Abstract
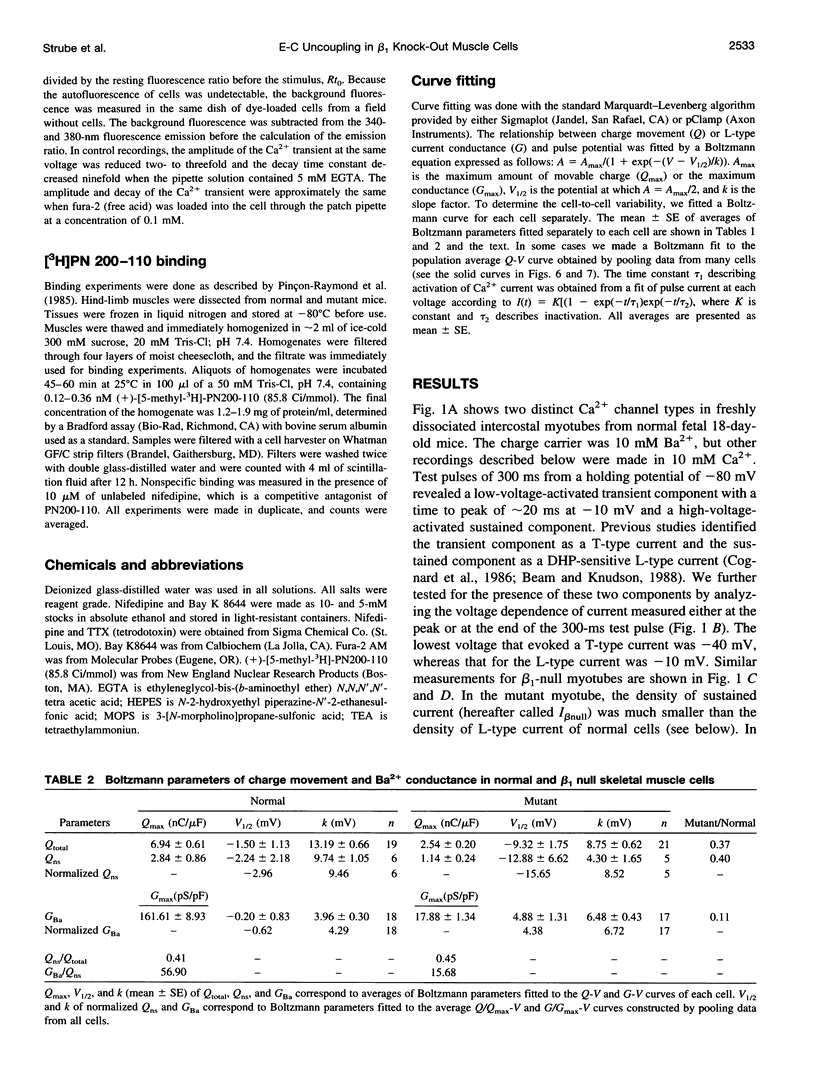
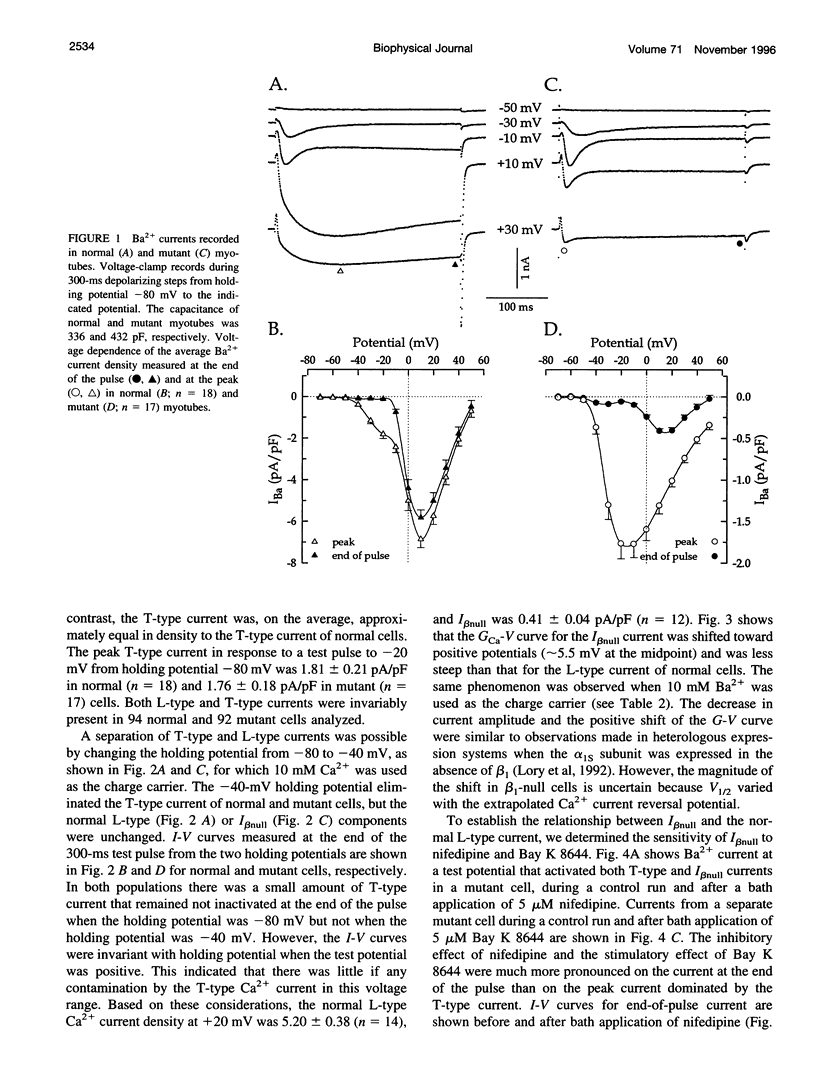
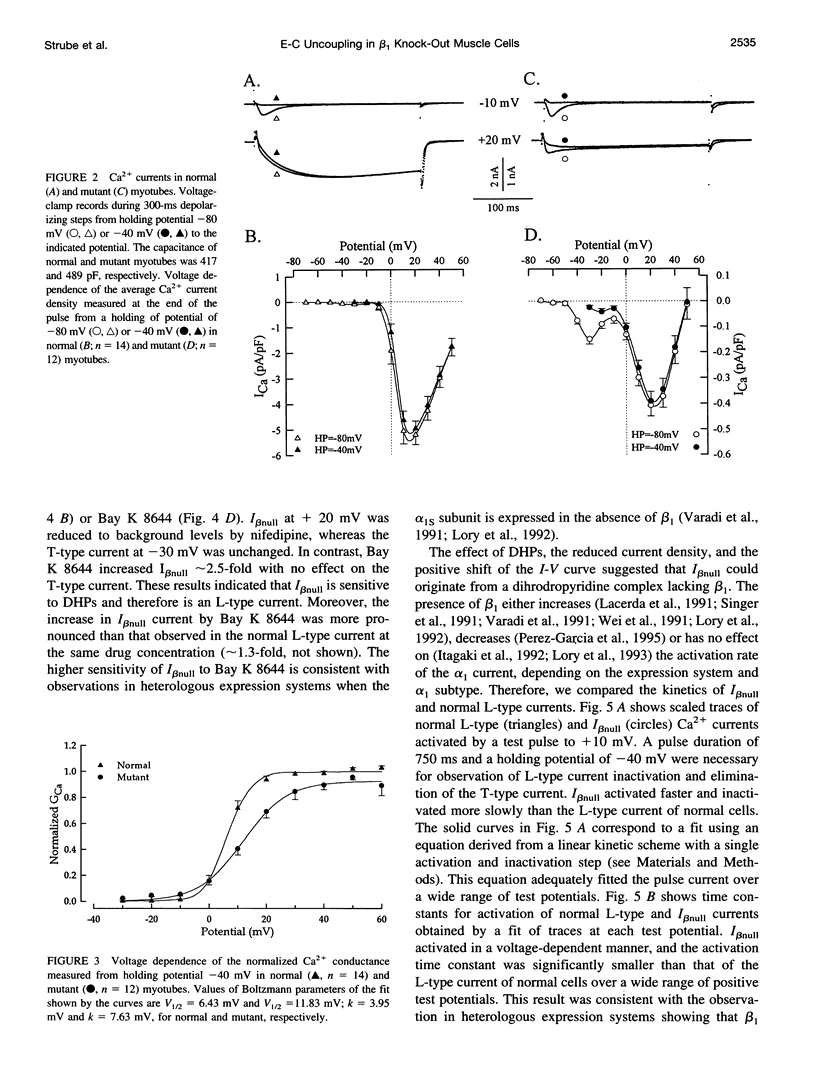
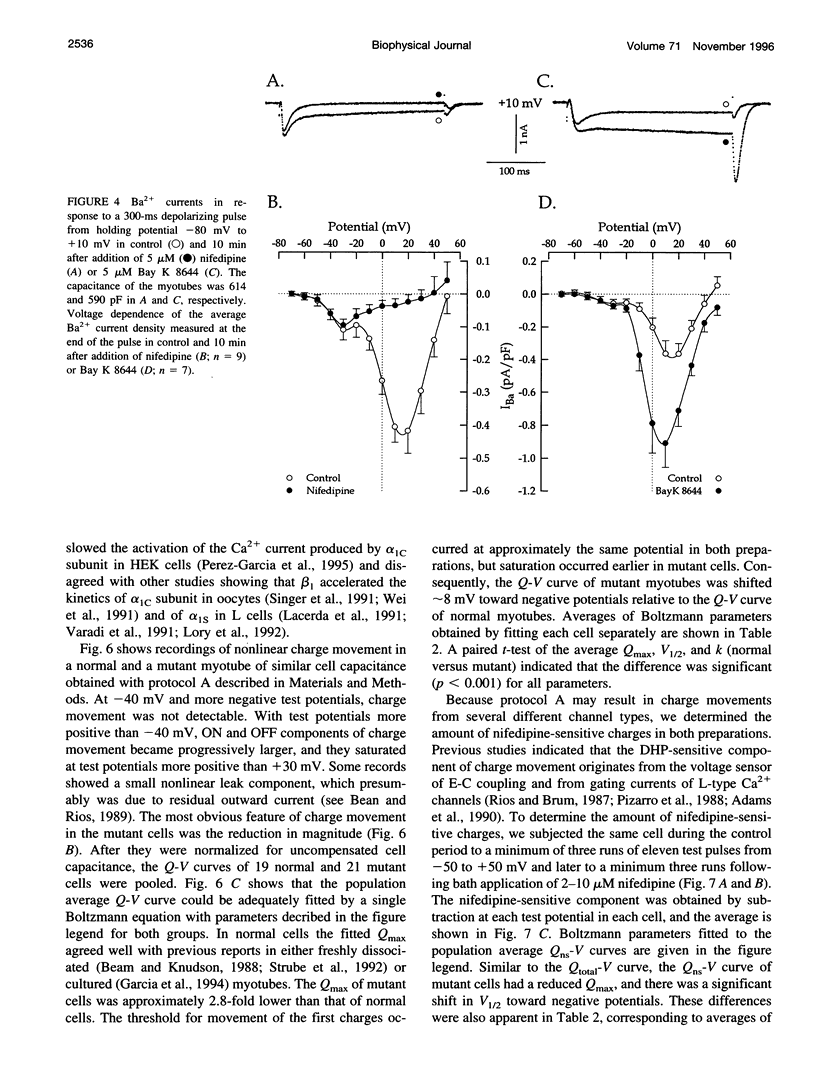
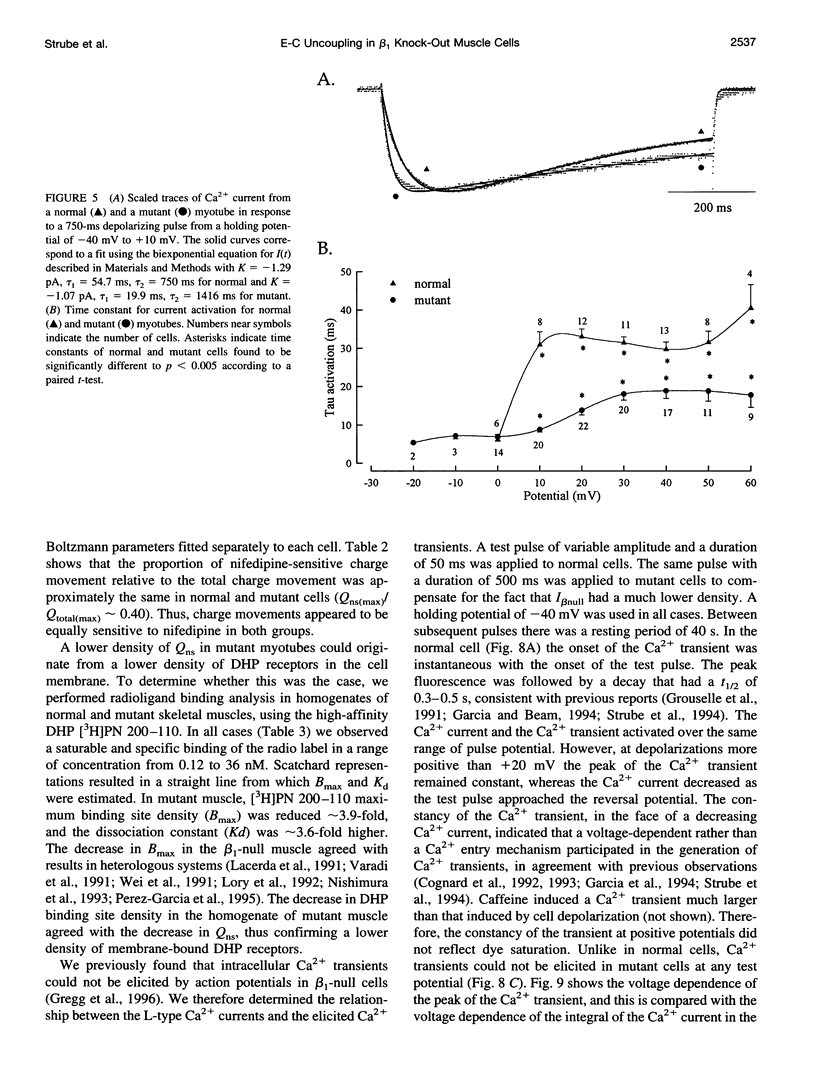
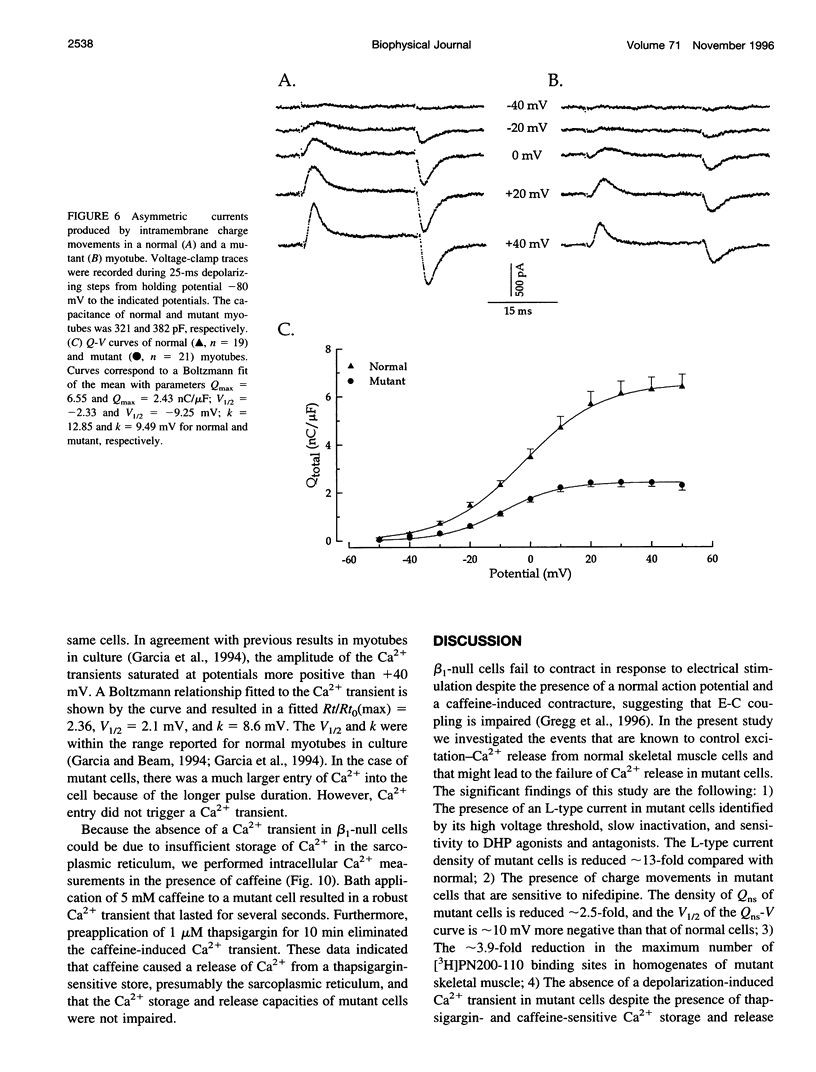
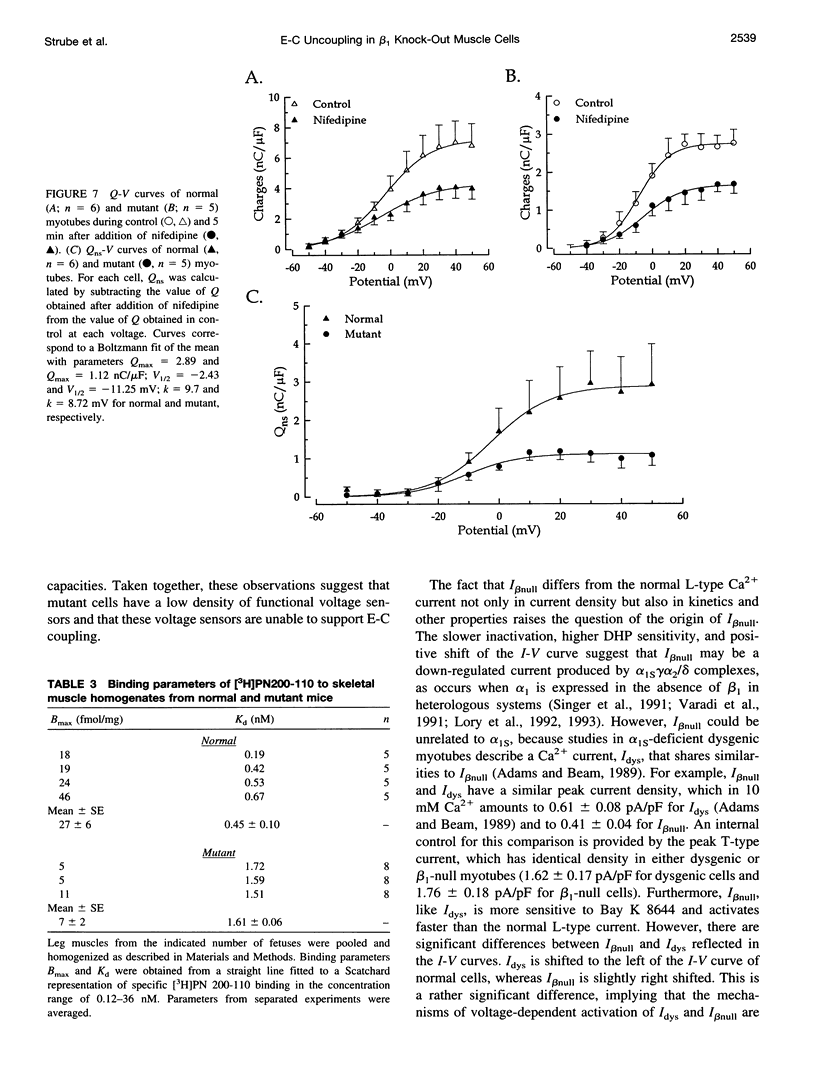
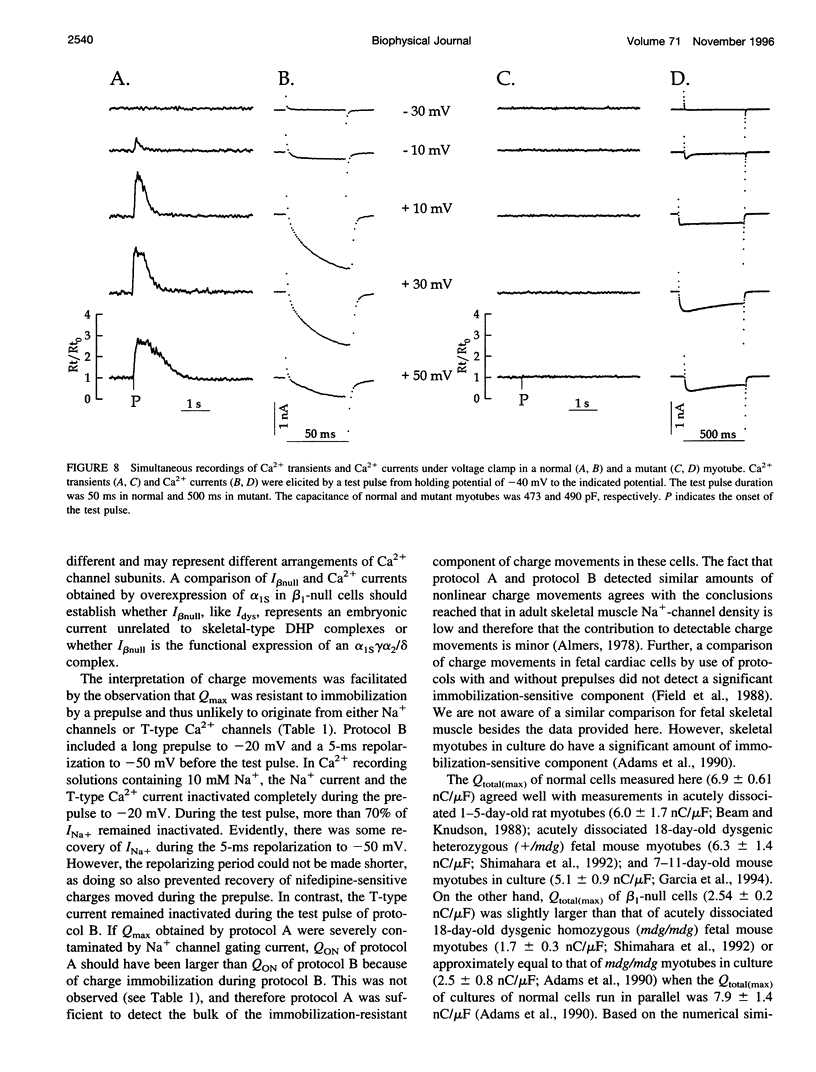
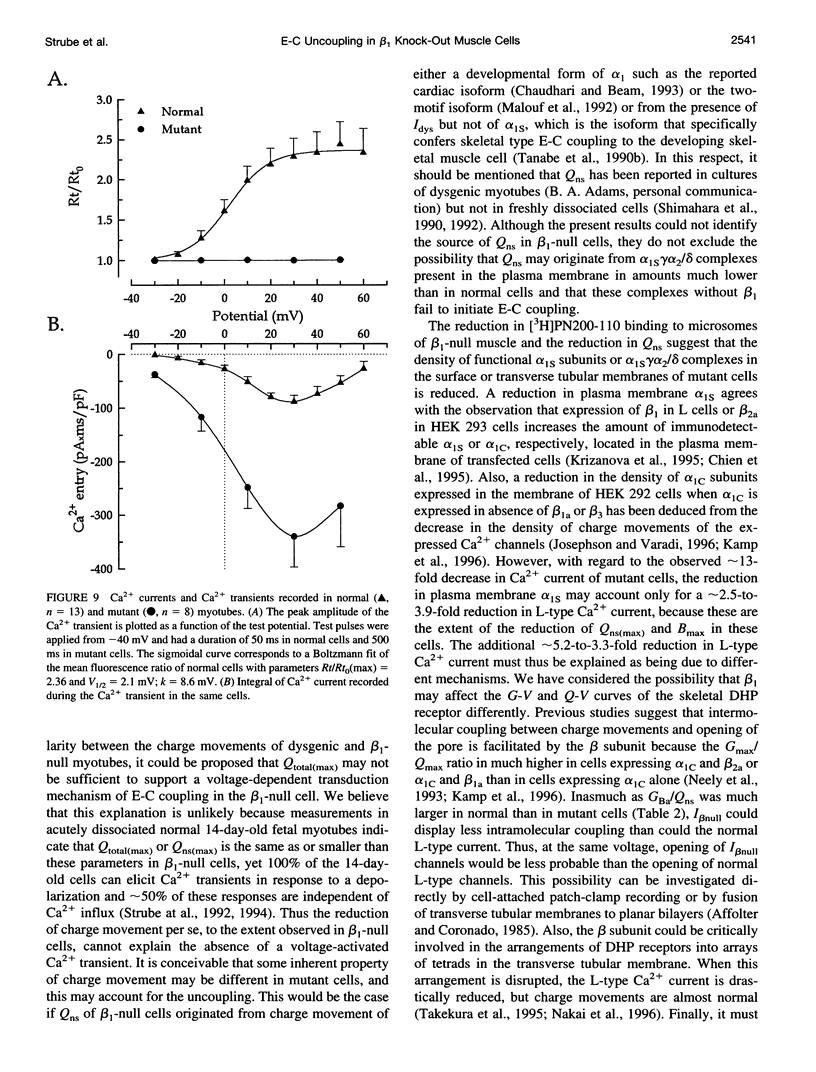
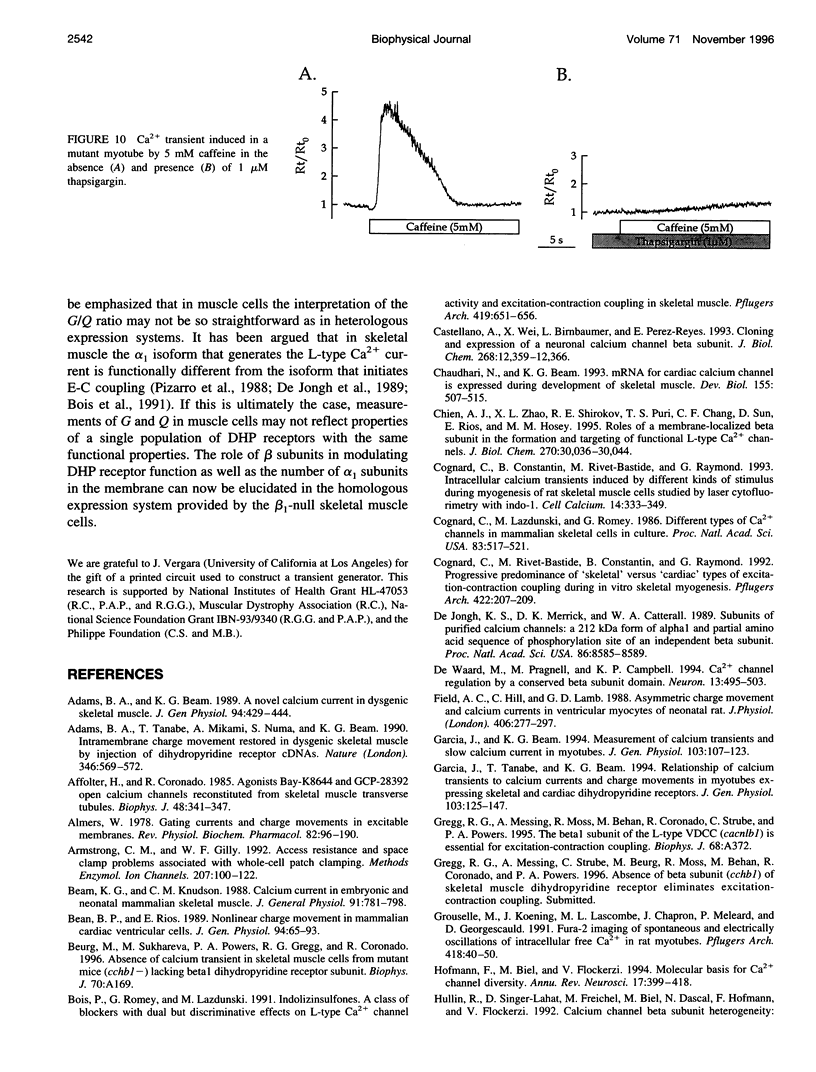
The Ca2+ currents, charge movements, and intracellular Ca2+ transients in mouse skeletal muscle cells homozygous for a null mutation in the cchb1 gene encoding the beta 1 subunit of the dihydropyridine receptor have been characterized. I beta null, the L-type Ca2+ current of mutant cells, had a approximately 13-fold lower density than the L-type current of normal cells (0.41 +/- 0.042 pA/pF at + 20 mV, compared with 5.2 +/- 0.38 pA/pF in normal cells). I beta null was sensitive to dihydropyridines and had faster kinetics of activation and slower kinetics of inactivation than the L-type current of normal cells. Charge movement was reduced approximately 2.8-fold, with Qmax = 6.9 +/- 0.61 and Qmax = 2.5 +/- 0.2 nC/microF in normal and mutant cells, respectively. Approximately 40% of Qmax was nifedipine sensitive in both groups. In contrast to normal cells, Ca2+ transients could not be detected in mutant cells at any test potential; however, caffeine induced a robust Ca2+ transient. In homogenates of mutant muscle, the maximum density of [3H]PN200-110 binding sites (Bmax) was reduced approximately 3.9-fold. The results suggest that the excitation-contraction uncoupling of beta 1-null skeletal muscle involves a failure of the transduction mechanism that is due to either a reduced amount of alpha 1S subunits in the membrane or the specific absence of beta 1 from the voltage-sensor complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Beam K. G. A novel calcium current in dysgenic skeletal muscle. J Gen Physiol. 1989 Sep;94(3):429–444. doi: 10.1085/jgp.94.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B. A., Tanabe T., Mikami A., Numa S., Beam K. G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990 Aug 9;346(6284):569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Affolter H., Coronado R. Agonists Bay-K8644 and CGP-28392 open calcium channels reconstituted from skeletal muscle transverse tubules. Biophys J. 1985 Aug;48(2):341–347. doi: 10.1016/S0006-3495(85)83789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Gilly W. F. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol. 1992;207:100–122. doi: 10.1016/0076-6879(92)07007-b. [DOI] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Rios E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol. 1989 Jul;94(1):65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois P., Romey G., Lazdunski M. Indolizinsulphones. A class of blockers with dual but discriminative effects on L-type Ca2+ channel activity and excitation-contraction coupling in skeletal muscle. Pflugers Arch. 1991 Dec;419(6):651–656. doi: 10.1007/BF00370310. [DOI] [PubMed] [Google Scholar]
- Chaudhari N., Beam K. G. mRNA for cardiac calcium channel is expressed during development of skeletal muscle. Dev Biol. 1993 Feb;155(2):507–515. doi: 10.1006/dbio.1993.1048. [DOI] [PubMed] [Google Scholar]
- Cognard C., Constantin B., Rivet-Bastide M., Raymond G. Intracellular calcium transients induced by different kinds of stimulus during myogenesis of rat skeletal muscle cells studied by laser cytofluorimetry with Indo-1. Cell Calcium. 1993 Apr;14(4):333–348. doi: 10.1016/0143-4160(93)90054-a. [DOI] [PubMed] [Google Scholar]
- Cognard C., Lazdunski M., Romey G. Different types of Ca2+ channels in mammalian skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1986 Jan;83(2):517–521. doi: 10.1073/pnas.83.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognard C., Rivet-Bastide M., Constantin B., Raymond G. Progressive predominance of 'skeletal' versus 'cardiac' types of excitation-contraction coupling during in vitro skeletal myogenesis. Pflugers Arch. 1992 Nov;422(2):207–209. doi: 10.1007/BF00370424. [DOI] [PubMed] [Google Scholar]
- De Jongh K. S., Merrick D. K., Catterall W. A. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard M., Pragnell M., Campbell K. P. Ca2+ channel regulation by a conserved beta subunit domain. Neuron. 1994 Aug;13(2):495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Field A. C., Hill C., Lamb G. D. Asymmetric charge movement and calcium currents in ventricular myocytes of neonatal rat. J Physiol. 1988 Dec;406:277–297. doi: 10.1113/jphysiol.1988.sp017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J., Beam K. G. Measurement of calcium transients and slow calcium current in myotubes. J Gen Physiol. 1994 Jan;103(1):107–123. doi: 10.1085/jgp.103.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J., Tanabe T., Beam K. G. Relationship of calcium transients to calcium currents and charge movements in myotubes expressing skeletal and cardiac dihydropyridine receptors. J Gen Physiol. 1994 Jan;103(1):125–147. doi: 10.1085/jgp.103.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouselle M., Koenig J., Lascombe M. L., Chapron J., Méléard P., Georgescauld D. Fura-2 imaging of spontaneous and electrically induced oscillations of intracellular free Ca2+ in rat myotubes. Pflugers Arch. 1991 Mar;418(1-2):40–50. doi: 10.1007/BF00370450. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992 Mar;11(3):885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki K., Koch W. J., Bodi I., Klöckner U., Slish D. F., Schwartz A. Native-type DHP-sensitive calcium channel currents are produced by cloned rat aortic smooth muscle and cardiac alpha 1 subunits expressed in Xenopus laevis oocytes and are regulated by alpha 2- and beta-subunits. FEBS Lett. 1992 Feb 10;297(3):221–225. doi: 10.1016/0014-5793(92)80542-o. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Varadi G. The beta subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+ channels. Biophys J. 1996 Mar;70(3):1285–1293. doi: 10.1016/S0006-3495(96)79685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp T. J., Pérez-García M. T., Marban E. Enhancement of ionic current and charge movement by coexpression of calcium channel beta 1A subunit with alpha 1C subunit in a human embryonic kidney cell line. J Physiol. 1996 Apr 1;492(Pt 1):89–96. doi: 10.1113/jphysiol.1996.sp021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizanova O., Varadi M., Schwartz A., Varadi G. Coexpression of skeletal muscle voltage-dependent calcium channel alpha 1 and beta cDNAs in mouse Ltk- cells increases the amount of alpha 1 protein in the cell membrane. Biochem Biophys Res Commun. 1995 Jun 26;211(3):921–927. doi: 10.1006/bbrc.1995.1900. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Kim H. S., Ruth P., Perez-Reyes E., Flockerzi V., Hofmann F., Birnbaumer L., Brown A. M. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991 Aug 8;352(6335):527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Lory P., Varadi G., Schwartz A. The beta subunit controls the gating and dihydropyridine sensitivity of the skeletal muscle Ca2+ channel. Biophys J. 1992 Nov;63(5):1421–1424. doi: 10.1016/S0006-3495(92)81705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory P., Varadi G., Slish D. F., Varadi M., Schwartz A. Characterization of beta subunit modulation of a rabbit cardiac L-type Ca2+ channel alpha 1 subunit as expressed in mouse L cells. FEBS Lett. 1993 Jan 4;315(2):167–172. doi: 10.1016/0014-5793(93)81156-t. [DOI] [PubMed] [Google Scholar]
- Malouf N. N., McMahon D. K., Hainsworth C. N., Kay B. K. A two-motif isoform of the major calcium channel subunit in skeletal muscle. Neuron. 1992 May;8(5):899–906. doi: 10.1016/0896-6273(92)90204-q. [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J., Froschmayr M., Grabner M., Striessnig J., Glossmann H. Calcium channels: the beta-subunit increases the affinity of dihydropyridine and Ca2+ binding sites of the alpha 1-subunit. FEBS Lett. 1994 Sep 26;352(2):141–145. doi: 10.1016/0014-5793(94)00938-4. [DOI] [PubMed] [Google Scholar]
- Nakai J., Dirksen R. T., Nguyen H. T., Pessah I. N., Beam K. G., Allen P. D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996 Mar 7;380(6569):72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Neely A., Wei X., Olcese R., Birnbaumer L., Stefani E. Potentiation by the beta subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993 Oct 22;262(5133):575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Takeshima H., Hofmann F., Flockerzi V., Imoto K. Requirement of the calcium channel beta subunit for functional conformation. FEBS Lett. 1993 Jun 21;324(3):283–286. doi: 10.1016/0014-5793(93)80135-h. [DOI] [PubMed] [Google Scholar]
- Pinçon-Raymond M., Rieger F., Fosset M., Lazdunski M. Abnormal transverse tubule system and abnormal amount of receptors for Ca2+ channel inhibitors of the dihydropyridine family in skeletal muscle from mice with embryonic muscular dysgenesis. Dev Biol. 1985 Dec;112(2):458–466. doi: 10.1016/0012-1606(85)90418-x. [DOI] [PubMed] [Google Scholar]
- Powers P. A., Liu S., Hogan K., Gregg R. G. Skeletal muscle and brain isoforms of a beta-subunit of human voltage-dependent calcium channels are encoded by a single gene. J Biol Chem. 1992 Nov 15;267(32):22967–22972. [PubMed] [Google Scholar]
- Pérez-García M. T., Kamp T. J., Marbán E. Functional properties of cardiac L-type calcium channels transiently expressed in HEK293 cells. Roles of alpha 1 and beta subunits. J Gen Physiol. 1995 Feb;105(2):289–305. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Shimahara T., Bournaud R., Inoue I., Strube C. Charge movement and Ca2+ release in normal and dysgenic foetal myotubes. J Physiol Paris. 1992;86(1-3):117–121. doi: 10.1016/s0928-4257(05)80015-4. [DOI] [PubMed] [Google Scholar]
- Shimahara T., Bournaud R., Inoue I., Strube C. Reduced intramembrane charge movement in the dysgenic skeletal muscle cell. Pflugers Arch. 1990 Sep;417(1):111–113. doi: 10.1007/BF00370778. [DOI] [PubMed] [Google Scholar]
- Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991 Sep 27;253(5027):1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Stea A., Dubel S. J., Pragnell M., Leonard J. P., Campbell K. P., Snutch T. P. A beta-subunit normalizes the electrophysiological properties of a cloned N-type Ca2+ channel alpha 1-subunit. Neuropharmacology. 1993 Nov;32(11):1103–1116. doi: 10.1016/0028-3908(93)90005-n. [DOI] [PubMed] [Google Scholar]
- Strube C., Beurg M., Georgescauld D., Bournaud R., Shimahara T. Extracellular Ca(2+)-dependent and independent calcium transient in fetal myotubes. Pflugers Arch. 1994 Jul;427(5-6):517–523. doi: 10.1007/BF00374269. [DOI] [PubMed] [Google Scholar]
- Strube C., Bournaud R., Inoue I., Shimahara T. Intramembrane charge movement in developing skeletal muscle cells from fetal mice. Pflugers Arch. 1992 Sep;421(6):572–577. doi: 10.1007/BF00375053. [DOI] [PubMed] [Google Scholar]
- Takekura H., Nishi M., Noda T., Takeshima H., Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Adams B. A., Niidome T., Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990 Aug 9;346(6284):567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Mikami A., Numa S., Beam K. G. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990 Mar 29;344(6265):451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]



