Abstract
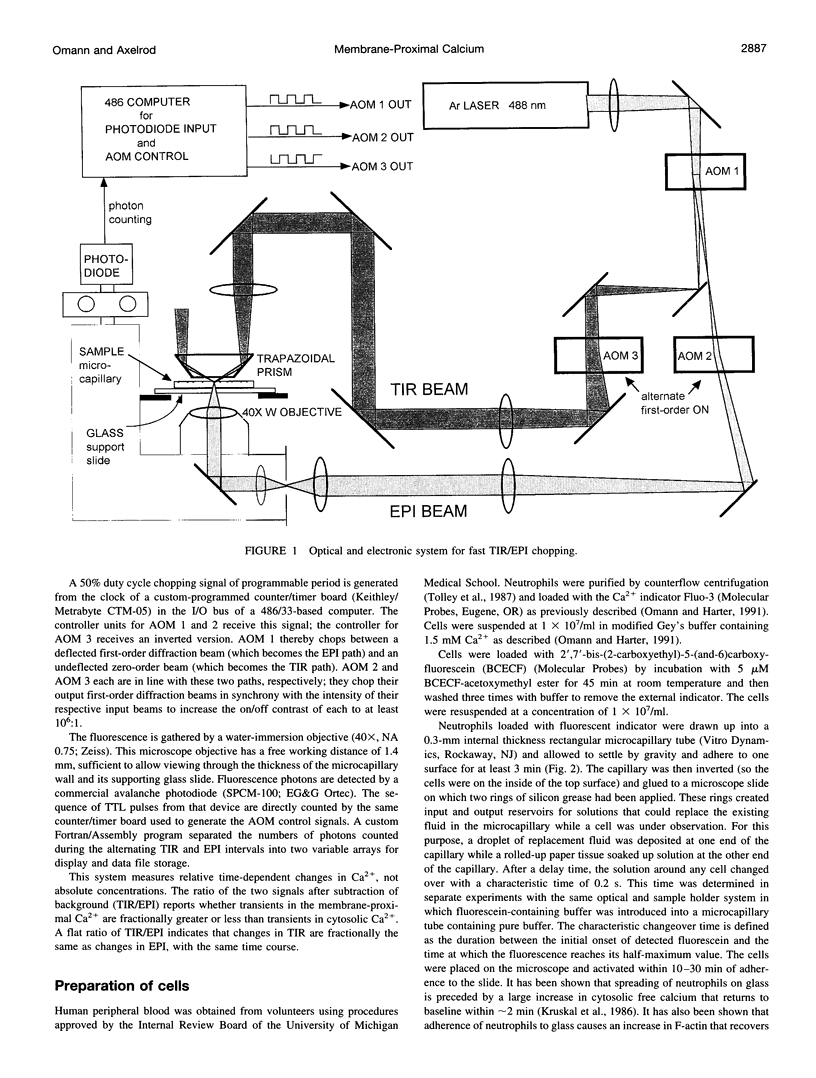
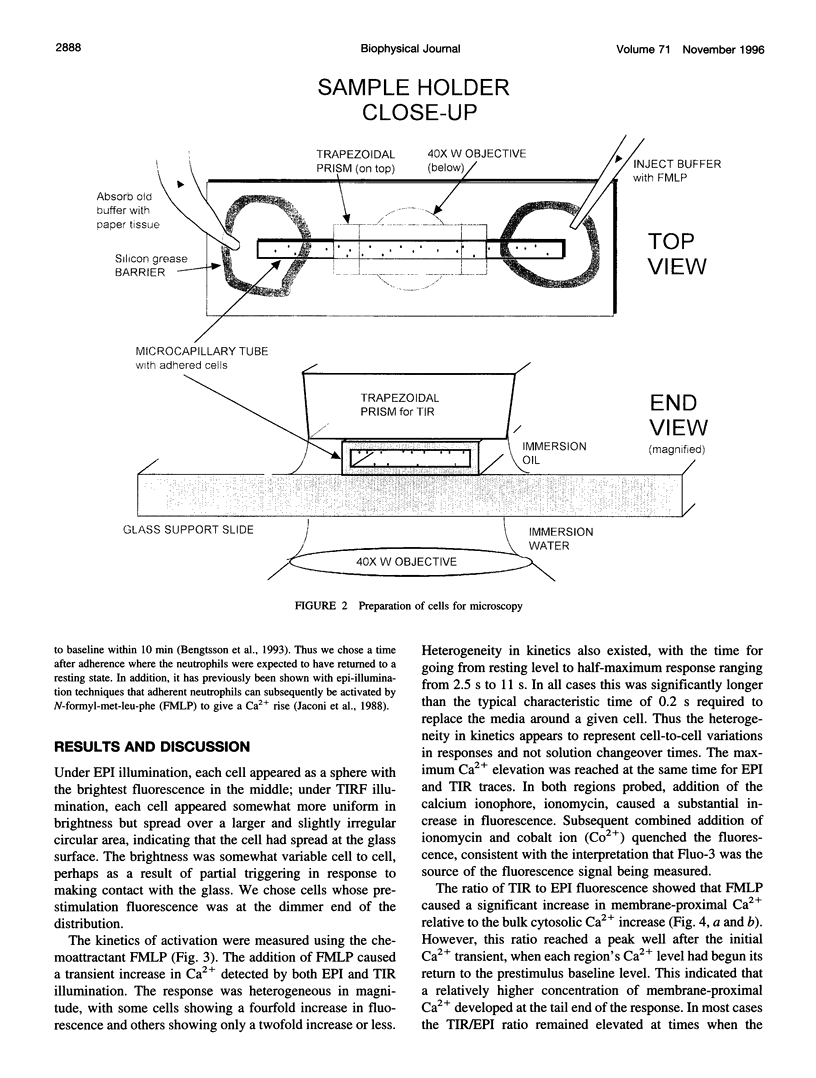
A novel fluorescence microscope/laser optical system was developed to measure fast transients of membrane-proximal versus bulk cytoplasmic intracellular calcium levels in cells labeled with a fluorescent calcium indicator. The method is based on the rapid chopping of illumination of the cells between optical configurations for epifluorescence, which excites predominantly the bulk intracellular region, and total internal reflection fluorescence, which excites only the region within approximately 100 nm of the cell-substrate contact. This method was applied to Fluo-3-loaded neutrophils that were activated by the chemoattractant N-formyl-met-leu-phe. Chemoattractant-activated cells showed 1) transient increases in both membrane-proximal and bulk cytosolic Ca2+ that peaked simultaneously; 2) a larger fractional change (20-60%) in membrane-proximal Ca2+ relative to bulk cytosolic Ca2+ that peaked at a time when the main Ca2+ transient was decreasing in both regions and that persisted well after the main transient was over. This method should be applicable to a wide variety of cell types and fluorescent ion indicators in which membrane-proximal ionic transients may be different from those deeper within the cytosol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T. Correlation between chemotactic peptide-induced changes in chlorotetracycline fluorescence and F-actin content in human neutrophils: a role for membrane-associated calcium in the regulation of actin polymerization? Exp Cell Res. 1990 Nov;191(1):57–63. doi: 10.1016/0014-4827(90)90035-9. [DOI] [PubMed] [Google Scholar]
- Bengtsson T., Jaconi M. E., Gustafson M., Magnusson K. E., Theler J. M., Lew D. P., Stendahl O. Actin dynamics in human neutrophils during adhesion and phagocytosis is controlled by changes in intracellular free calcium. Eur J Cell Biol. 1993 Oct;62(1):49–58. [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J. The elemental principles of calcium signaling. Cell. 1995 Dec 1;83(5):675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Hutchison J. D. Selectivity of cation chelation to tetracyclines: evidence for special conformation of calcium chelate. Biochem Biophys Res Commun. 1971 May 7;43(3):625–630. doi: 10.1016/0006-291x(71)90660-7. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Hutchison J. D. Visualization of membrane bound cations by a fluorescent technique. Biochem Biophys Res Commun. 1971 Jan 8;42(1):43–49. doi: 10.1016/0006-291x(71)90359-7. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Kazilek C. J. Calcium signals in neutrophils can be divided into three distinct phases. Biochim Biophys Acta. 1987 Nov 12;931(2):175–179. doi: 10.1016/0167-4889(87)90204-7. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular divalent cation release in pancreatic acinar cells during stimulus-secretion coupling. I. Use of chlorotetracycline as fluorescent probe. J Cell Biol. 1978 Feb;76(2):371–385. doi: 10.1083/jcb.76.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Barry V. A. Stimulus-secretion coupling in excitable cells: a central role for calcium. J Exp Biol. 1993 Nov;184:183–196. doi: 10.1242/jeb.184.1.183. [DOI] [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Cramer E. B., Gallin J. I. Localization of submembranous cations to the leading end of human neutrophils during chemotaxis. J Cell Biol. 1979 Aug;82(2):369–379. doi: 10.1083/jcb.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Etter E. F., Kuhn M. A., Fay F. S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J Biol Chem. 1994 Apr 1;269(13):10141–10149. [PubMed] [Google Scholar]
- Felder C. C., Singer-Lahat D., Mathes C. Voltage-independent calcium channels. Regulation by receptors and intracellular calcium stores. Biochem Pharmacol. 1994 Nov 29;48(11):1997–2004. doi: 10.1016/0006-2952(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Greenberg M. E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995 Apr 14;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Jaconi M. E., Rivest R. W., Schlegel W., Wollheim C. B., Pittet D., Lew P. D. Spontaneous and chemoattractant-induced oscillations of cytosolic free calcium in single adherent human neutrophils. J Biol Chem. 1988 Aug 5;263(22):10557–10560. [PubMed] [Google Scholar]
- Kruskal B. A., Shak S., Maxfield F. R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 May;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Kaptein J. S., Lin C. K., Kalunta C. I., Scott S. J., Gu D. G. G-proteins and the role of second messengers in the regulation of the human neutrophil. Immunol Ser. 1992;57:107–136. [PubMed] [Google Scholar]
- Lagast H., Lew P. D., Waldvogel F. A. Adenosine triphosphate-dependent calcium pump in the plasma membrane of guinea pig and human neutrophils. J Clin Invest. 1984 Jan;73(1):107–115. doi: 10.1172/JCI111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Lloyd Q. P., Kuhn M. A., Gay C. V. Characterization of calcium translocation across the plasma membrane of primary osteoblasts using a lipophilic calcium-sensitive fluorescent dye, calcium green C18. J Biol Chem. 1995 Sep 22;270(38):22445–22451. doi: 10.1074/jbc.270.38.22445. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Volpi M., Showell H. J., Becker E. L., Sha'afi R. I. Chemotactic factor-induced release of membrane calcium in rabbit neutrophils. Science. 1979 Feb 2;203(4379):461–463. doi: 10.1126/science.760200. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Harter J. M. Pertussis toxin effects on chemoattractant-induced response heterogeneity in human PMNs utilizing Fluo-3 and flow cytometry. Cytometry. 1991;12(3):252–259. doi: 10.1002/cyto.990120308. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Omann G. M., Painter R. G. Relationship of actin polymerization and depolymerization to light scattering in human neutrophils: dependence on receptor occupancy and intracellular Ca++. J Cell Biol. 1985 Sep;101(3):1161–1166. doi: 10.1083/jcb.101.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. E., Eisenstat B. A., Weissmann G. The fluorescence response of chlorotetracycline-loaded human neutrophils. Correlations with lysosomal enzyme release and evidence for a 'trigger pool' of calcium. Biochim Biophys Acta. 1982 Aug 27;717(3):422–431. doi: 10.1016/0304-4165(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Stoehr S. J., Boxer L. A. Human neutrophils permeabilized with digitonin respond with lysosomal enzyme release when exposed to micromolar levels of free calcium. Biochim Biophys Acta. 1986 Apr 8;886(1):1–17. doi: 10.1016/0167-4889(86)90205-3. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Stoehr S. J. Guanine nucleotides reduce the free calcium requirement for secretion of granule constituents from permeabilized human neutrophils. Biochim Biophys Acta. 1986 Nov 28;889(2):171–178. doi: 10.1016/0167-4889(86)90101-1. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Stoehr S. J. Micromolar concentrations of free calcium provoke secretion of lysozyme from human neutrophils permeabilized with saponin. J Immunol. 1985 Mar;134(3):1859–1865. [PubMed] [Google Scholar]
- Tolley J. O., Omann G. M., Jesaitis A. J. A high-yield, high-purity elutriation method for preparing human granulocytes demonstrating enhanced experimental lifetimes. J Leukoc Biol. 1987 Jul;42(1):43–50. doi: 10.1002/jlb.42.1.43. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Täljedal I. B. Chlorotetracycline as a fluorescent Ca2+ probe in pancreatic islet cells. J Cell Biol. 1978 Mar;76(3):652–674. doi: 10.1083/jcb.76.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Pearce F. L. Characterization of chlortetracycline (aureomycin) as a calcium ionophore. Biochemistry. 1982 Nov 23;21(24):6309–6312. doi: 10.1021/bi00267a041. [DOI] [PubMed] [Google Scholar]
- White J. R., Pearce F. L. Use of chlortetracycline to monitor calcium mobilization during histamine secretion from the mast cell: a cautionary note. Anal Biochem. 1983 Jul 1;132(1):1–5. doi: 10.1016/0003-2697(83)90417-7. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]