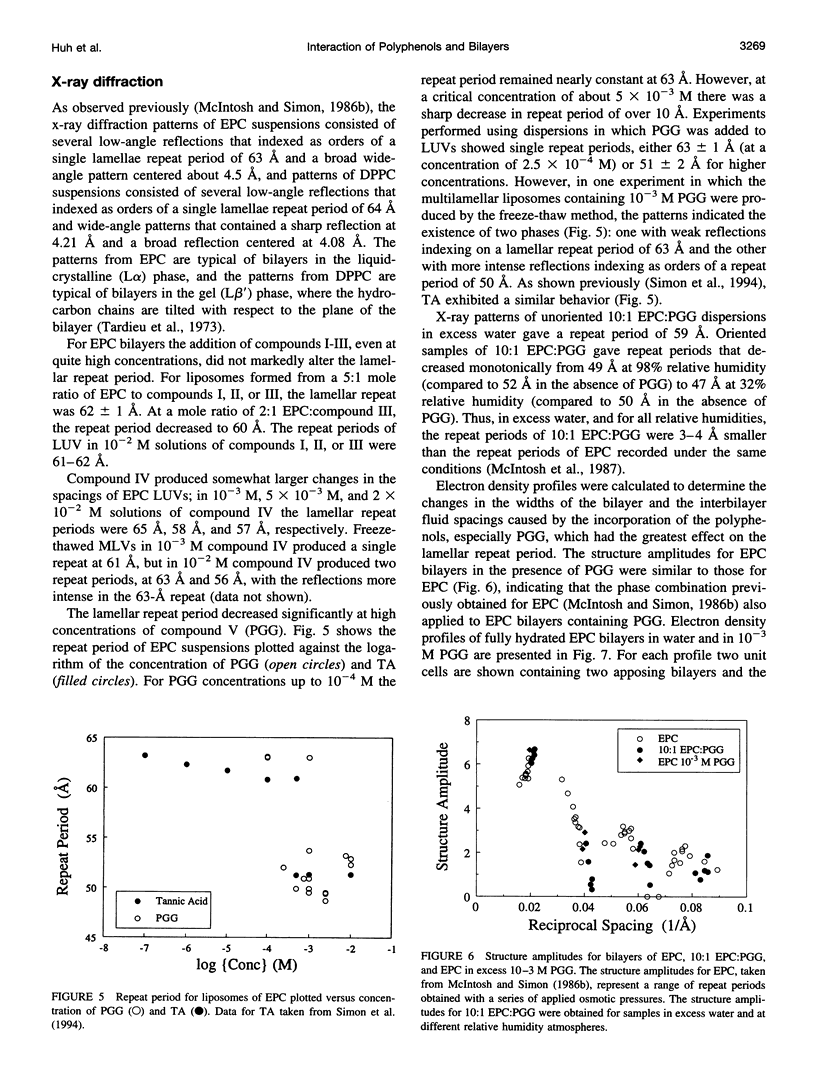
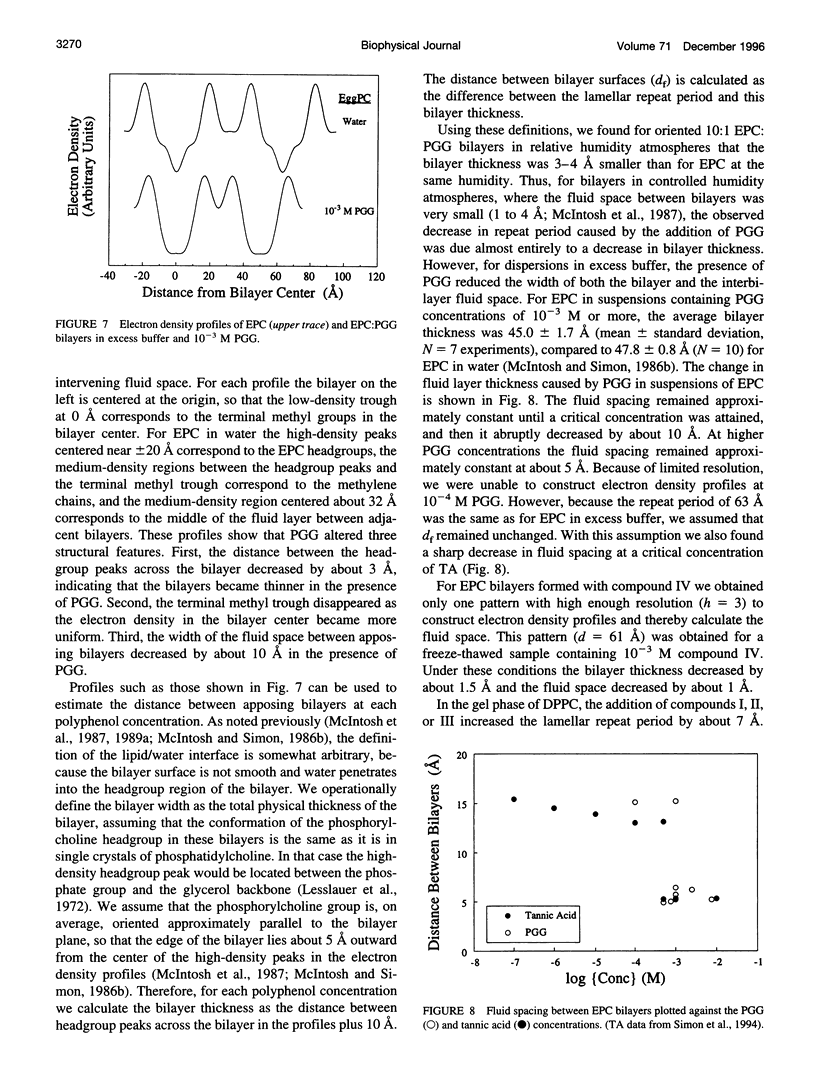
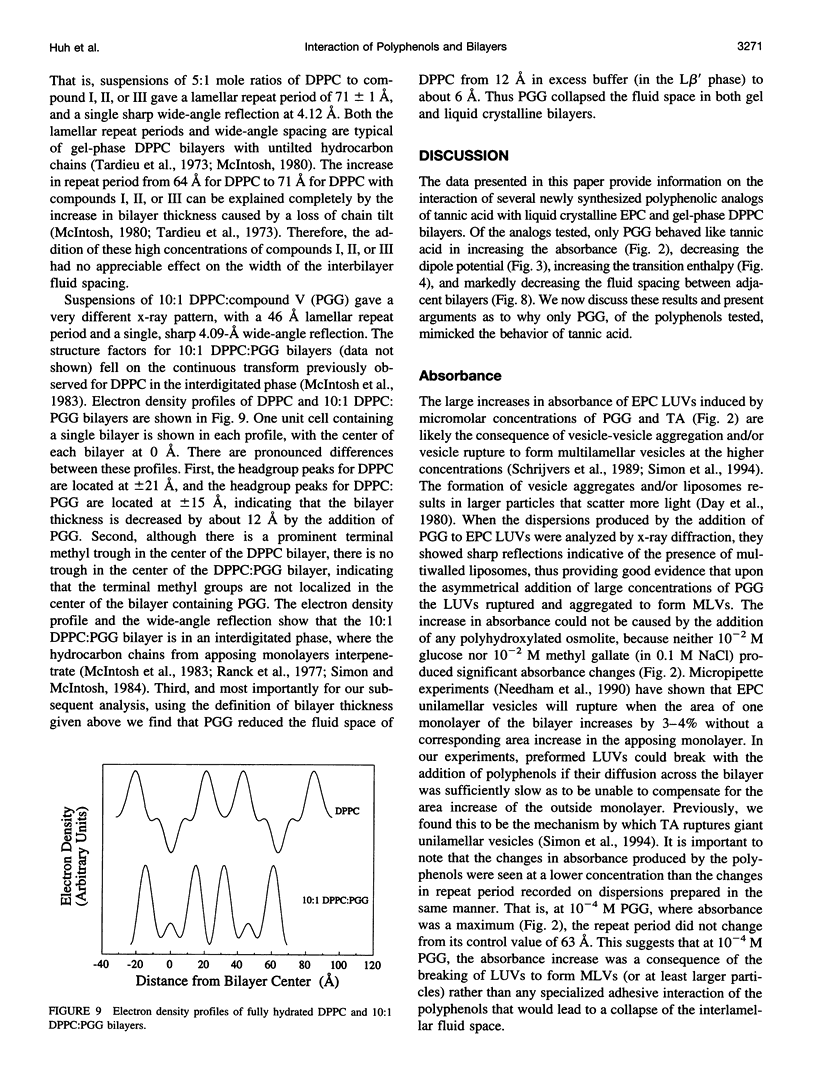
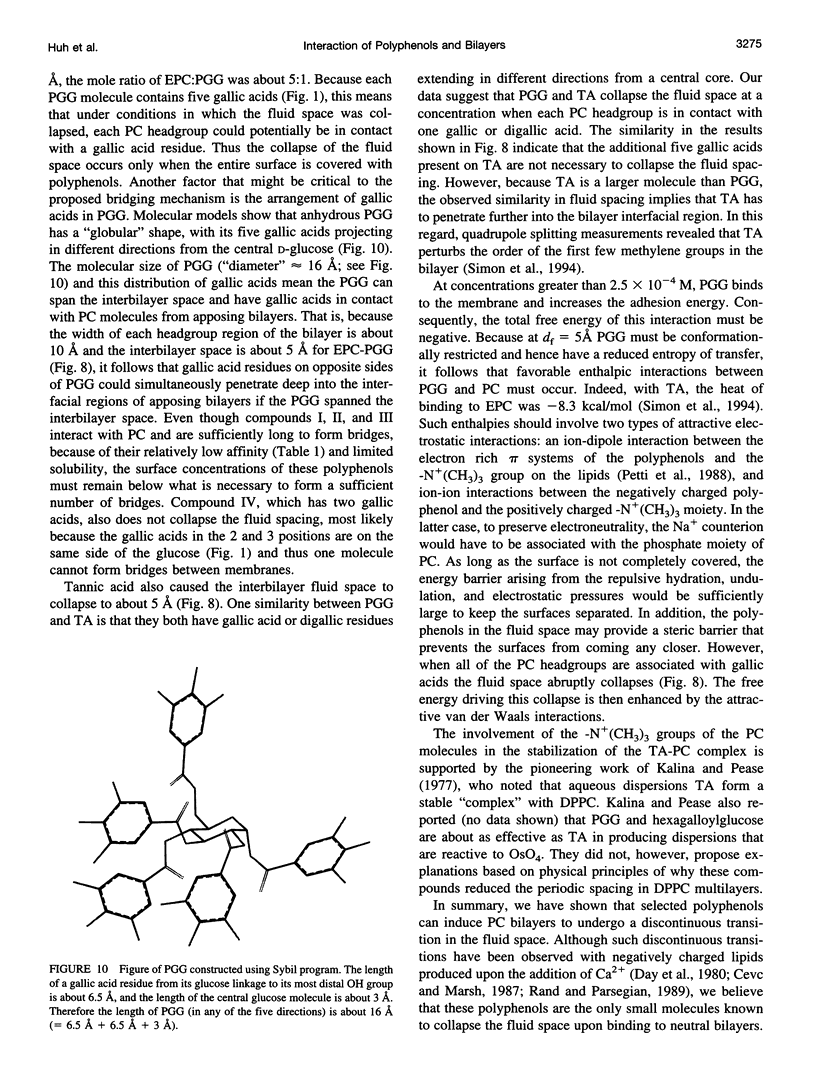
Abstract
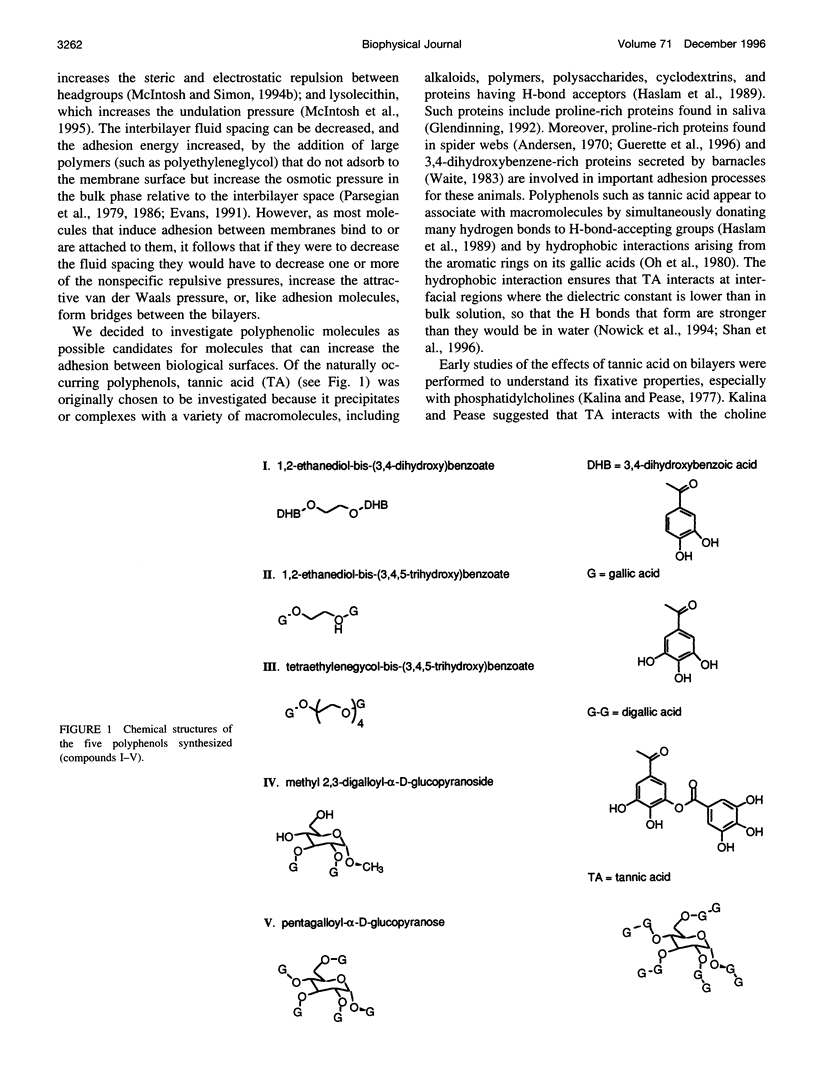
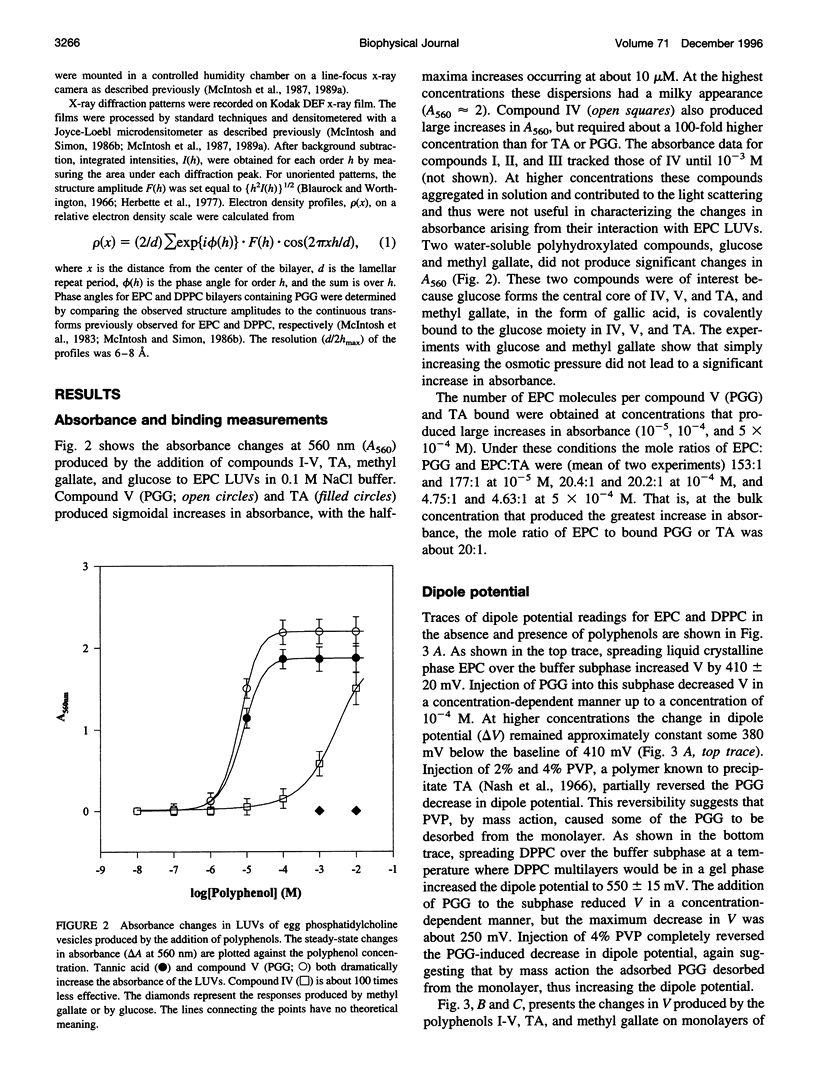
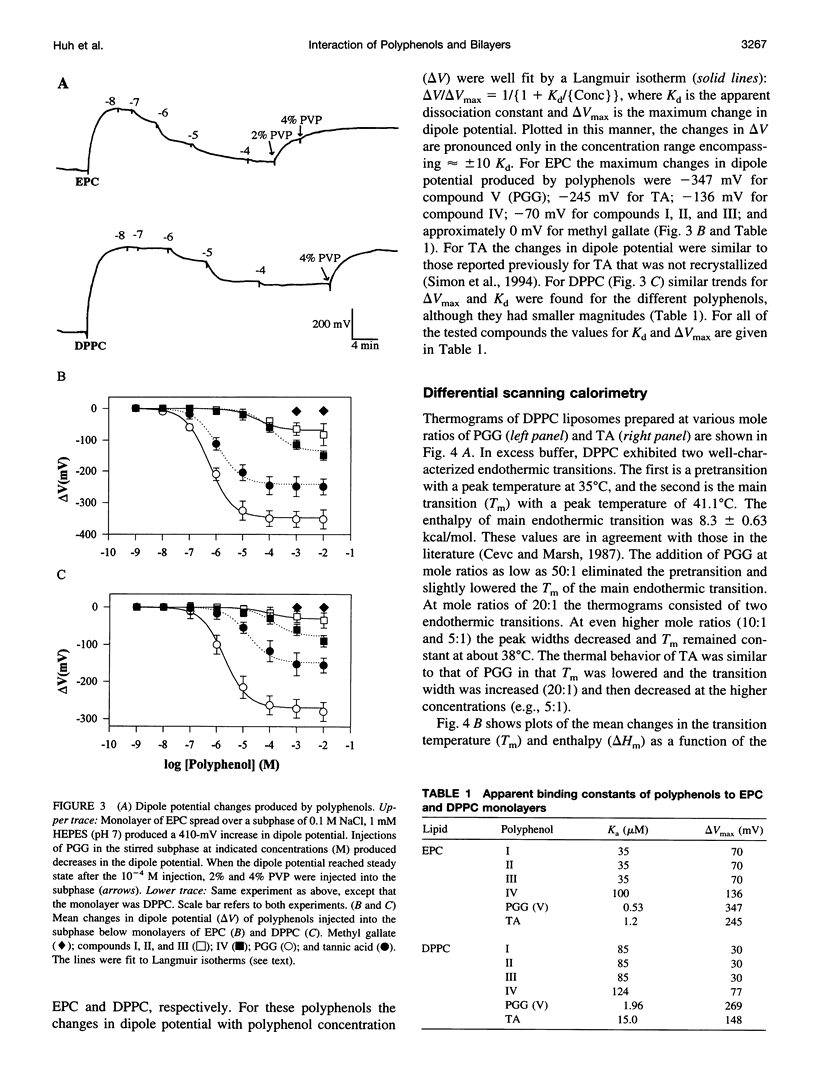
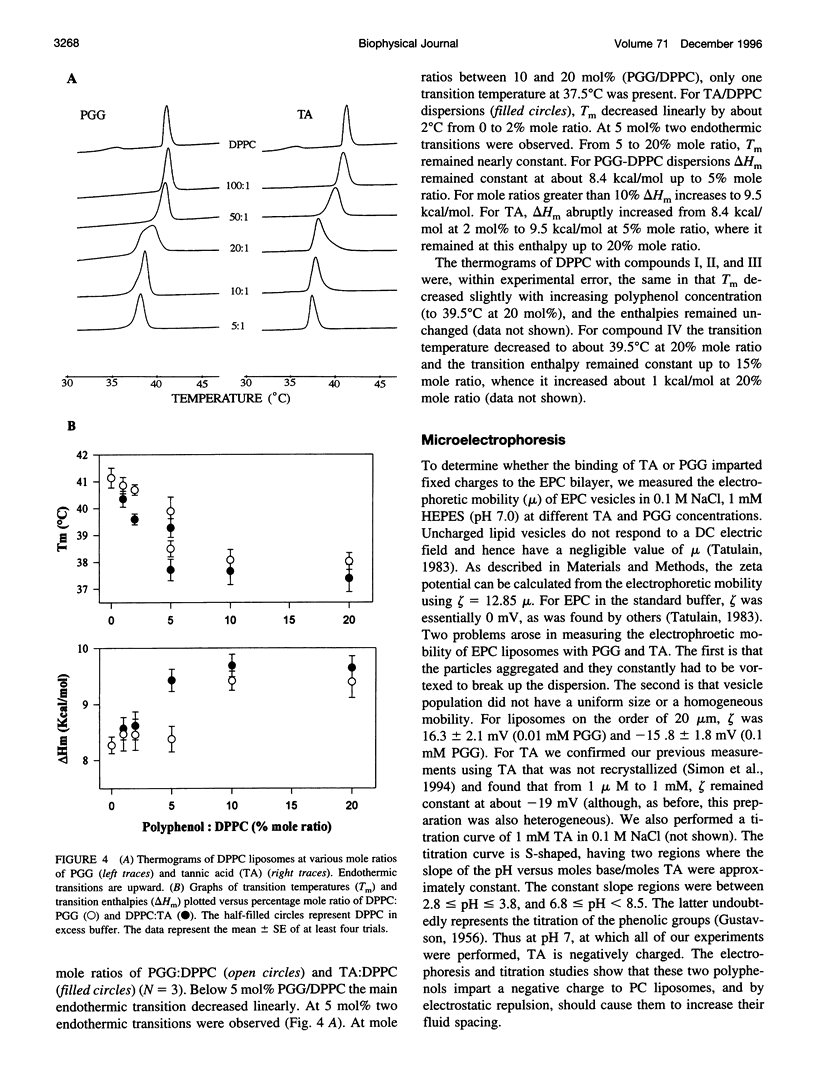
Because proteins and other molecules with a high polyphenol content are commonly involved in adhesion processes, we are investigating the interactions between polyphenols and biological materials. A naturally occurring polyphenol that binds a variety of proteins and lipids is tannic acid (TA), which contains five digallic acid residues covalently linked to a central D-glucose. A previous study has shown that TA increases the adhesion between apposing phosphatidylcholine (PC) bilayers and over a very narrow concentration range collapses the interbilayer fluid space from about 15 A to 5 A. To determine the chemical requirements a polyphenolic molecule must possess to increase bilayer adhesion, we have synthesized several simpler TA analogs that vary in their size, shape, and number of gallic acid and hydroxyl groups. X-ray diffraction, absorbance, binding, and differential scanning calorimetry measurements were used to investigate the interaction of these polyphenolic molecules with egg PC (EPC) and dipalmitoyl PC (DPPC) bilayers. Of these synthetic polyphenols, only penta-O-galloyl-alpha-D-glucose (PGG) was able to completely mimic the effects of TA by collapsing the interbilayer fluid space from 15 A to 5 A, decreasing the dipole potential by about 300 mV, increasing the transition enthalpy of DPPC liposomes, and inducing an interdigitated phase in DPPC. Binding studies indicated that the fluid space was reduced to 5 A at an EPC:PGG mole ratio of 5:1. We conclude that these polyphenols collapse the fluid space of PC bilayers because they 1) are amphipathic and partition into the bilayers interfacial region, 2) are long enough to span the interbilayer space, 3) contain several gallic acids distributed so that they can partition simultaneously into apposing bilayers, and 4) have sufficient gallic acid residues to interact with all lipid headgroups and cover the bilayer surface. Under these conditions we conclude that the polyphenols from interbilayer bridges. We argue that these bridges are stabilized by increased adhesion arising from an increased van der Waals interaction between apposing bilayers, electrostatic interactions between the pi electrons in the phenol ring and the -(N+CH3)3 groups on the PC headgroups, decreased hydration repulsion between bilayers, and hydrogen bonds between the H-bond-donating moieties on the polyphenols and H-bond-accepting groups in the bilayer.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attard P., Mitchell D. J., Ninham B. W. The attractive forces between polar lipid bilayers. Biophys J. 1988 Mar;53(3):457–460. doi: 10.1016/S0006-3495(88)83122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCKMAN H. Dipole potential of lipid membranes. Chem Phys Lipids. 1994 Sep 6;73(1-2):57–79. doi: 10.1016/0009-3084(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Worthington C. R. Treatment of low angle x-ray data from planar and concentric multilayered structures. Biophys J. 1966 May;6(3):305–312. doi: 10.1016/S0006-3495(66)86658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry B. Z., Lipka G., Sturtevant J. M. Thermodynamics of phospholipid-sucrose interactions. Biophys J. 1984 Sep;46(3):419–422. doi: 10.1016/S0006-3495(84)84038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran K. V., Merz K. M., Jr A comparison of DMPC- and DLPE-based lipid bilayers. Biophys J. 1994 Apr;66(4):1076–1087. doi: 10.1016/S0006-3495(94)80889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day E. P., Kwok A. Y., Hark S. K., Ho J. T., Vail W. J., Bentz J., Nir S. Reversibility of sodium-induced aggregation of sonicated phosphatidylserine vesicles. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4026–4029. doi: 10.1073/pnas.77.7.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Thermal-mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerette P. A., Ginzinger D. G., Weber B. H., Gosline J. M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science. 1996 Apr 5;272(5258):112–115. doi: 10.1126/science.272.5258.112. [DOI] [PubMed] [Google Scholar]
- Haslam E., Lilley T. H., Cai Y., Martin R., Magnolato D. Traditional herbal medicines--the role of polyphenols. Planta Med. 1989 Feb;55(1):1–8. doi: 10.1055/s-2006-961764. [DOI] [PubMed] [Google Scholar]
- Haslam E. Polyphenol-protein interactions. Biochem J. 1974 Apr;139(1):285–288. doi: 10.1042/bj1390285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Herbette L., Marquardt J., Scarpa A., Blasie J. K. A direct analysis of lamellar x-ray diffraction from hydrated oriented multilayers of fully functional sarcoplasmic reticulum. Biophys J. 1977 Nov;20(2):245–272. doi: 10.1016/S0006-3495(77)85547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert J. P., Jr, Kiernan P. D., Beahrs O. H., McConahey W. M., Woolner L. B. Occult papillary carcinoma of the thyroid. Arch Surg. 1980 Apr;115(4):394–398. doi: 10.1001/archsurg.1980.01380040028004. [DOI] [PubMed] [Google Scholar]
- Kalina M., Pease D. C. The preservation of ultrastructure in saturated phosphatidyl cholines by tannic acid in model systems and type II pneumocytes. J Cell Biol. 1977 Sep;74(3):726–741. doi: 10.1083/jcb.74.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminoh Y., Tashiro C., Kamaya H., Ueda I. Depression of phase-transition temperature by anesthetics: nonzero solid membrane binding. Biochim Biophys Acta. 1988 Dec 22;946(2):215–220. doi: 10.1016/0005-2736(88)90395-1. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesslauer W., Cain J. E., Blasie J. K. X-ray diffraction studies of lecithin bimolecular leaflets with incorporated fluorescent probes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1499–1503. doi: 10.1073/pnas.69.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A. Lipid monolayer states and their relationships to bilayers. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Advani S., Burton R. E., Zhelev D. V., Needham D., Simon S. A. Experimental tests for protrusion and undulation pressures in phospholipid bilayers. Biochemistry. 1995 Jul 11;34(27):8520–8532. doi: 10.1021/bi00027a002. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. Differences in hydrocarbon chain tilt between hydrated phosphatidylethanolamine and phosphatidylcholine bilayers. A molecular packing model. Biophys J. 1980 Feb;29(2):237–245. doi: 10.1016/S0006-3495(80)85128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry. 1989 Jan 10;28(1):17–25. doi: 10.1021/bi00427a004. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Range of the solvation pressure between lipid membranes: dependence on the packing density of solvent molecules. Biochemistry. 1989 Sep 19;28(19):7904–7912. doi: 10.1021/bi00445a053. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Repulsive interactions between uncharged bilayers. Hydration and fluctuation pressures for monoglycerides. Biophys J. 1989 May;55(5):897–904. doi: 10.1016/S0006-3495(89)82888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Steric repulsion between phosphatidylcholine bilayers. Biochemistry. 1987 Nov 17;26(23):7325–7332. doi: 10.1021/bi00397a020. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 1986 Aug 26;25(17):4948–4952. doi: 10.1021/bi00365a034. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Contributions of hydration and steric (entropic) pressures to the interactions between phosphatidylcholine bilayers: experiments with the subgel phase. Biochemistry. 1993 Aug 17;32(32):8374–8384. doi: 10.1021/bi00083a042. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Ellington J. C., Jr, Porter N. A. New structural model for mixed-chain phosphatidylcholine bilayers. Biochemistry. 1984 Aug 28;23(18):4038–4044. doi: 10.1021/bi00313a005. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration and steric pressures between phospholipid bilayers. Annu Rev Biophys Biomol Struct. 1994;23:27–51. doi: 10.1146/annurev.bb.23.060194.000331. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Long- and short-range interactions between phospholipid/ganglioside GM1 bilayers. Biochemistry. 1994 Aug 30;33(34):10477–10486. doi: 10.1021/bi00200a032. [DOI] [PubMed] [Google Scholar]
- Nash T., Allison A. C., Harington J. S. Physico-chemical properties of silica in relation to its toxicity. Nature. 1966 Apr 16;210(5033):259–261. doi: 10.1038/210259a0. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Ranck J. L., Keira T., Luzzati V. A novel packing of the hydrocarbon chains in lipids. The low temperature phases of dipalmitoyl phosphatidyl-glycerol. Biochim Biophys Acta. 1977 Sep 28;488(3):432–441. doi: 10.1016/0005-2760(77)90201-6. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Rudolph A. S., Goins B. The effect of hydration stress solutes on the phase behavior of hydrated dipalmitoylphosphatidylcholine. Biochim Biophys Acta. 1991 Jul 1;1066(1):90–94. doi: 10.1016/0005-2736(91)90255-7. [DOI] [PubMed] [Google Scholar]
- Schrijvers A. H., Frederik P. M., Stuart M. C., Burger K. N., Heijnen V. V., Van der Vusse G. J., Reneman R. S. Formation of multilamellar vesicles by addition of tannic acid to phosphatidylcholine-containing small unilamellar vesicles. J Histochem Cytochem. 1989 Nov;37(11):1635–1643. doi: 10.1177/37.11.2809174. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Shan S. O., Loh S., Herschlag D. The energetics of hydrogen bonds in model systems: implications for enzymatic catalysis. Science. 1996 Apr 5;272(5258):97–101. doi: 10.1126/science.272.5258.97. [DOI] [PubMed] [Google Scholar]
- Simon S. A., Advani S., McIntosh T. J. Temperature dependence of the repulsive pressure between phosphatidylcholine bilayers. Biophys J. 1995 Oct;69(4):1473–1483. doi: 10.1016/S0006-3495(95)80017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Disalvo E. A., Gawrisch K., Borovyagin V., Toone E., Schiffman S. S., Needham D., McIntosh T. J. Increased adhesion between neutral lipid bilayers: interbilayer bridges formed by tannic acid. Biophys J. 1994 Jun;66(6):1943–1958. doi: 10.1016/S0006-3495(94)80988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Fink C. A., Kenworthy A. K., McIntosh T. J. The hydration pressure between lipid bilayers. Comparison of measurements using x-ray diffraction and calorimetry. Biophys J. 1991 Mar;59(3):538–546. doi: 10.1016/S0006-3495(91)82270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim Biophys Acta. 1984 Jun 13;773(1):169–172. doi: 10.1016/0005-2736(84)90562-5. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Magid A. D., Needham D. Modulation of the interbilayer hydration pressure by the addition of dipoles at the hydrocarbon/water interface. Biophys J. 1992 Mar;61(3):786–799. doi: 10.1016/S0006-3495(92)81883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Magnitude of the solvation pressure depends on dipole potential. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9263–9267. doi: 10.1073/pnas.86.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejtek P., Wang S. R. Adsorption to dipalmitoylphosphatidylcholine membranes in gel and fluid state: pentachlorophenolate, dipicrylamine, and tetraphenylborate. Biophys J. 1990 Nov;58(5):1285–1294. doi: 10.1016/S0006-3495(90)82468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Swamy M. J., Marsh D. Thermodynamics of interdigitated phases of phosphatidylcholine in glycerol. Biophys J. 1995 Oct;69(4):1402–1408. doi: 10.1016/S0006-3495(95)80009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Tatulian S. A. Effect of lipid phase transition on the binding of anions to dimyristoylphosphatidylcholine liposomes. Biochim Biophys Acta. 1983 Dec 21;736(2):189–195. doi: 10.1016/0005-2736(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Waite J. H. Evidence for a repeating 3,4-dihydroxyphenylalanine- and hydroxyproline-containing decapeptide in the adhesive protein of the mussel, Mytilus edulis L. J Biol Chem. 1983 Mar 10;258(5):2911–2915. [PubMed] [Google Scholar]
- Zheng C., Vanderkooi G. Molecular origin of the internal dipole potential in lipid bilayers: calculation of the electrostatic potential. Biophys J. 1992 Oct;63(4):935–941. doi: 10.1016/S0006-3495(92)81673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


