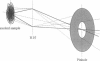
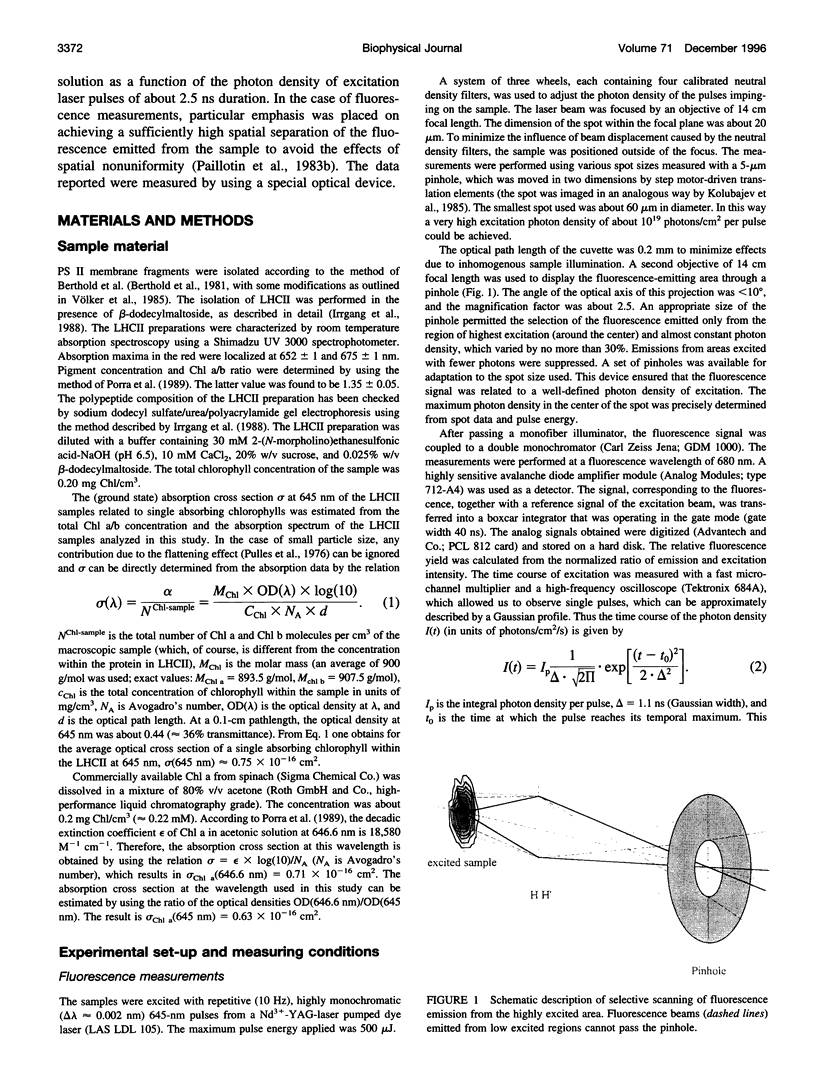
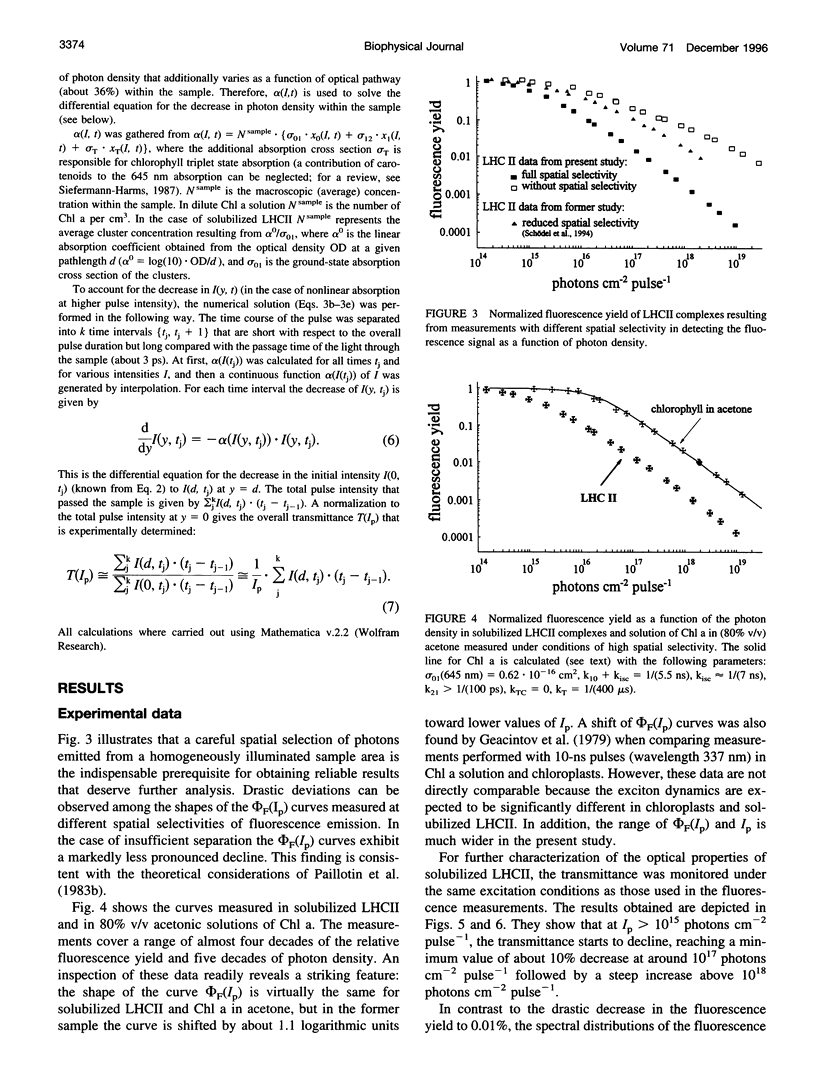
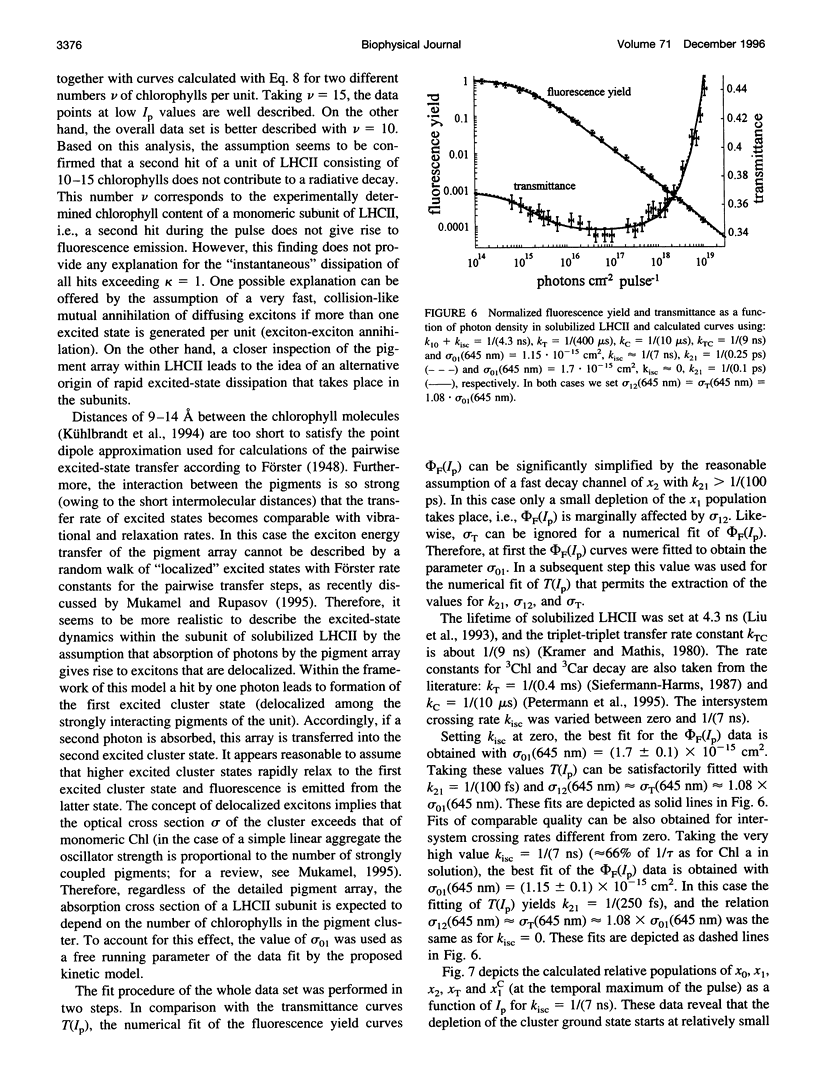
Abstract
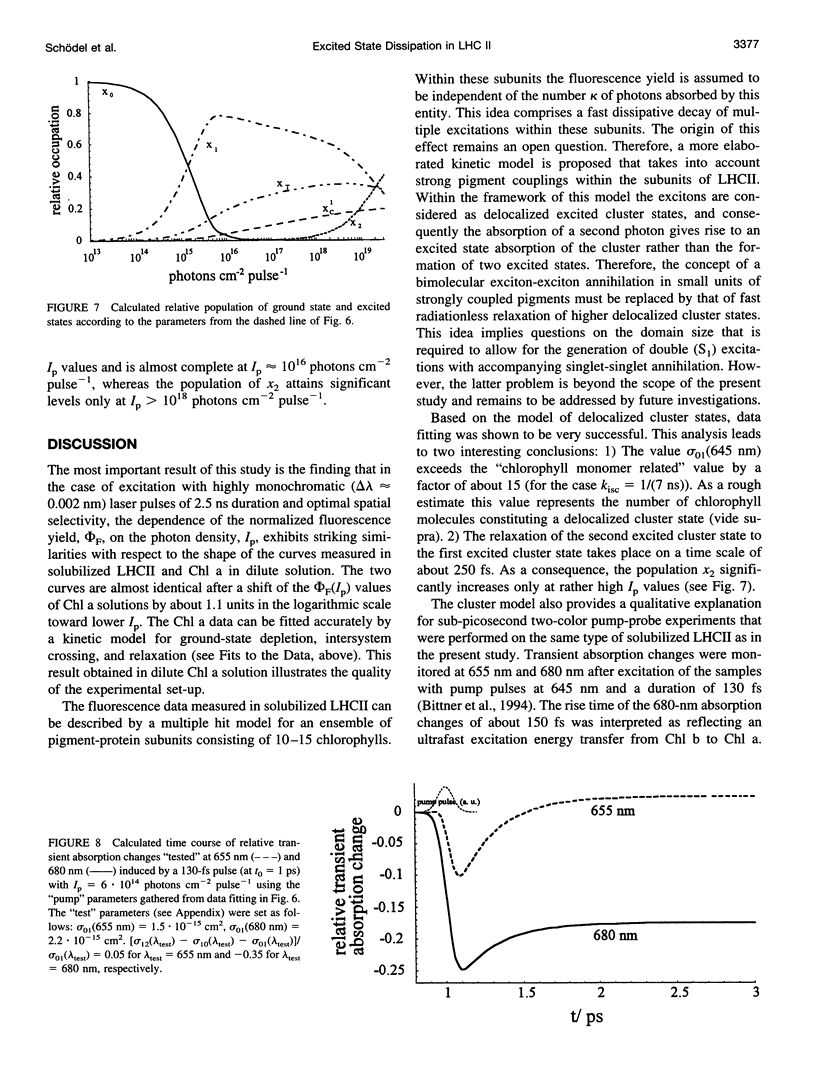
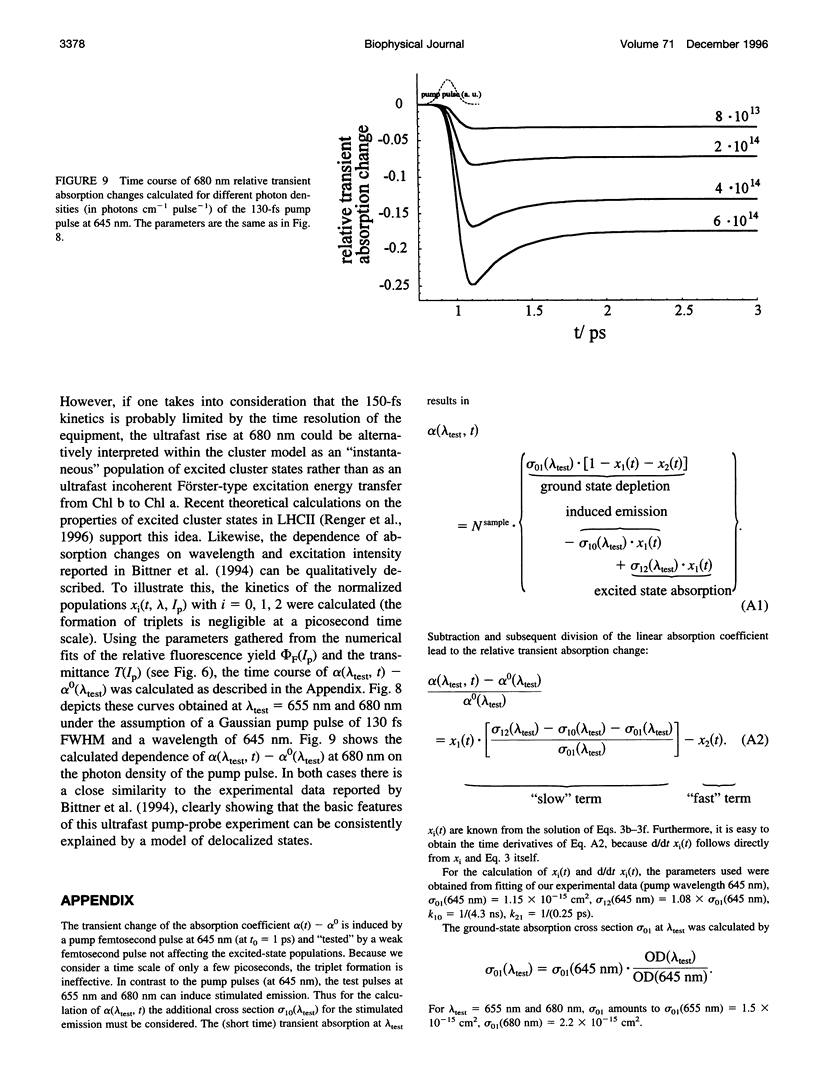
Relative fluorescence yield, phi F, and transmittance, T, were measured in solubilized light-harvesting complex II (LHCII) as a function of photon density, Ip, of monochromatic 645-nm laser pulses (duration: approximately 2.5 ns). Special efforts were made in constructing an optical set-up that allows the accurate determination of the fluorescence from an area of constant Ip, phi F(Ip) starts to decline at approximately 10(14) and drops to values below 0.01% at maximum Ip (approximately 10(19) photons cm-2 pulse-1). T(Ip) decreases only slightly at photon densities of approximately 10(15) but increases steeply at values of > 10(17) photons cm-2 pulse-1. The interpretation of the phi F(Ip) data using the saturation limit of Mauzerall's multiple hit model leads to a unit size of about 10-15 chlorophyll molecules. One interpretation is to attribute this result to a very fast exciton-exciton annihilation of multiple excited states generated within this small domain. Alternatively, based on the assumption that delocalized cluster states within the monomeric/trimeric subunit of LHCII exist, the results can be consistently described by a kinetic model comprising ground, monoexcitonic, and biexcitonic states of clusters and a triplet state that is quenched by carotenoids in LHCII. Within the framework of this model the annihilation of multiple excitations is explained as ultrafast radiationless relaxation of higher excited cluster states. Comparative measurements in diluted acetonic Chl a solution are consistently described by the depletion of the ground state, taking the absorption cross section at the used wavelength.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breton J., Geacintov N. E. Picosecond fluorescence kinetics and fast energy transfer processes in photosynthetic membranes. Biochim Biophys Acta. 1980 Dec 22;594(1):1–32. doi: 10.1016/0304-4173(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Breton J., Geacintov N. E. Quenching of fluorescence of chlorophyll in vivo by long-lived excited states. FEBS Lett. 1976 Oct 15;69(1):86–89. doi: 10.1016/0014-5793(76)80659-x. [DOI] [PubMed] [Google Scholar]
- Campillo A. J., Kollman V. H., Shapiro S. L. Intensity Dependence of the Fluorescence Lifetime of in vivo Chlorophyll Excited by a Picosecond Light Pulse. Science. 1976 Jul 16;193(4249):227–229. doi: 10.1126/science.193.4249.227. [DOI] [PubMed] [Google Scholar]
- Campillo A. J., Shapiro S. L., Kollman V. H., Winn K. R., Hyer R. C. Picosecond exciton annihilation in photosynthetic systems. Biophys J. 1976 Jan;16(1):93–97. doi: 10.1016/S0006-3495(76)85666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geacintov N. E., Breton J., Swenberg C., Campillo A. J., Hyer R. C., Shapiro S. L. Picosecond and microsecond pulse laser studies of exciton quenching and exciton distribution in spinach chloroplasts at low temperatures. Biochim Biophys Acta. 1977 Aug 10;461(2):306–312. doi: 10.1016/0005-2728(77)90180-3. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., Swenberg C. E., Campillo A. J., Hyer R. C., Shapiro S. L., Winn K. R. A picosecond pulse train study of exciton dynamics in photosynthetic membranes. Biophys J. 1978 Oct;24(1):347–359. doi: 10.1016/S0006-3495(78)85382-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülen D., Wittmershaus B. P., Knox R. S. Theory of picosecond-laser-induced fluorescence from highly excited complexes with small numbers of chromophores. Biophys J. 1986 Feb;49(2):469–477. doi: 10.1016/S0006-3495(86)83656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth A. R. Applications of ultrafast laser spectroscopy for the study of biological systems. Q Rev Biophys. 1989 Aug;22(3):239–326. doi: 10.1017/s0033583500002985. [DOI] [PubMed] [Google Scholar]
- Irrgang K. D., Boekema E. J., Vater J., Renger G. Structural determination of the photosystem II core complex from spinach. Eur J Biochem. 1988 Dec 1;178(1):209–217. doi: 10.1111/j.1432-1033.1988.tb14445.x. [DOI] [PubMed] [Google Scholar]
- Jansson S. The light-harvesting chlorophyll a/b-binding proteins. Biochim Biophys Acta. 1994 Feb 8;1184(1):1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Kramer H., Mathis P. Quantum yield and rate of formation of the carotenoid triplet state in photosynthetic structures. Biochim Biophys Acta. 1980 Dec 3;593(2):319–329. doi: 10.1016/0005-2728(80)90069-9. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Mauzerall D. Multiple excitations in photosynthetic systems. Biophys J. 1976 Jan;16(1):87–91. doi: 10.1016/S0006-3495(76)85665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund T. M., Knox W. H. Lifetime of fluorescence from light-harvesting chlorophyll a/b proteins. Excitation intensity dependence. Biophys J. 1981 Oct;36(1):193–201. doi: 10.1016/S0006-3495(81)84723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussberger S., Dekker J. P., Kühlbrandt W., van Bolhuis B. M., van Grondelle R., van Amerongen H. Spectroscopic characterization of three different monomeric forms of the main chlorophyll a/b binding protein from chloroplast membranes. Biochemistry. 1994 Dec 13;33(49):14775–14783. doi: 10.1021/bi00253a016. [DOI] [PubMed] [Google Scholar]
- Paillotin G., Geacintov N. E., Breton J. A master equation theory of fluorescence induction, photochemical yield, and singlet-triplet exciton quenching in photosynthetic systems. Biophys J. 1983 Oct;44(1):65–77. doi: 10.1016/S0006-3495(83)84278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillotin G., Swenberg C. E., Breton J., Geacintov N. E. Analysis of picosecond laser induced fluorescence phenomena in photosynthetic membranes utilizing a master equation approach. Biophys J. 1979 Mar;25(3):513–533. doi: 10.1016/S0006-3495(79)85320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschenko V. Z., Protasov S. P., Rubin A. B., Timofeev K. N., Zamazova L. M., Rubin L. B. Probing the kinetics of photosystem I and photosystem II fluorescence in pea chloroplasts on a picosecond pulse fluorometer. Biochim Biophys Acta. 1975 Nov 11;408(2):143–153. doi: 10.1016/0005-2728(75)90006-7. [DOI] [PubMed] [Google Scholar]
- Peterman E. J., Dukker F. M., van Grondelle R., van Amerongen H. Chlorophyll a and carotenoid triplet states in light-harvesting complex II of higher plants. Biophys J. 1995 Dec;69(6):2670–2678. doi: 10.1016/S0006-3495(95)80138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Seibert M., Alfano R. R. Probing photosynthesis on a picosecond time scale. Evidence for photosystem I and photosystem II fluorescence in chloroplasts. Biophys J. 1974 Apr;14(4):269–283. doi: 10.1016/S0006-3495(74)85915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenberg C. E., Geacintov N. E., Pope M. Bimolecular quenching of excitons and fluorescence in the photosynthetic unit. Biophys J. 1976 Dec;16(12):1447–1452. doi: 10.1016/S0006-3495(76)85786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkunas L., Trinkunas G., Liuolia V., van Grondelle R. Nonlinear annihilation of excitations in photosynthetic systems. Biophys J. 1995 Sep;69(3):1117–1129. doi: 10.1016/S0006-3495(95)79986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]