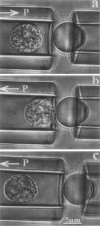
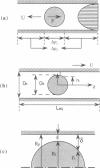
Abstract
A new method for measuring piconewton-scale forces that employs micropipette suction is presented here. Spherical cells or beads are used directly as force transducers, and forces as small as 10-20 pN can be imposed. When the transducer is stationary in the pipette, the force is simply the product of the suction pressure and the cross-sectional area of the pipette minus a small correction for the narrow gap that exists between the transducer and the pipette wall. When the transducer is moving along the pipette, the force on it is corrected by a factor that is proportional to the ratio of its velocity relative to its drag-free velocity. With this technique, the minimum force required to form a membrane tether from neutrophils is determined (45 pN), and the length of the microvilli on the surface of neutrophils is inferred. The strength of this technique is in its simplicity and its ability to measure forces between cells without requiring a separate theory or a calibration against an external standard and without requiring the use of a solid surface.
Full text
PDF

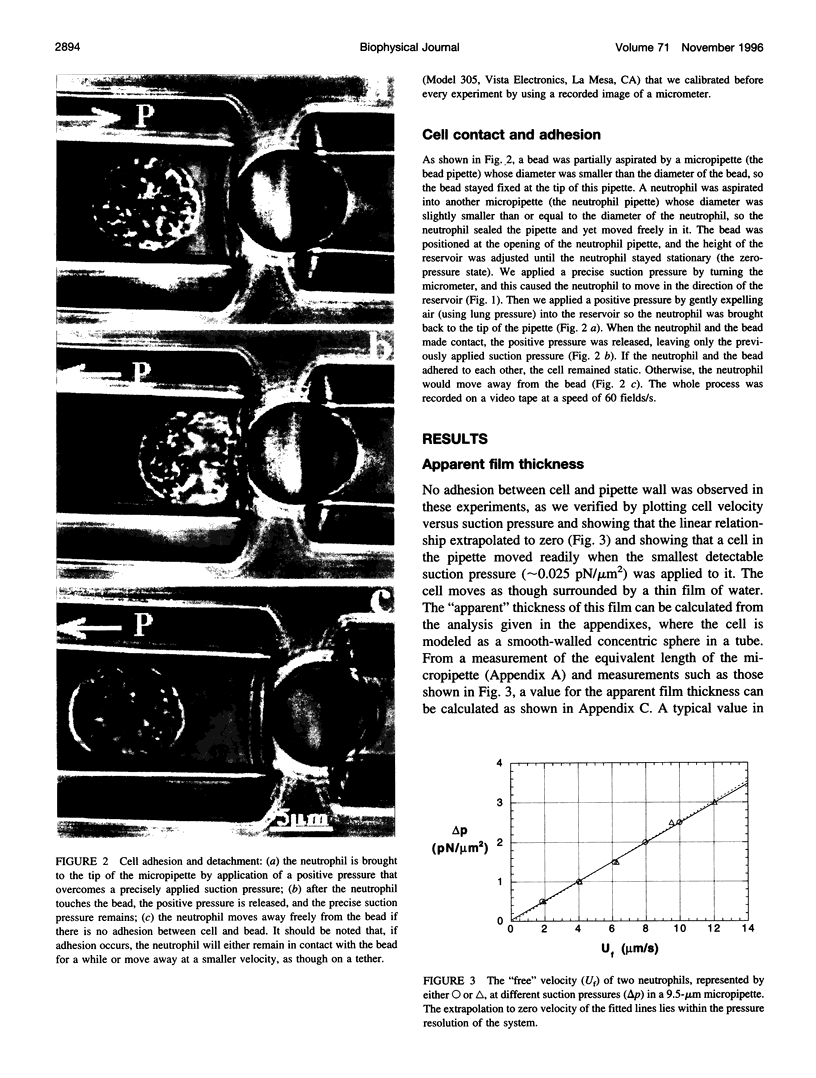
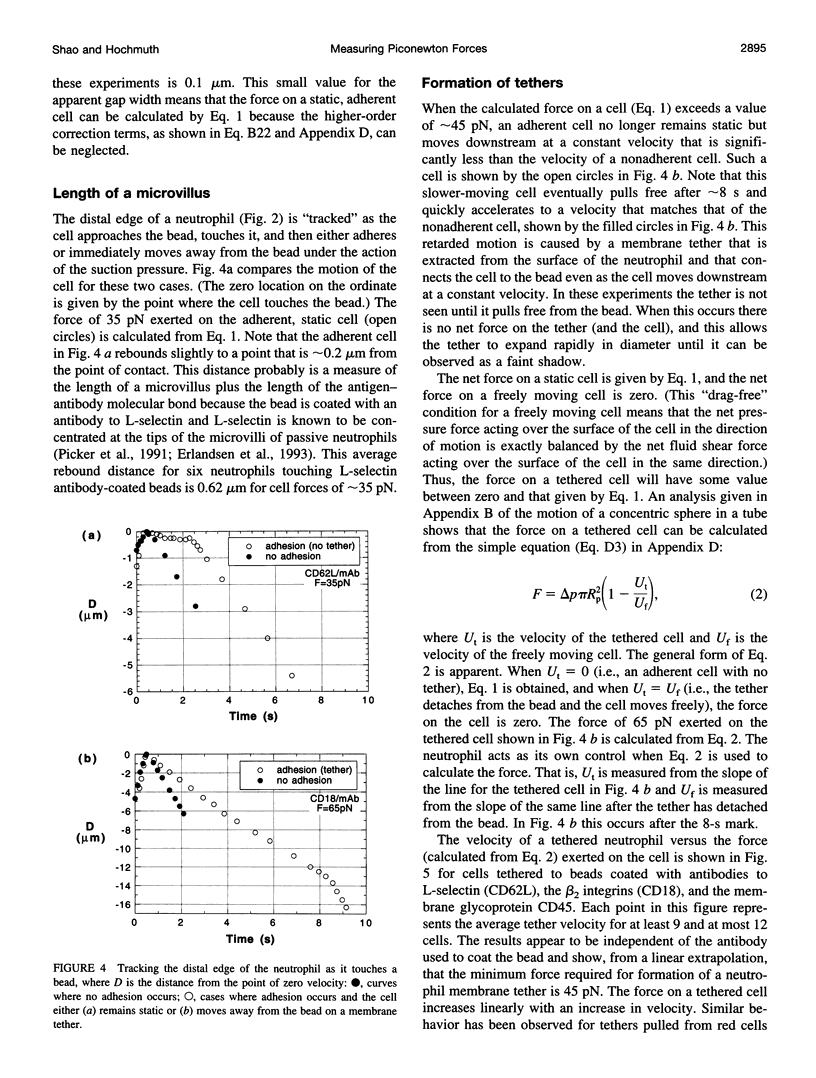




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon R., Hammer D. A., Springer T. A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995 Apr 6;374(6522):539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Ashkin A. Forces of a single-beam gradient laser trap on a dielectric sphere in the ray optics regime. Biophys J. 1992 Feb;61(2):569–582. doi: 10.1016/S0006-3495(92)81860-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Dai J., Sheetz M. P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys J. 1995 Mar;68(3):988–996. doi: 10.1016/S0006-3495(95)80274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammer U., Popescu O., Wagner P., Anselmetti D., Güntherodt H. J., Misevic G. N. Binding strength between cell adhesion proteoglycans measured by atomic force microscopy. Science. 1995 Feb 24;267(5201):1173–1175. doi: 10.1126/science.7855599. [DOI] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Erlandsen S. L., Hasslen S. R., Nelson R. D. Detection and spatial distribution of the beta 2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J Histochem Cytochem. 1993 Mar;41(3):327–333. doi: 10.1177/41.3.7679125. [DOI] [PubMed] [Google Scholar]
- Evans E. A. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys J. 1973 Sep;13(9):941–954. doi: 10.1016/S0006-3495(73)86036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A. Detachment of agglutinin-bonded red blood cells. I. Forces to rupture molecular-point attachments. Biophys J. 1991 Apr;59(4):838–848. doi: 10.1016/S0006-3495(91)82296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984 Nov;64(5):1028–1035. [PubMed] [Google Scholar]
- Evans E., Ritchie K., Merkel R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys J. 1995 Jun;68(6):2580–2587. doi: 10.1016/S0006-3495(95)80441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Florin E. L., Moy V. T., Gaub H. E. Adhesion forces between individual ligand-receptor pairs. Science. 1994 Apr 15;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Hochmuth F. M., Shao J. Y., Dai J., Sheetz M. P. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys J. 1996 Jan;70(1):358–369. doi: 10.1016/S0006-3495(96)79577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans C. A., Wiles H. C., McCown J. T. Mechanical measurement of red cell membrane thickness. Science. 1983 Apr 1;220(4592):101–102. doi: 10.1126/science.6828875. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Kamimura S., Takahashi K. Direct measurement of the force of microtubule sliding in flagella. Nature. 1981 Oct 15;293(5833):566–568. doi: 10.1038/293566a0. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Kojima H., Ishijima A., Yanagida T. Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12962–12966. doi: 10.1073/pnas.91.26.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Optical tweezers in cell biology. Trends Cell Biol. 1992 Apr;2(4):116–118. doi: 10.1016/0962-8924(92)90016-g. [DOI] [PubMed] [Google Scholar]
- Moy V. T., Florin E. L., Gaub H. E. Intermolecular forces and energies between ligands and receptors. Science. 1994 Oct 14;266(5183):257–259. doi: 10.1126/science.7939660. [DOI] [PubMed] [Google Scholar]
- Needham D., Armstrong M., Hatchell D. L., Nunn R. S. Rapid deformation of "passive" polymorphonuclear leukocytes: the effects of pentoxifylline. J Cell Physiol. 1989 Sep;140(3):549–557. doi: 10.1002/jcp.1041400321. [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. A sensitive measure of surface stress in the resting neutrophil. Biophys J. 1992 Jun;61(6):1664–1670. doi: 10.1016/S0006-3495(92)81970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. I. MEMBRANE STIFFNESS AND INTRACELLULAR PRESSURE. Biophys J. 1964 Mar;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Bauserman R. G. Physical measurements of bilayer-skeletal separation forces. Ann Biomed Eng. 1995 May-Jun;23(3):308–321. doi: 10.1007/BF02584431. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Hochmuth R. M. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys J. 1987 Sep;52(3):391–400. doi: 10.1016/S0006-3495(87)83227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev D. V., Needham D., Hochmuth R. M. Role of the membrane cortex in neutrophil deformation in small pipets. Biophys J. 1994 Aug;67(2):696–705. doi: 10.1016/S0006-3495(94)80529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev D. V., Needham D. Tension-stabilized pores in giant vesicles: determination of pore size and pore line tension. Biochim Biophys Acta. 1993 Apr 8;1147(1):89–104. doi: 10.1016/0005-2736(93)90319-u. [DOI] [PubMed] [Google Scholar]