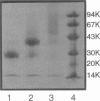
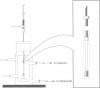
Abstract
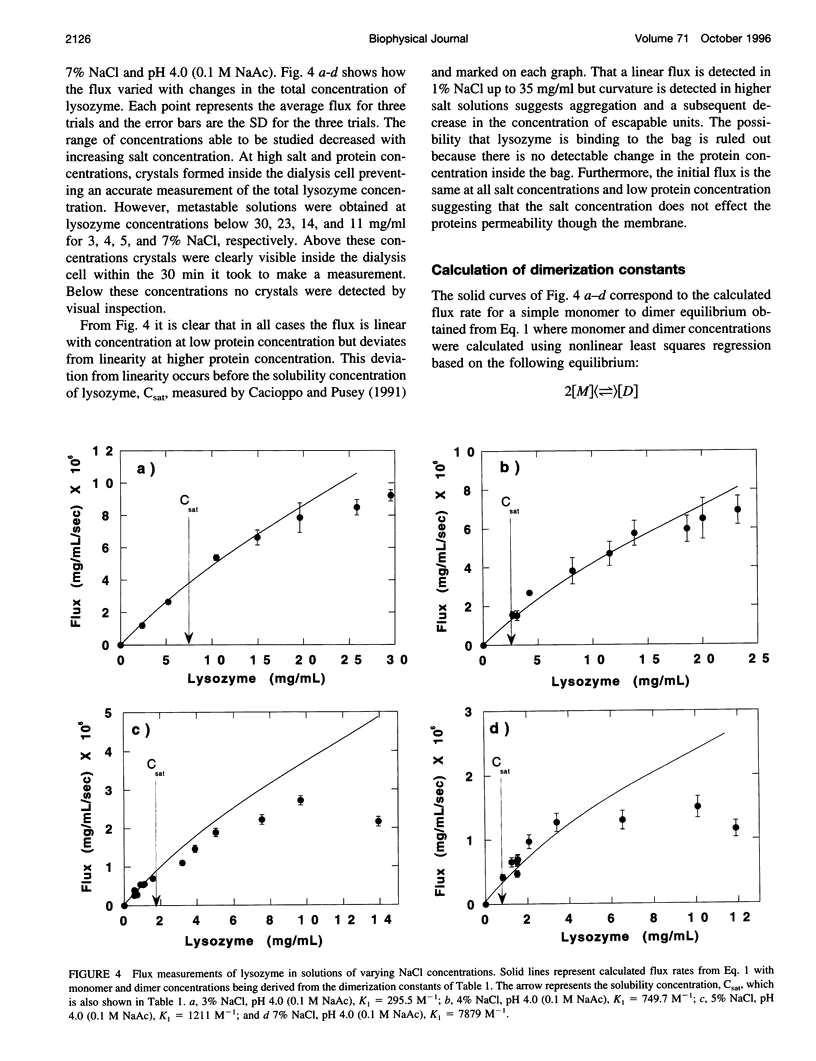
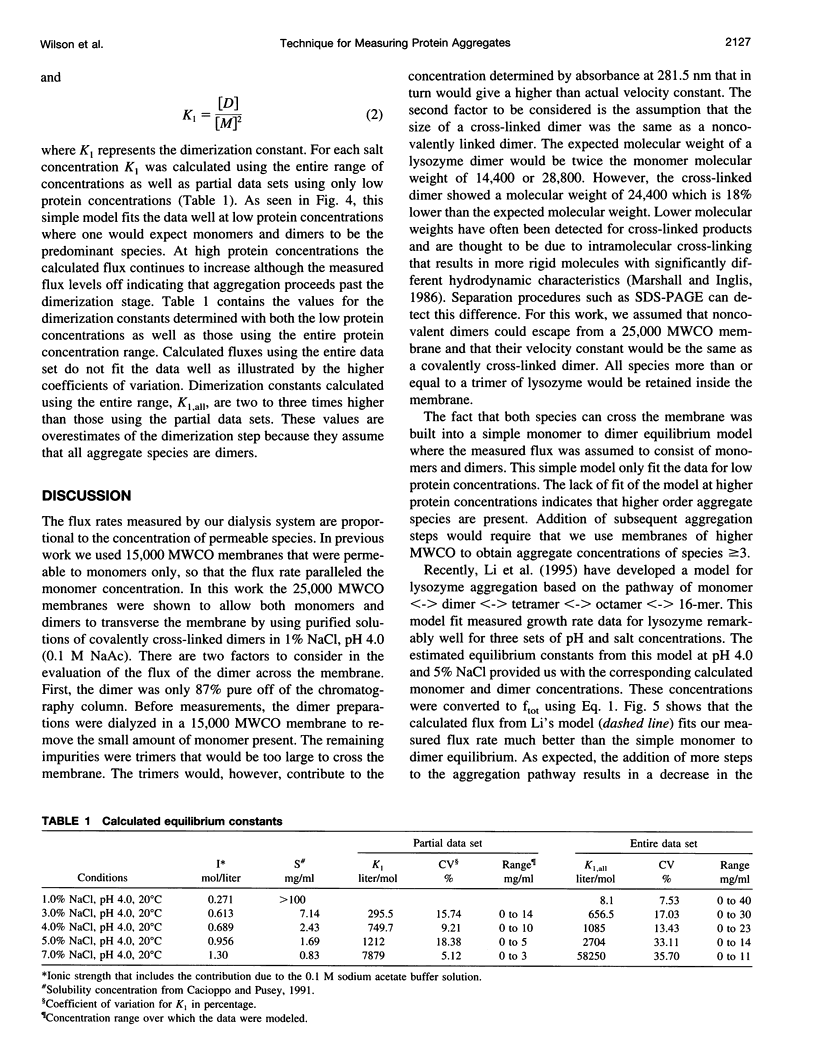
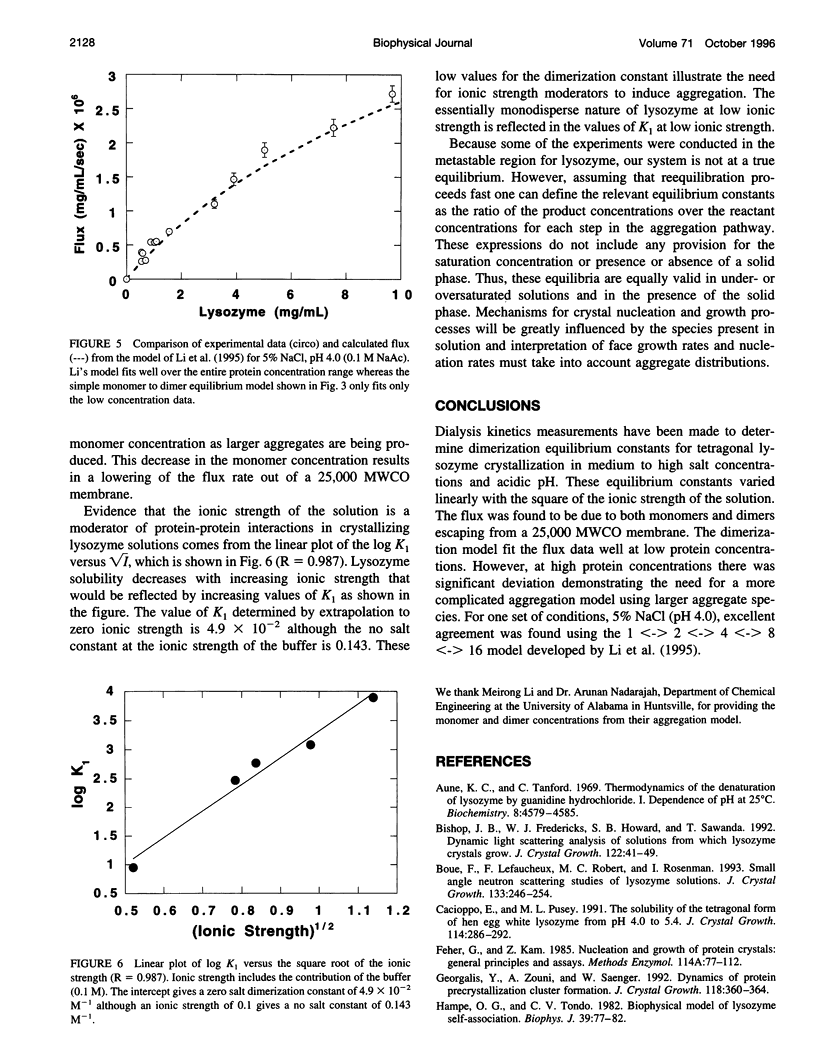
Dialysis kinetics measurements have been made to study the effect of ionic strength on the dimerization of lysozyme in acidic solutions that lead to the growth of tetragonal lysozyme crystals. Using glutaraldehyde cross-linked dimers of lysozyme, we have determined that both monomers and dimers can escape from 25,000 molecular weight cutoff dialysis membranes with velocity constants of 5.1 x 10(-7) and 1.0 x 10(-7) s(-1) for the monomer and dimer species, respectively. The flux from 25K MWCO membranes has been measured for lysozyme in pH 4.0 buffered solutions of 1, 3, 4, 5, and 7% NaCl over a wide range of protein concentrations. Assuming that dimerization is the first step in crystallization, a simple monomer to dimer equilibrium was used to model the flux rates. Dimerization constants calculated at low protein concentrations were 265, 750, 1212, and 7879 M(-1) for 3, 4, 5, and 7% NaCl, respectively. These values indicate that dimerization increases with the ionic strength of the solution suggesting that aggregation is moderated by electrostatic interactions. At high protein concentrations and high supersaturation, the dimerization model does not describe the data well. However, the Li model that uses a pathway of monomer <-> dimer <-> tetramer <-> octamer <-> 16-mer fits the measured flux data remarkably well suggesting the presence of higher order aggregates in crystallizing solutions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune K. C., Tanford C. Thermodynamics of the denaturation of lysozyme by guanidine hydrochloride. I. Depdendence on pH at 25 degrees. Biochemistry. 1969 Nov;8(11):4579–4585. doi: 10.1021/bi00839a052. [DOI] [PubMed] [Google Scholar]
- Feher G., Kam Z. Nucleation and growth of protein crystals: general principles and assays. Methods Enzymol. 1985;114:77–112. doi: 10.1016/0076-6879(85)14006-1. [DOI] [PubMed] [Google Scholar]
- Hampe O. G., Tondo C. V., Hasson-Voloch A. A biophysical model of lysozyme self-association. Biophys J. 1982 Oct;40(1):77–82. doi: 10.1016/S0006-3495(82)84460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol V., Hirsch E., Giegé R. Diagnostic of precipitant for biomacromolecule crystallization by quasi-elastic light-scattering. J Mol Biol. 1990 May 5;213(1):187–195. doi: 10.1016/S0022-2836(05)80130-5. [DOI] [PubMed] [Google Scholar]
- Ries-Kautt M. M., Ducruix A. F. Relative effectiveness of various ions on the solubility and crystal growth of lysozyme. J Biol Chem. 1989 Jan 15;264(2):745–748. [PubMed] [Google Scholar]
- Skouri M., Delsanti M., Munch J. P., Lorber B., Giegé R. Dynamic light scattering studies of the aggregation of lysozyme under crystallization conditions. FEBS Lett. 1991 Dec 16;295(1-3):84–88. doi: 10.1016/0014-5793(91)81391-k. [DOI] [PubMed] [Google Scholar]