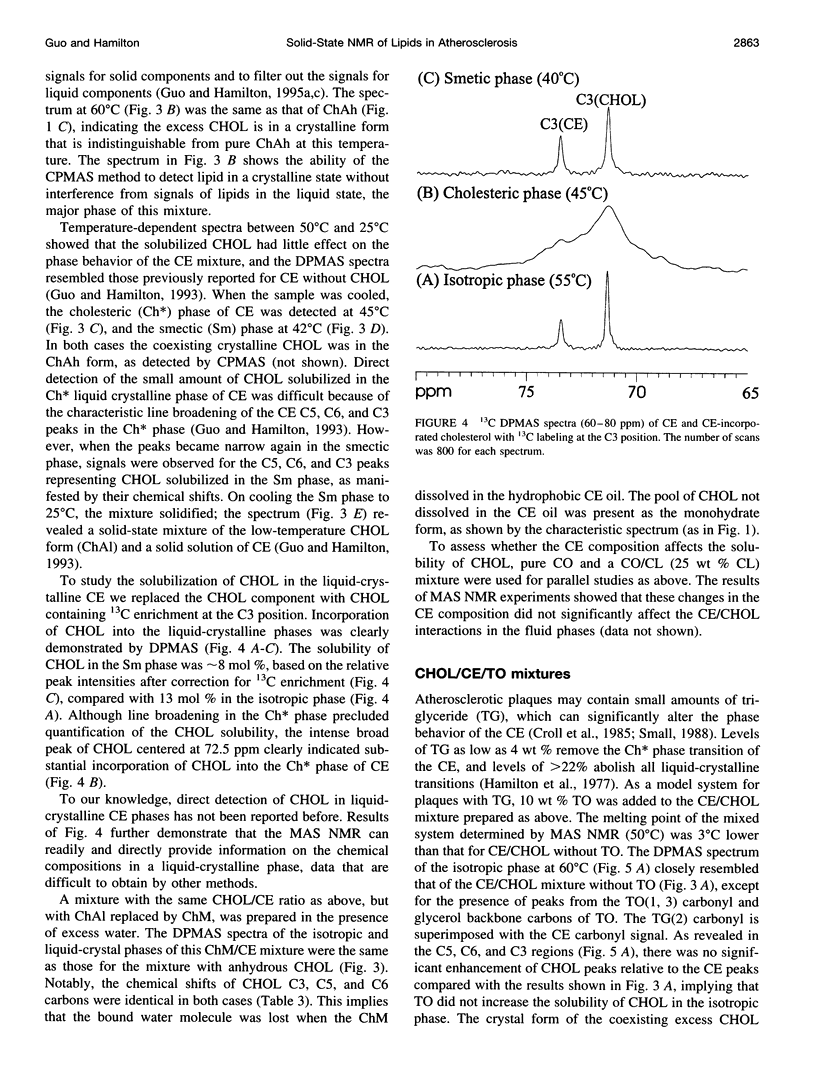
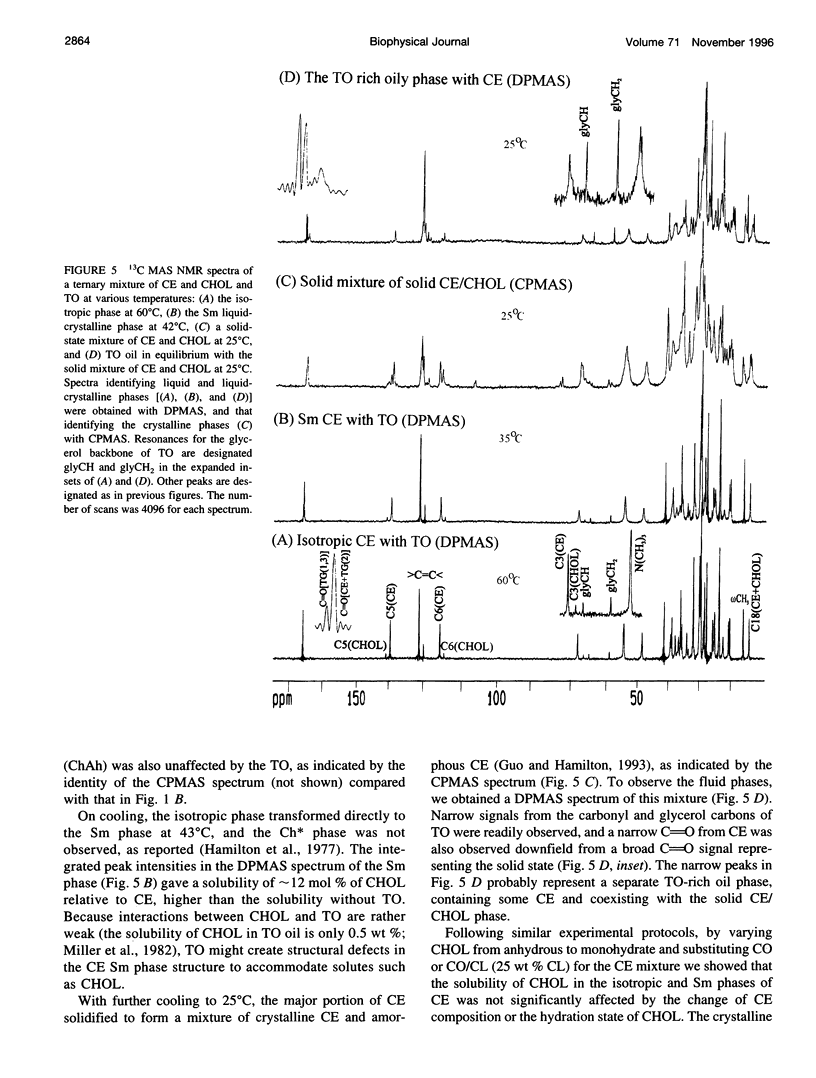
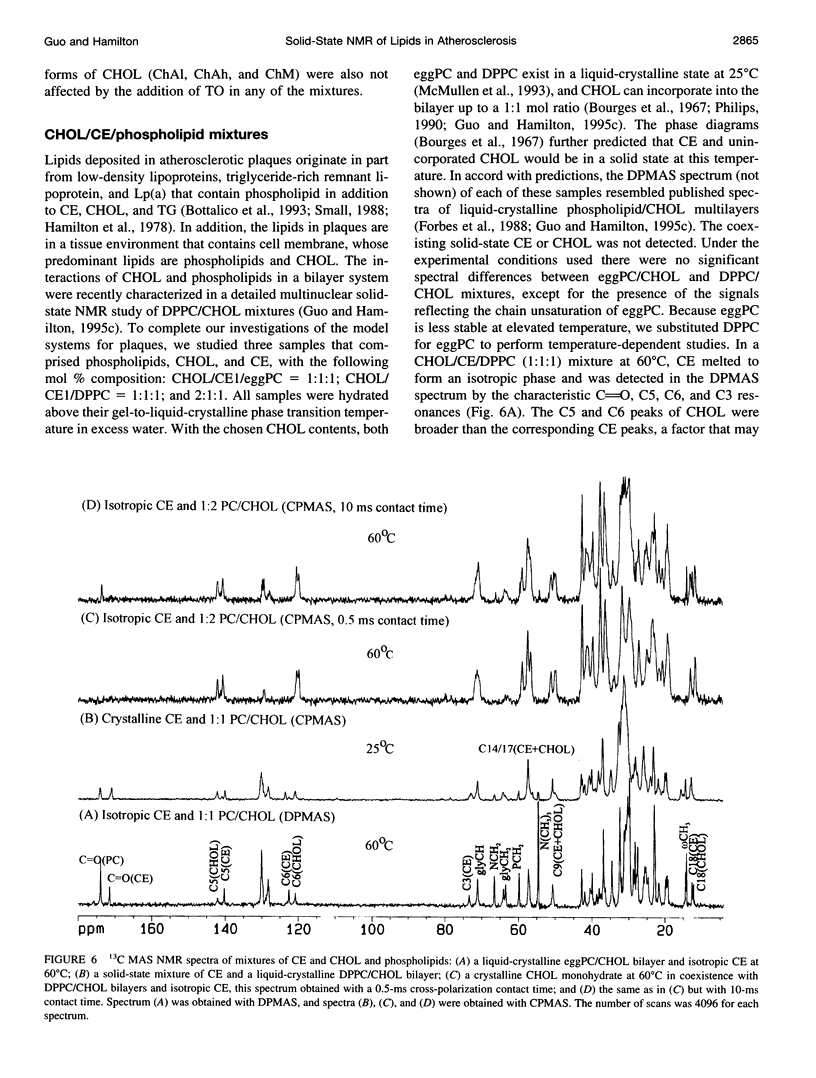
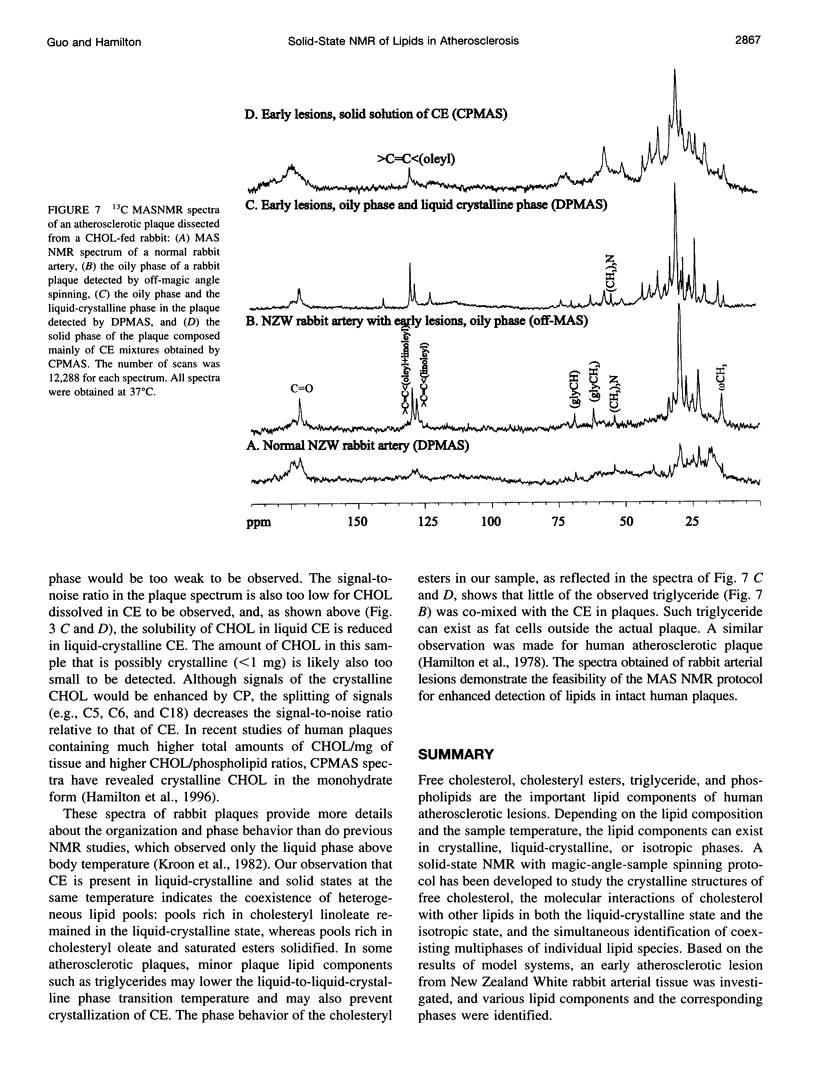
Abstract
Cholesterol and cholesteryl esters are the predominant lipids of atherosclerotic plaques. To provide fundamental data for the quantitative study of plaque lipids in situ, crystalline cholesterol (CHOL) and CHOL/cholesteryl ester (CE) mixtures with other lipids were studied by solid-state nuclear magnetic resonance with magic-angle-sample spinning. Highly distinctive spectra for three different crystalline structures of CHOL were obtained. When CHOL crystals were mixed with isotropic CE oil, solubilized CHOL (approximately 13 mol % CHOL) was detected by characteristic resonances such as C5, C6, and C3; the excess crystalline CHOL (either anhydrous or monohydrate) remained in its original crystalline structure, without being affected by the coexisting CE. By use of 13C-enriched CHOL, the solubility of CHOL in the CE liquid-crystalline phase (approximately 8 mol %) was measured. When phosphatidylcholine was hydrated in presence of CHOL and CE, magic-angle-sampling nuclear magnetic resonance revealed liquid-crystalline CHOL/phosphatidylcholine multilayers with approximately an equal molar ratio of CHOL/phosphatidylcholine. Excess CHOL existed in the monohydrate crystalline form, and CE in separate oil or crystalline phases, depending on the temperature. The magic-angle-sampling nuclear magnetic resonance protocol for identifying different lipid phases was applied to intact (ex vivo) atherosclerotic plaques of cholesterol-fed rabbits. Liquid, liquid-crystalline, and solid phases of CE were characterized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottalico L. A., Keesler G. A., Fless G. M., Tabas I. Cholesterol loading of macrophages leads to marked enhancement of native lipoprotein(a) and apoprotein(a) internalization and degradation. J Biol Chem. 1993 Apr 25;268(12):8569–8573. [PubMed] [Google Scholar]
- Bourgès M., Small D. M., Dervichian D. G. Biophysics of lipidic associations. II. The ternary systems: cholesterol-lecithin-water. Biochim Biophys Acta. 1967 Feb 14;137(1):157–167. doi: 10.1016/0005-2760(67)90019-7. [DOI] [PubMed] [Google Scholar]
- Craven B. M. Crystal structure of cholesterol monohydrate. Nature. 1976 Apr 22;260(5553):727–729. doi: 10.1038/260727a0. [DOI] [PubMed] [Google Scholar]
- Croll D. H., Small D. M., Hamilton J. A. Molecular motions and thermotropic phase behavior of cholesteryl esters with triolein. Biochemistry. 1985 Dec 31;24(27):7971–7980. doi: 10.1021/bi00348a020. [DOI] [PubMed] [Google Scholar]
- Guo W., Hamilton J. A. Molecular organization and motions of cholesteryl esters in crystalline and liquid crystalline phases: a 13C and 1H magic angle spinning NMR study. Biochemistry. 1993 Sep 7;32(35):9038–9052. doi: 10.1021/bi00086a009. [DOI] [PubMed] [Google Scholar]
- Guo W., Hamilton J. A. Molecular organization and motions of crystalline monoacylglycerols and diacylglycerols: a C-13 MASNMR study. Biophys J. 1995 Apr;68(4):1383–1395. doi: 10.1016/S0006-3495(95)80311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Hamilton J. A. Phase behavior and crystalline structures of cholesteryl ester mixtures: a C-13 MASNMR study. Biophys J. 1995 Jun;68(6):2376–2386. doi: 10.1016/S0006-3495(95)80420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Cordes E. H., Glueck C. J. Lipid dynamics in human low density lipoproteins and human aortic tissue with fibrous plaques. A study by high field 13C NMR spectroscopy. J Biol Chem. 1979 Jun 25;254(12):5435–5441. [PubMed] [Google Scholar]
- Hamilton J. A., Cordes E. H. Molecular dynamics of lipids in human plasma high density lipoproteins. A high field 13C NMR study. J Biol Chem. 1978 Jul 25;253(14):5193–5198. [PubMed] [Google Scholar]
- Hamilton J. A., Oppenheimer N., Cordes E. H. Carbon-13 nuclear magnetic resonance studies of cholesteryl esters and cholesteryl ester/triglyceride mixtures. J Biol Chem. 1977 Nov 25;252(22):8071–8080. [PubMed] [Google Scholar]
- Kroon P. A., Quinn D. M., Cordes E. H. A carbon-13 nuclear magnetic resonance study of aortic lesions and cholesteryl ester rich lipoproteins from atherosclerotic rabbits. Biochemistry. 1982 May 25;21(11):2745–2753. doi: 10.1021/bi00540a026. [DOI] [PubMed] [Google Scholar]
- Loomis C. R., Shipley G. G., Small D. M. The phase behavior of hydrated cholesterol. J Lipid Res. 1979 May;20(4):525–535. [PubMed] [Google Scholar]
- McMullen T. P., Lewis R. N., McElhaney R. N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993 Jan 19;32(2):516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- Nolte C. J., Tercyak A. M., Wu H. M., Small D. M. Chemical and physiochemical comparison of advanced atherosclerotic lesions of similar size and cholesterol content in cholesterol-fed New Zealand White and Watanabe Heritable Hyperlipidemic rabbits. Lab Invest. 1990 Feb;62(2):213–222. [PubMed] [Google Scholar]
- North B. E., Katz S. S., Small D. M. The dissolution of cholesterol monohydrate crystals in atherosclerotic plaque lipids. Atherosclerosis. 1978 Jul;30(3):211–217. doi: 10.1016/0021-9150(78)90047-3. [DOI] [PubMed] [Google Scholar]
- Phillips M. C. Cholesterol packing, crystallization and exchange properties in phosphatidylcholine vesicle systems. Hepatology. 1990 Sep;12(3 Pt 2):75S–82S. [PubMed] [Google Scholar]
- Shieh H. S., Hoard L. G., Nordman C. E. Crystal structure of anhydrous cholesterol. Nature. 1977 May 19;267(5608):287–289. doi: 10.1038/267287a0. [DOI] [PubMed] [Google Scholar]
- Small D. M. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988 Mar-Apr;8(2):103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Spooner P. J., Hamilton J. A., Gantz D. L., Small D. M. The effect of free cholesterol on the solubilization of cholesteryl oleate in phosphatidylcholine bilayers: A 13C-NMR study. Biochim Biophys Acta. 1986 Aug 21;860(2):345–353. doi: 10.1016/0005-2736(86)90531-6. [DOI] [PubMed] [Google Scholar]
- Spooner P. J., Small D. M. Effect of free cholesterol on incorporation of triolein in phospholipid bilayers. Biochemistry. 1987 Sep 8;26(18):5820–5825. doi: 10.1021/bi00392a036. [DOI] [PubMed] [Google Scholar]
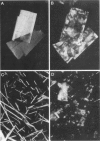
- Tangirala R. K., Jerome W. G., Jones N. L., Small D. M., Johnson W. J., Glick J. M., Mahlberg F. H., Rothblat G. H. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J Lipid Res. 1994 Jan;35(1):93–104. [PubMed] [Google Scholar]
- Toussaint J. F., Southern J. F., Fuster V., Kantor H. L. 13C-NMR spectroscopy of human atherosclerotic lesions. Relation between fatty acid saturation, cholesteryl ester content, and luminal obstruction. Arterioscler Thromb. 1994 Dec;14(12):1951–1957. doi: 10.1161/01.atv.14.12.1951. [DOI] [PubMed] [Google Scholar]