] [PubMed] [Google Scholar]
- Ortells M. O., Lunt G. G. A mixed helix-beta-sheet model of the transmembrane region of the nicotinic acetylcholine receptor. Protein Eng. 1996 Jan;9(1):51–59. doi: 10.1093/protein/9.1.51. [DOI] [PubMed] [Google Scholar]
- Ortells M. O., Lunt G. G. The transmembrane region of the nicotinic acetylcholine receptor: is it an all-helix bundle? Receptors Channels. 1994;2(1):53–59. [PubMed] [Google Scholar]
- Peitzsch R. M., Eisenberg M., Sharp K. A., McLaughlin S. Calculations of the electrostatic potential adjacent to model phospholipid bilayers. Biophys J. 1995 Mar;68(3):729–738. doi: 10.1016/S0006-3495(95)80253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll D. R., Faerman C. H., Axelsen P. H., Silman I., Sussman J. L. An electrostatic mechanism for substrate guidance down the aromatic gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5128–5132. doi: 10.1073/pnas.90.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Karplus M. Molecular dynamics simulations of the gramicidin channel. Annu Rev Biophys Biomol Struct. 1994;23:731–761. doi: 10.1146/annurev.bb.23.060194.003503. [DOI] [PubMed] [Google Scholar]
- Sankararamakrishnan R., Sansom M. S. Modelling packing interactions in parallel helix bundles: pentameric bundles of nicotinic receptor M2 helices. Biochim Biophys Acta. 1995 Nov 1;1239(2):122–132. doi: 10.1016/0005-2736(95)00165-y. [DOI] [PubMed] [Google Scholar]
- Sankararamakrishnan R., Sansom M. S. Structural features of isolated M2 helices of nicotinic receptors. Simulated annealing via molecular dynamics studies. Biophys Chem. 1995 Aug;55(3):215–230. doi: 10.1016/0301-4622(95)00006-j. [DOI] [PubMed] [Google Scholar]
- Sansom M. S. Ion-channel gating. Twist to open. Curr Biol. 1995 Apr 1;5(4):373–375. doi: 10.1016/s0960-9822(95)00076-5. [DOI] [PubMed] [Google Scholar]
- Sansom M. S., Kerr I. D., Breed J., Sankararamakrishnan R. Water in channel-like cavities: structure and dynamics. Biophys J. 1996 Feb;70(2):693–702. doi: 10.1016/S0006-3495(96)79609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom M. S., Kerr I. D. Transbilayer pores formed by beta-barrels: molecular modeling of pore structures and properties. Biophys J. 1995 Oct;69(4):1334–1343. doi: 10.1016/S0006-3495(95)80000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom M. S., Sankararamakrishnan R., Kerr I. D. Modelling membrane proteins using structural restraints. Nat Struct Biol. 1995 Aug;2(8):624–631. doi: 10.1038/nsb0895-624. [DOI] [PubMed] [Google Scholar]
- Sansom M. S., Son H. S., Sankararamakrishnan R., Kerr I. D., Breed J. Seven-helix bundles: molecular modeling via restrained molecular dynamics. Biophys J. 1995 Apr;68(4):1295–1310. doi: 10.1016/S0006-3495(95)80303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp K. A., Honig B. Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Colquhoun D. Acetylcholine receptors: too many channels, too few functions. Science. 1995 Sep 22;269(5231):1681–1682. doi: 10.1126/science.7569892. [DOI] [PubMed] [Google Scholar]
- Smart O. S., Goodfellow J. M., Wallace B. A. The pore dimensions of gramicidin A. Biophys J. 1993 Dec;65(6):2455–2460. doi: 10.1016/S0006-3495(93)81293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud R. M., McCarthy M. P., Shuster M. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry. 1990 Dec 18;29(50):11009–11023. doi: 10.1021/bi00502a001. [DOI] [PubMed] [Google Scholar]
- Treutlein H. R., Lemmon M. A., Engelman D. M., Brünger A. T. The glycophorin A transmembrane domain dimer: sequence-specific propensity for a right-handed supercoil of helices. Biochemistry. 1992 Dec 29;31(51):12726–12732. doi: 10.1021/bi00166a003. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995 Jan 5;373(6509):37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Unwin N. Projection structure of the nicotinic acetylcholine receptor: distinct conformations of the alpha subunits. J Mol Biol. 1996 Apr 5;257(3):586–596. doi: 10.1006/jmbi.1996.0187. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Herlitze S., Koenen M., Sakmann B. Location of a threonine residue in the alpha-subunit M2 transmembrane segment that determines the ion flow through the acetylcholine receptor channel. Proc Biol Sci. 1991 Jan 22;243(1306):69–74. doi: 10.1098/rspb.1991.0012. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Herlitze S., Witzemann V., Koenen M., Sakmann B. Asymmetry of the rat acetylcholine receptor subunits in the narrow region of the pore. Proc Biol Sci. 1992 Sep 22;249(1326):317–324. doi: 10.1098/rspb.1992.0121. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Sakmann B. Threonine in the selectivity filter of the acetylcholine receptor channel. Biophys J. 1992 Apr;62(1):196–208. doi: 10.1016/S0006-3495(92)81805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]