Abstract
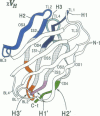
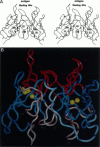
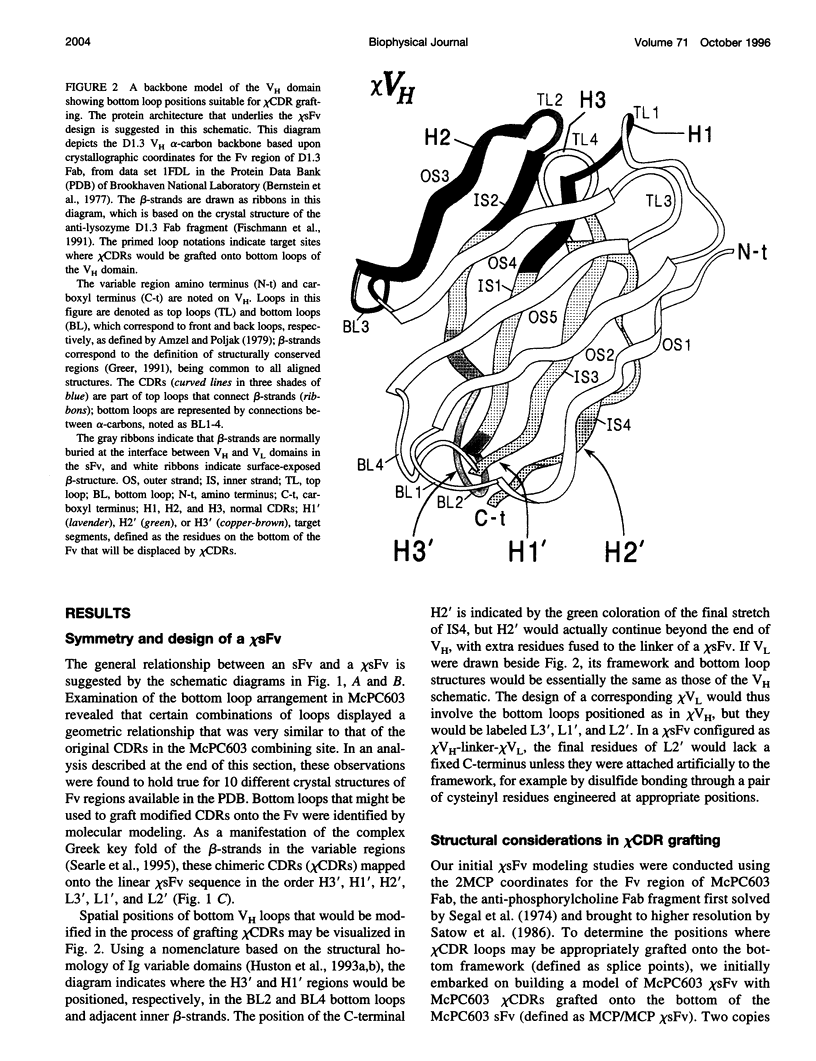
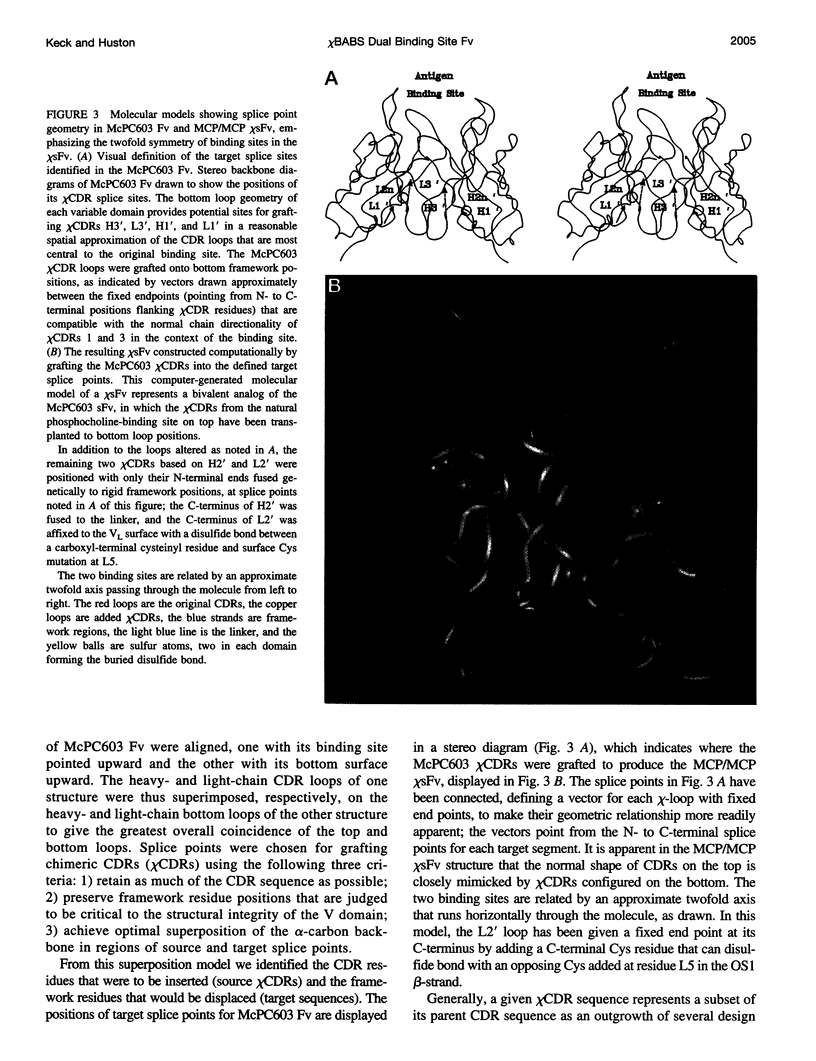
Molecular modeling studies on antibody Fv regions have been pursued to design a second antigen-binding site (chi-site) in a chimeric single-chain Fv (chi sFv) species of about 30 kDa. This analysis has uncovered an architectural basis common to many Fv regions that permits grafting a chi-site onto the Fv surface that diametrically opposes the normal combining site. By using molecular graphics analysis, chimeric complementarity-determining regions (chi CDRs) were defined that comprised most of the CDRs from an antibody binding site of interest. The chain directionality of chi CDRs was consistent with that of specific bottom loops of the sFv, which allowed for grafting of chi CDRs with an overall geometry approximating CDRs in the parent combining site. Analysis of 10 different Fv crystal structures indicates that the positions for inserting chi CDRs are very highly conserved, as are the corresponding chi CDR boundaries in the parent binding site. The results of this investigation suggest that it should be possible to generally apply this approach to the development of chimeric bispecific antibody binding site (chi BABS) proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair J. R. Engineering antibodies for therapy. Immunol Rev. 1992 Dec;130:5–40. doi: 10.1111/j.1600-065x.1992.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Adams G. P., McCartney J. E., Tai M. S., Oppermann H., Huston J. S., Stafford W. F., 3rd, Bookman M. A., Fand I., Houston L. L., Weiner L. M. Highly specific in vivo tumor targeting by monovalent and divalent forms of 741F8 anti-c-erbB-2 single-chain Fv. Cancer Res. 1993 Sep 1;53(17):4026–4034. [PubMed] [Google Scholar]
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Billetta R., Zanetti M. Antigenicity and immunogenicity of antigenized antibodies. Studies on B and T cells. Int Rev Immunol. 1993;10(2-3):251–263. doi: 10.3109/08830189309061700. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. Protein configurations. Science. 1994 Sep 9;265(5178):1511–1511. [PubMed] [Google Scholar]
- Brunet A. P., Huang E. S., Huffine M. E., Loeb J. E., Weltman R. J., Hecht M. H. The role of turns in the structure of an alpha-helical protein. Nature. 1993 Jul 22;364(6435):355–358. doi: 10.1038/364355a0. [DOI] [PubMed] [Google Scholar]
- Clarke B. L., Blalock J. E. Steroidogenic activity of a peptide specified by the reversed sequence of corticotropin mRNA. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9708–9711. doi: 10.1073/pnas.87.24.9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H., Yarranton G. T., Rees A. R. Expression of mouse immunoglobulin light and heavy chain variable regions in Escherichia coli and reconstitution of antigen-binding activity. Protein Eng. 1990 Jul;3(7):641–647. doi: 10.1093/protein/3.7.641. [DOI] [PubMed] [Google Scholar]
- Fischmann T. O., Bentley G. A., Bhat T. N., Boulot G., Mariuzza R. A., Phillips S. E., Tello D., Poljak R. J. Crystallographic refinement of the three-dimensional structure of the FabD1.3-lysozyme complex at 2.5-A resolution. J Biol Chem. 1991 Jul 15;266(20):12915–12920. [PubMed] [Google Scholar]
- Gelfand I. M., Kister A. E., Leshchiner D. The invariant system of coordinates of antibody molecules: prediction of the "standard" C alpha framework of VL and VH domains. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3675–3678. doi: 10.1073/pnas.93.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. J., Jamar F., Tai M. S., Heelan B. T., Adams G. P., McCartney J. E., Houston L. L., Weiner L. M., Oppermann H., Peters A. M. Radiometal labeling of recombinant proteins by a genetically engineered minimal chelation site: technetium-99m coordination by single-chain Fv antibody fusion proteins through a C-terminal cysteinyl peptide. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8358–8362. doi: 10.1073/pnas.92.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. J., Titus J. A., Jost C. R., Kurucz I., Perez P., Andrew S. M., Nicholls P. J., Huston J. S., Segal D. M. Redirection of T cell-mediated cytotoxicity by a recombinant single-chain Fv molecule. J Immunol. 1994 Feb 15;152(4):1802–1811. [PubMed] [Google Scholar]
- Greer J. Comparative modeling of homologous proteins. Methods Enzymol. 1991;202:239–252. doi: 10.1016/0076-6879(91)02014-z. [DOI] [PubMed] [Google Scholar]
- Hecht M. H. De novo design of beta-sheet proteins. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8729–8730. doi: 10.1073/pnas.91.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. S., Mudgett-Hunter M., Tai M. S., McCartney J., Warren F., Haber E., Oppermann H. Protein engineering of single-chain Fv analogs and fusion proteins. Methods Enzymol. 1991;203:46–88. doi: 10.1016/0076-6879(91)03005-2. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Tai M. S., McCartney J., Keck P., Oppermann H. Antigen recognition and targeted delivery by the single-chain Fv. Cell Biophys. 1993 Jan-Jun;22(1-3):189–224. doi: 10.1007/BF03033874. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. D., Strong R. K., Sieker L. C., Chang C. Y., Campbell R. L., Petsko G. A., Haber E., Margolies M. N., Sheriff S. 26-10 Fab-digoxin complex: affinity and specificity due to surface complementarity. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10310–10314. doi: 10.1073/pnas.90.21.10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kamtekar S., Hecht M. H. Protein Motifs. 7. The four-helix bundle: what determines a fold? FASEB J. 1995 Aug;9(11):1013–1022. doi: 10.1096/fasebj.9.11.7649401. [DOI] [PubMed] [Google Scholar]
- Kamtekar S., Schiffer J. M., Xiong H., Babik J. M., Hecht M. H. Protein design by binary patterning of polar and nonpolar amino acids. Science. 1993 Dec 10;262(5140):1680–1685. doi: 10.1126/science.8259512. [DOI] [PubMed] [Google Scholar]
- Knappik A., Plückthun A. Engineered turns of a recombinant antibody improve its in vivo folding. Protein Eng. 1995 Jan;8(1):81–89. doi: 10.1093/protein/8.1.81. [DOI] [PubMed] [Google Scholar]
- McCartney J. E., Tai M. S., Hudziak R. M., Adams G. P., Weiner L. M., Jin D., Stafford W. F., 3rd, Liu S., Bookman M. A., Laminet A. A. Engineering disulfide-linked single-chain Fv dimers [(sFv')2] with improved solution and targeting properties: anti-digoxin 26-10 (sFv')2 and anti-c-erbB-2 741F8 (sFv')2 made by protein folding and bonded through C-terminal cysteinyl peptides. Protein Eng. 1995 Mar;8(3):301–314. doi: 10.1093/protein/8.3.301. [DOI] [PubMed] [Google Scholar]
- Neri D., Momo M., Prospero T., Winter G. High-affinity antigen binding by chelating recombinant antibodies (CRAbs). J Mol Biol. 1995 Feb 24;246(3):367–373. doi: 10.1006/jmbi.1994.0091. [DOI] [PubMed] [Google Scholar]
- Novotny J., Bruccoleri R. E., Saul F. A. On the attribution of binding energy in antigen-antibody complexes McPC 603, D1.3, and HyHEL-5. Biochemistry. 1989 May 30;28(11):4735–4749. doi: 10.1021/bi00437a034. [DOI] [PubMed] [Google Scholar]
- Novotný J., Bruccoleri R., Newell J., Murphy D., Haber E., Karplus M. Molecular anatomy of the antibody binding site. J Biol Chem. 1983 Dec 10;258(23):14433–14437. [PubMed] [Google Scholar]
- Novotný J., Haber E. Structural invariants of antigen binding: comparison of immunoglobulin VL-VH and VL-VL domain dimers. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4592–4596. doi: 10.1073/pnas.82.14.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predki P. F., Agrawal V., Brünger A. T., Regan L. Amino-acid substitutions in a surface turn modulate protein stability. Nat Struct Biol. 1996 Jan;3(1):54–58. doi: 10.1038/nsb0196-54. [DOI] [PubMed] [Google Scholar]
- Quinn T. P., Tweedy N. B., Williams R. W., Richardson J. S., Richardson D. C. Betadoublet: de novo design, synthesis, and characterization of a beta-sandwich protein. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8747–8751. doi: 10.1073/pnas.91.19.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann L., Foote J., Winter G. Expression of an antibody Fv fragment in myeloma cells. J Mol Biol. 1988 Oct 5;203(3):825–828. doi: 10.1016/0022-2836(88)90214-8. [DOI] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T., Rajewsky K. A functional antibody mutant with an insertion in the framework region 3 loop of the VH domain: implications for antibody engineering. Protein Eng. 1992 Apr;5(3):229–234. doi: 10.1093/protein/5.3.229. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Tai M. S., McCartney J. E., Adams G. P., Jin D., Hudziak R. M., Oppermann H., Laminet A. A., Bookman M. A., Wolf E. J., Liu S. Targeting c-erbB-2 expressing tumors using single-chain Fv monomers and dimers. Cancer Res. 1995 Dec 1;55(23 Suppl):5983s–5989s. [PubMed] [Google Scholar]
- Tai M. S., Mudgett-Hunter M., Levinson D., Wu G. M., Haber E., Oppermann H., Huston J. S. A bifunctional fusion protein containing Fc-binding fragment B of staphylococcal protein A amino terminal to antidigoxin single-chain Fv. Biochemistry. 1990 Sep 4;29(35):8024–8030. doi: 10.1021/bi00487a005. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Milstein C., Winter G. Reshaping human antibodies: grafting an antilysozyme activity. Science. 1988 Mar 25;239(4847):1534–1536. doi: 10.1126/science.2451287. [DOI] [PubMed] [Google Scholar]
- Winter G., Griffiths A. D., Hawkins R. E., Hoogenboom H. R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- Xiong H., Buckwalter B. L., Shieh H. M., Hecht M. H. Periodicity of polar and nonpolar amino acids is the major determinant of secondary structure in self-assembling oligomeric peptides. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6349–6353. doi: 10.1073/pnas.92.14.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Erickson B. W. Engineering of betabellin 14D: disulfide-induced folding of a beta-sheet protein. Protein Sci. 1994 Jul;3(7):1069–1073. doi: 10.1002/pro.5560030709. [DOI] [PMC free article] [PubMed] [Google Scholar]