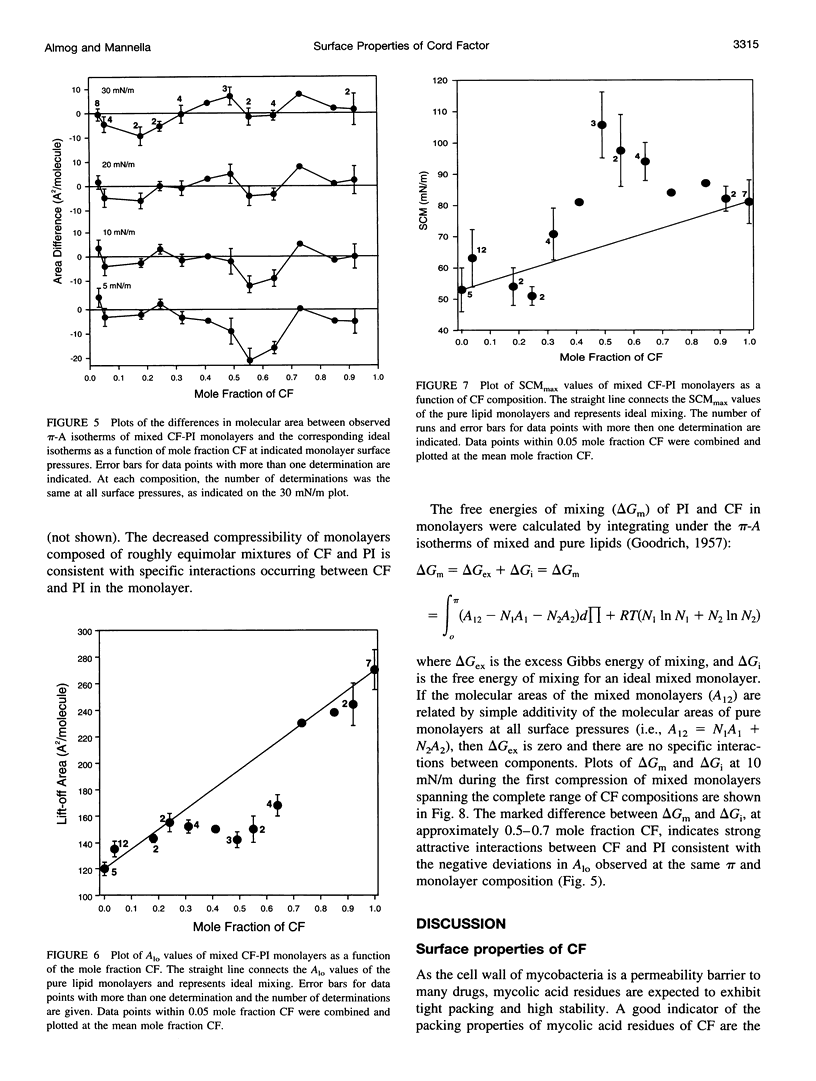
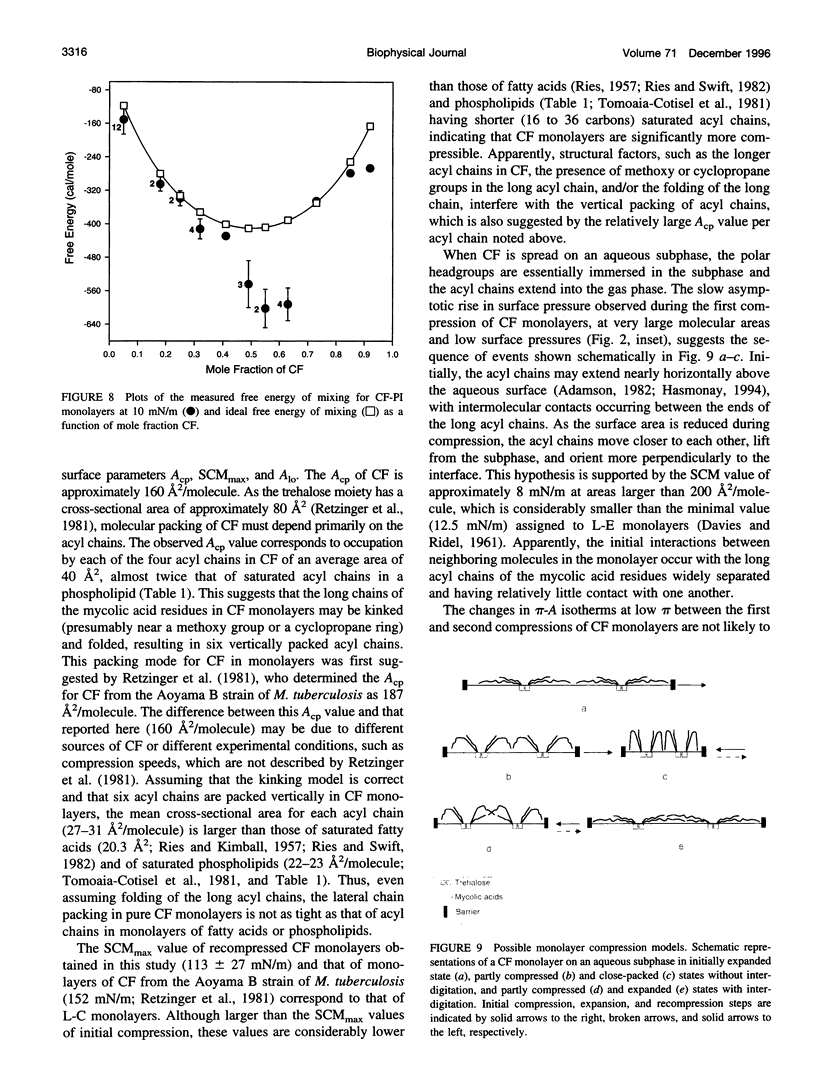
Abstract
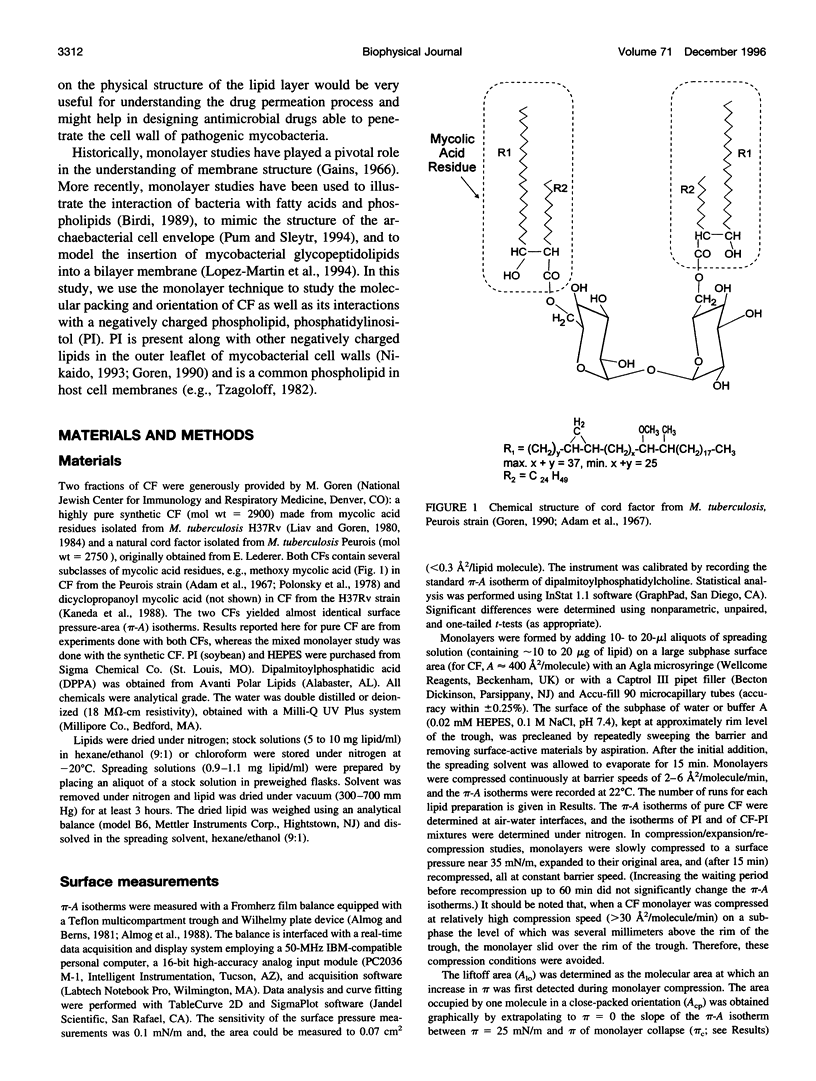
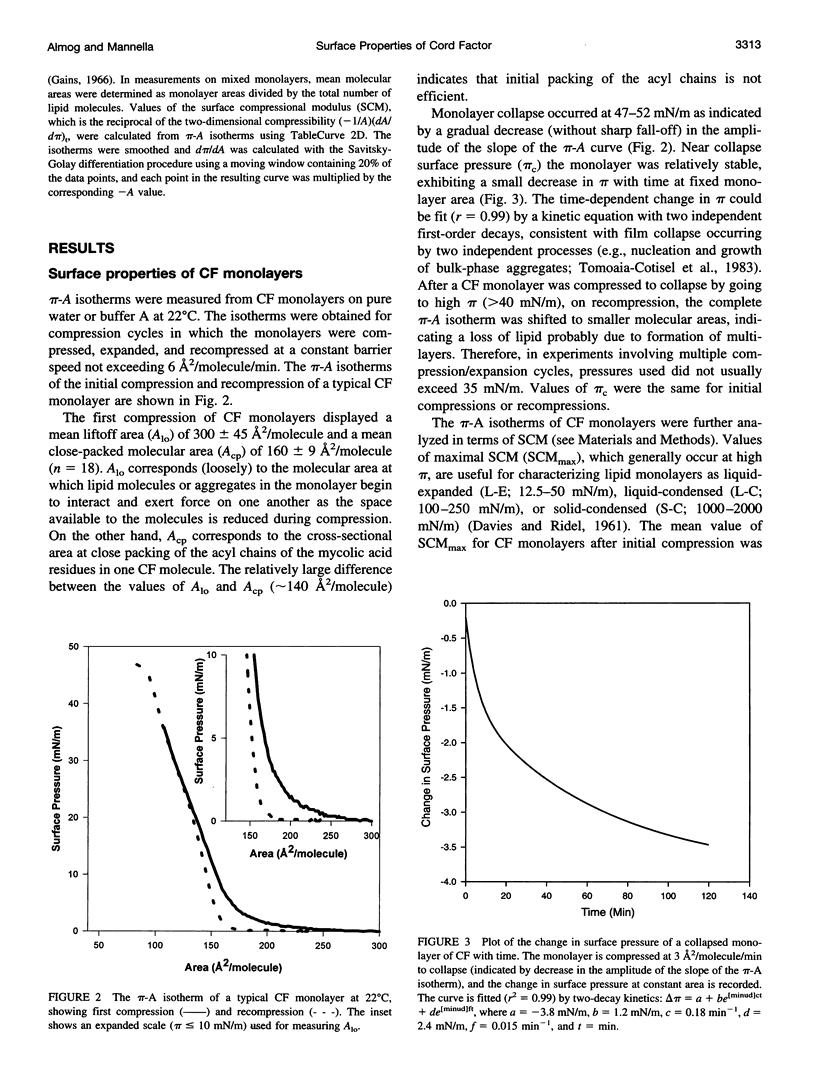
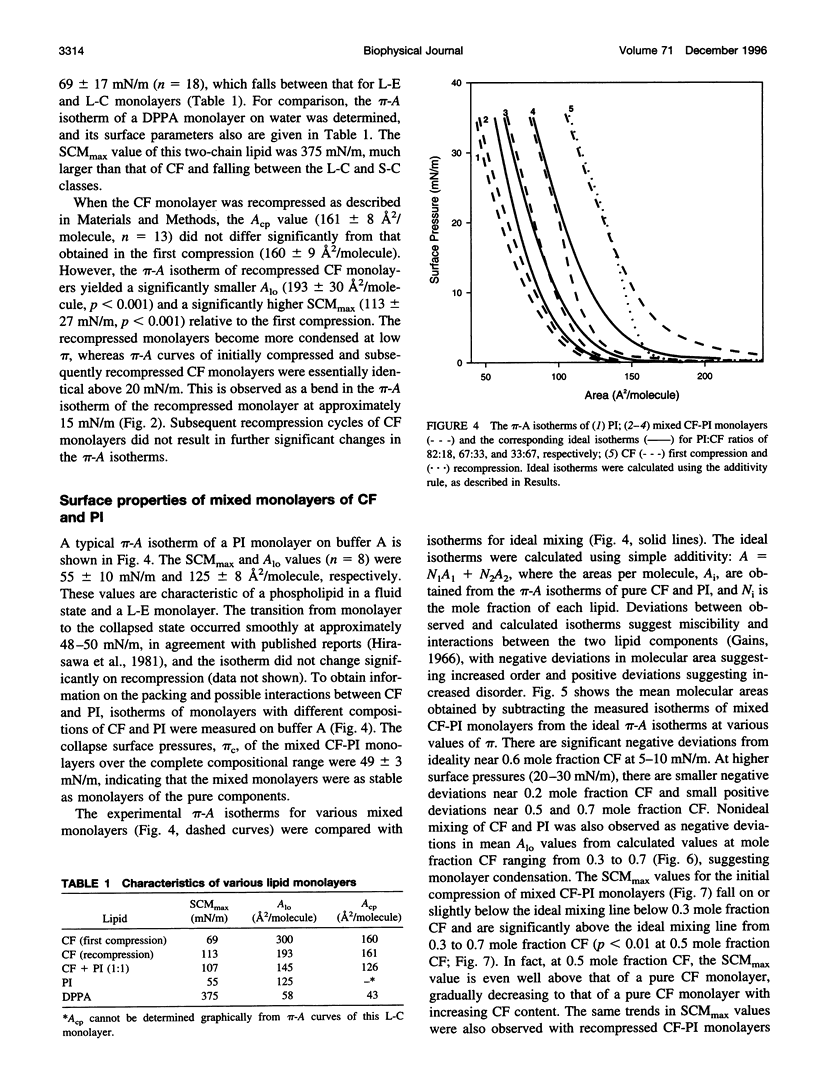
Cord factor (trehalose 6,6'-dimycolate, CF) is a glycolipid located in the outer mycobacterial cell wall that is implicated in the pathogenesis of mycobacteria. Furthermore, CF is a convenient model for studying mycolic acid residues, the major lipid constituents of the mycobacterial cell wall that are believed to form a barrier against drug penetration. The surface properties of CF and its interactions with phosphatidylinositol (PI) have been investigated using the monolayer technique. During compression/expansion/recompression cycles, CF monolayers switch from a loosely packed to a more tightly packed structure. The change in surface properties suggests a molecular rearrangement, perhaps involving interdigitation of long and short chains of the CF molecules. In CF-PI monolayers, maximal lateral packing density occurs between 0.5 and 0.7 mole fraction CF, which is close to the relative composition of mycolic acid residues and shorter-chain lipids in the mycobacterial cell wall. Low concentrations of CF increase the order in PI monolayers, consistent with CF toxicity involving rigidification of cell membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Senn M., Vilkas E., Lederer E. Spectrométrie de masse de glycolipides. 2. Diesters de tréhalose naturels et synthétiques. Eur J Biochem. 1967 Nov;2(4):460–468. doi: 10.1111/j.1432-1033.1967.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Almog R., Marsilio F., Berns D. S. Interaction of C-phycocyanin with lipid monolayers under nitrogen and in the presence of air. Arch Biochem Biophys. 1988 Jan;260(1):28–36. doi: 10.1016/0003-9861(88)90420-1. [DOI] [PubMed] [Google Scholar]
- Asselineau C., Asselineau J. Trehalose-containing glycolipids. Prog Chem Fats Other Lipids. 1978;16:59–99. doi: 10.1016/0079-6832(78)90037-x. [DOI] [PubMed] [Google Scholar]
- Behling C. A., Bennett B., Takayama K., Hunter R. L. Development of a trehalose 6,6'-dimycolate model which explains cord formation by Mycobacterium tuberculosis. Infect Immun. 1993 Jun;61(6):2296–2303. doi: 10.1128/iai.61.6.2296-2303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs J. M., Tümmler B. Interdigitated gel phase bilayers formed by unsaturated synthetic and bacterial glycerolipids in the presence of polymyxin B and glycerol. Biochim Biophys Acta. 1993 Jan 18;1145(1):42–50. doi: 10.1016/0005-2736(93)90379-e. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Crowe L. M., Spargo B. J., Ioneda T., Beaman B. L., Crowe J. H. Interaction of cord factor (alpha, alpha'-trehalose-6,6'-dimycolate) with phospholipids. Biochim Biophys Acta. 1994 Aug 24;1194(1):53–60. doi: 10.1016/0005-2736(94)90202-x. [DOI] [PubMed] [Google Scholar]
- David H. L., Lévy-Frébault V., Thorel M. F. Characterization of distinct layers of the Mycobacterium avium envelope in respect of their composition by fatty acids, proteins, oligosaccharides and antigens. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):193–208. doi: 10.1016/s0176-6724(88)80003-8. [DOI] [PubMed] [Google Scholar]
- Durand E., Gillois M., Tocanne J. F., Laneelle G. Property and activity of mycoloyl esters of methyl glucoside and trehalose. Effect on mitochondrial oxidative phosphorylation related to organization of suspensions and to acyl-chain structures. Eur J Biochem. 1979 Feb 15;94(1):109–118. doi: 10.1111/j.1432-1033.1979.tb12877.x. [DOI] [PubMed] [Google Scholar]
- Durand E., Welby M., Laneelle G., Tocanne J. F. Phase behaviour of cord factor and related bacterial glycolipid toxins. A monolayer study. Eur J Biochem. 1979 Jan 2;93(1):103–112. doi: 10.1111/j.1432-1033.1979.tb12799.x. [DOI] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. The hydrolysis of phosphatidylinositol monolayers at an air/water interface by the calcium-ion-dependent phosphatidylinositol phosphodiesterase of pig brain. Biochem J. 1981 Feb 1;193(2):607–614. doi: 10.1042/bj1930607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Mason J. T. Structure and properties of mixed-chain phospholipid assemblies. Biochim Biophys Acta. 1986 Dec 22;864(3-4):423–470. doi: 10.1016/0304-4157(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Kaneda K., Imaizumi S., Mizuno S., Baba T., Tsukamura M., Yano I. Structure and molecular species composition of three homologous series of alpha-mycolic acids from Mycobacterium spp. J Gen Microbiol. 1988 Aug;134(8):2213–2229. doi: 10.1099/00221287-134-8-2213. [DOI] [PubMed] [Google Scholar]
- Lanéelle G., Daffé M. Mycobacterial cell wall and pathogenicity: a lipodologist's view. Res Microbiol. 1991 May;142(4):433–437. doi: 10.1016/0923-2508(91)90116-r. [DOI] [PubMed] [Google Scholar]
- Lopez-Marin L. M., Quesada D., Lakhdar-Ghazal F., Tocanne J. F., Lanéelle G. Interactions of mycobacterial glycopeptidolipids with membranes: influence of carbohydrate on induced alterations. Biochemistry. 1994 Jun 14;33(23):7056–7061. doi: 10.1021/bi00189a006. [DOI] [PubMed] [Google Scholar]
- McNeil M. R., Brennan P. J. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol. 1991 May;142(4):451–463. doi: 10.1016/0923-2508(91)90120-y. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Kim S. H., Rosenberg E. Y. Physical organization of lipids in the cell wall of Mycobacterium chelonae. Mol Microbiol. 1993 Jun;8(6):1025–1030. doi: 10.1111/j.1365-2958.1993.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Retzinger G. S., Meredith S. C., Takayama K., Hunter R. L., Kézdy F. J. The role of surface in the biological activities of trehalose 6,6'-dimycolate. Surface properties and development of a model system. J Biol Chem. 1981 Aug 10;256(15):8208–8216. [PubMed] [Google Scholar]
- Sut A., Sirugue S., Sixou S., Lakhdar-Ghazal F., Tocanne J. F., Lanéelle G. Mycobacteria glycolipids as potential pathogenicity effectors: alteration of model and natural membranes. Biochemistry. 1990 Sep 11;29(36):8498–8502. doi: 10.1021/bi00488a042. [DOI] [PubMed] [Google Scholar]
- Woodbury J. L., Barrow W. W. Radiolabelling of Mycobacterium avium oligosaccharide determinant and use in macrophage studies. J Gen Microbiol. 1989 Jul;135(7):1875–1884. doi: 10.1099/00221287-135-7-1875. [DOI] [PubMed] [Google Scholar]