Abstract
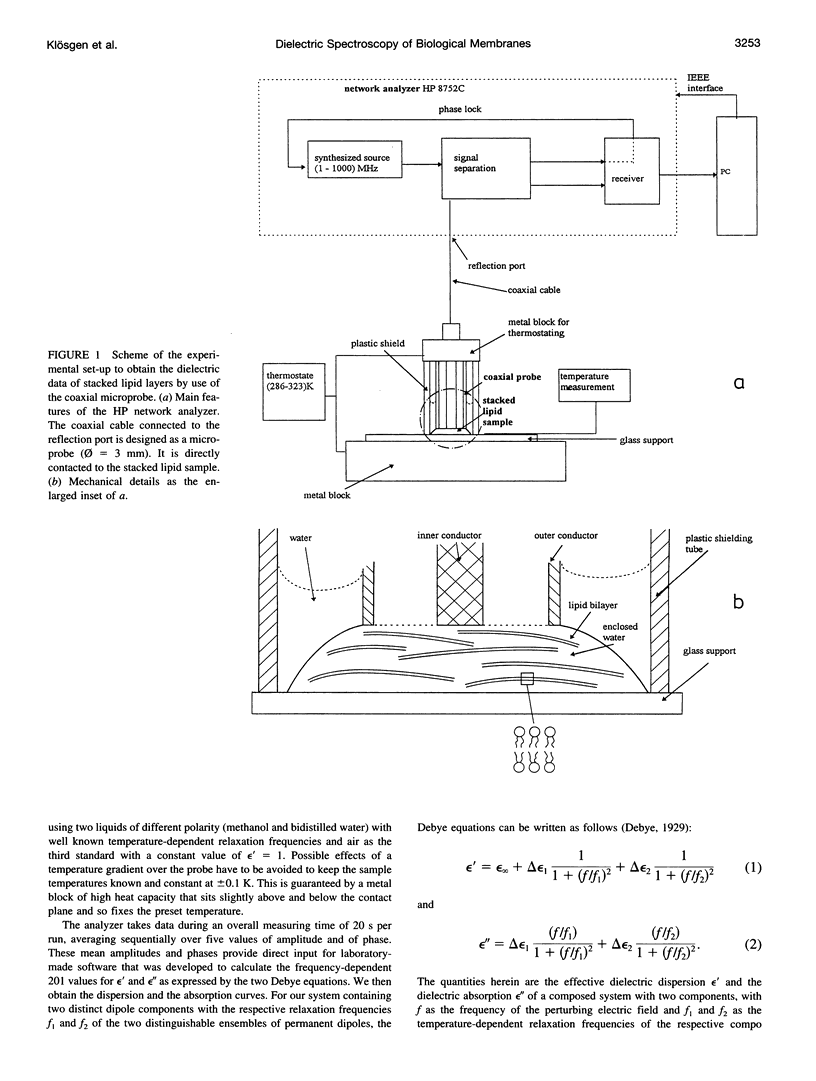
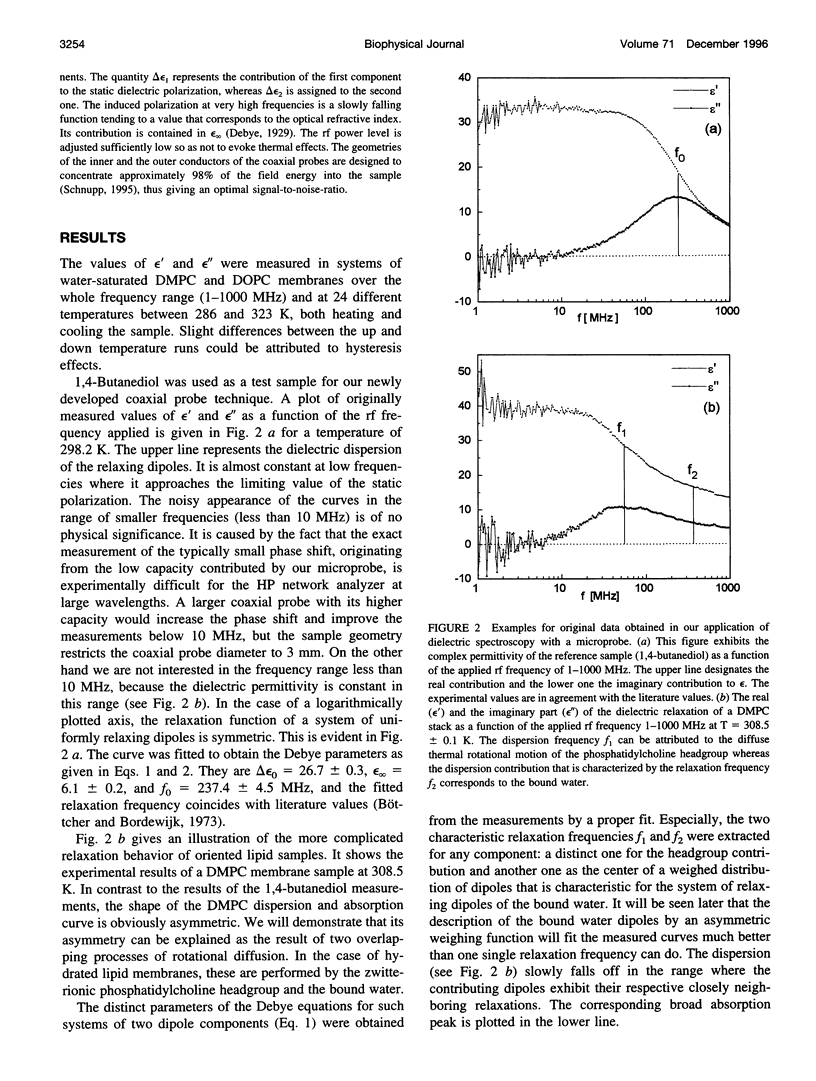
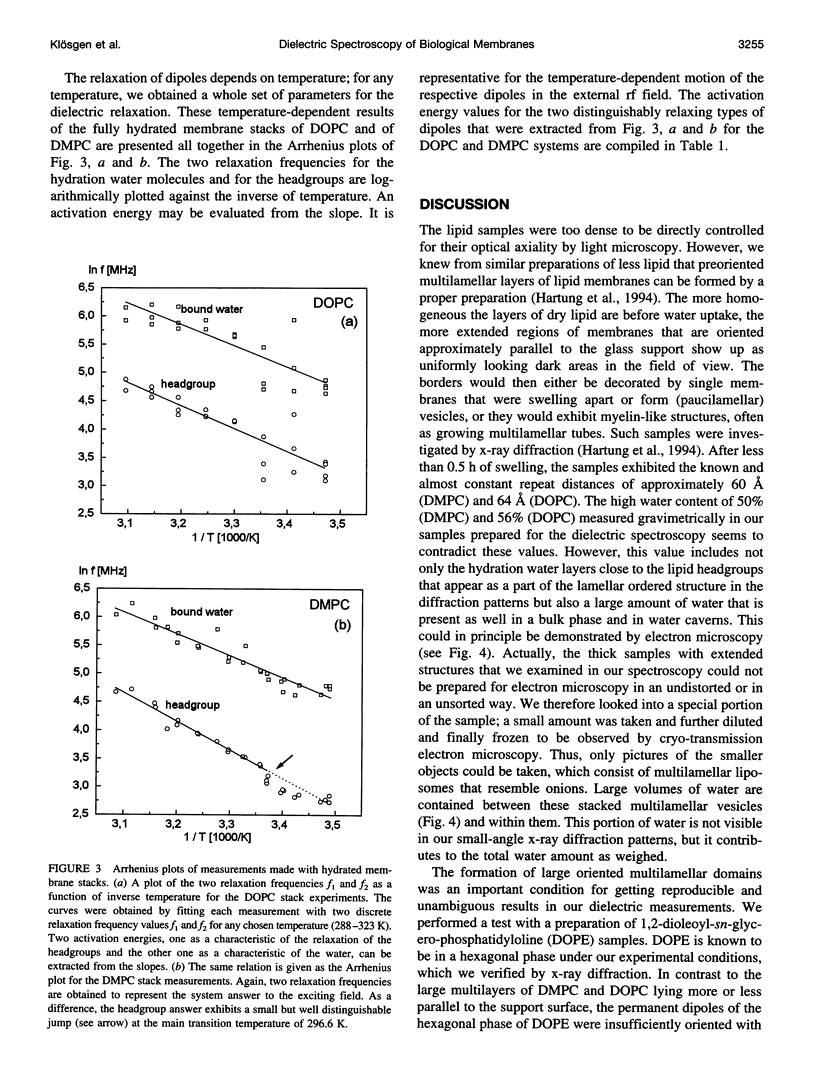
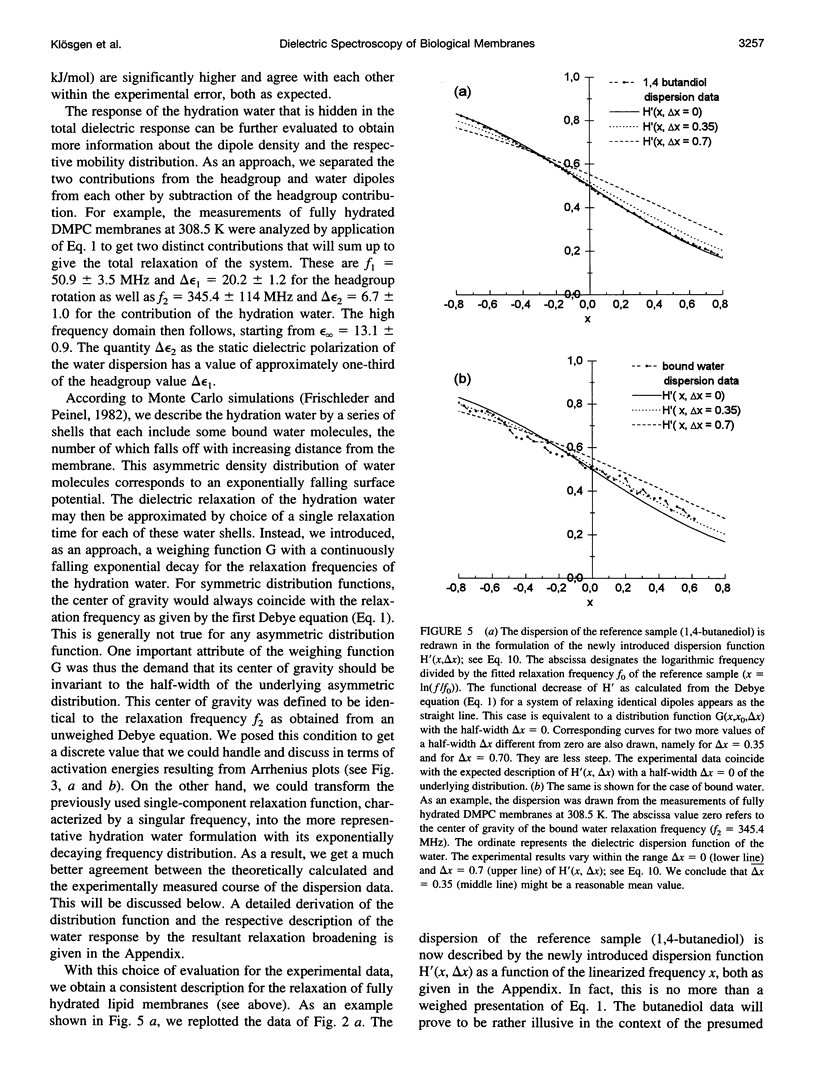
Dielectric spectroscopy is based on the response of the permanent dipoles to a driving electric field. The phospholipid membrane systems of dimyristoylphosphatidylcholine and dioleoylphosphatidylcholine can be prepared as samples of multilamellar liposomes with a well known amount of interlamellar water. For optimal resolution in dielectric spectroscopy one has to design the experimental set-up so that the direction of the permanent headgroup dipole moment is mostly parallel to the field vector of the external radio frequency (rf) electric field in this layered system. A newly developed coaxial probe technique makes it possible to sweep the measuring frequency between 1 and 1000 MHz in the temperature range 286-323 K. The response yields both the dispersion (epsilon') and the absorption part (epsilon") of the complex dielectric permittivity, which are attributed to the rotational diffusions of the zwitterionic phosphatidylcholine headgroup and the hydration water, respectively. Although the contributions of the headgroup and the hydration dipole moments to the dielectric relaxation are found to be situated close together, we succeeded in separating them. In the language of the Debye description, we propose to assign the lower frequency portion of the signal response to the relaxation contributed by the headgroups. The respective relaxation frequency is a discrete value in the range of 15-100 MHz and it shows normal temperature dependence. The contribution of the hydration water molecules exhibits a similar behavior in the range of 100-500 MHz but with the attributed relaxation frequency as the center of an asymmetric distribution of frequencies in analogy to simulation models known from the literature. Activation energies are derived for each of these relaxation processes from the Arrhenius plots of the temperature-dependent relaxation frequencies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caffrey M., Hogan J., Mencke A. Kinetics of the barotropic ripple (P beta')/lamellar liquid crystal (L alpha) phase transition in fully hydrated dimyristoylphosphatidylcholine (DMPC) monitored by time-resolved x-ray diffraction. Biophys J. 1991 Aug;60(2):456–466. doi: 10.1016/S0006-3495(91)82072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M. The study of lipid phase transition kinetics by time-resolved X-ray diffraction. Annu Rev Biophys Biophys Chem. 1989;18:159–186. doi: 10.1146/annurev.bb.18.060189.001111. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Mayer C., Stohrer J., Althoff G., Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys J. 1992 Jan;61(1):42–57. doi: 10.1016/S0006-3495(92)81814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K., Ruston D., Zimmerberg J., Parsegian V. A., Rand R. P., Fuller N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys J. 1992 May;61(5):1213–1223. doi: 10.1016/S0006-3495(92)81931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klösgen B., Helfrich W. Special features of phosphatidylcholine vesicles as seen in cryo-transmission electron microscopy. Eur Biophys J. 1993;22(5):329–340. doi: 10.1007/BF00213556. [DOI] [PubMed] [Google Scholar]
- Murthy N. S., Worthington C. R. X-ray diffraction evidence for the presence of discrete water layers on the surface of membranes. Biochim Biophys Acta. 1991 Feb 25;1062(2):172–176. doi: 10.1016/0005-2736(91)90389-p. [DOI] [PubMed] [Google Scholar]
- Pottel R., Göpel K. D., Henze R., Kaatze U., Uhlendorf V. The dielectric permittivity spectrum of aqueous colloidal phospholipid solutions between 1 kHz and 60 GHz. Biophys Chem. 1984 May;19(3):233–244. doi: 10.1016/0301-4622(84)87005-2. [DOI] [PubMed] [Google Scholar]
- Ulrich A. S., Watts A. Molecular response of the lipid headgroup to bilayer hydration monitored by 2H-NMR. Biophys J. 1994 May;66(5):1441–1449. doi: 10.1016/S0006-3495(94)80934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volke F., Eisenblätter S., Galle J., Klose G. Dynamic properties of water at phosphatidylcholine lipid-bilayer surfaces as seen by deuterium and pulsed field gradient proton NMR. Chem Phys Lipids. 1994 Apr 19;70(2):121–131. doi: 10.1016/0009-3084(94)90080-9. [DOI] [PubMed] [Google Scholar]



