Abstract
The taurine transporter, TauT, regulates various taurine-mediated physiological and pathological functions by facilitating taurine uptake in a sodium- and chloride-dependent manner. Dysfunction of TauT is associated with male infertility, retinal health and cancers. Despite extensive research efforts, the intricate structure of TauT, the molecular mechanisms underlying taurine transport, and the inhibition mechanisms involved, all remain elusive. Here, we present eleven cryo-electron microscopy (cryo-EM) structures of TauT. The structures TauT bound to substrate (taurine) and substrate analogues (β-alanine, guanidinoacetate, and γ-aminobutyric acid), are captured in distinct conformations. Combining with biochemical analyses, these structures reveal that amino acids Leu134 and Glu406 play a crucial role in substrate specificity within the GABA subfamily. Five distinct inhibitors, namely, piperidine-4-sulfonic acid, imidazole-4-acetatic acid, 5-aminovaleric acid, nipecotic acid and homotaurine, stabilize TauT in an inward-open conformation. Conversely, guanidinoethyl sulphonate stabilizes TauT in the occluded state. These structural insights offer a comprehensive understanding of how these inhibitors counteract taurine transport. Collectively, these findings advance our understanding of the substrate coordination and inhibitor recognition mechanisms of TauT.
Subject terms: Cryoelectron microscopy, Molecular biophysics, Electron microscopy
The taurine transporter TauT regulates taurine-mediated physiological and pathological functions by facilitating taurine uptake in a sodium- and chloride-dependent manner. Here, the authors use cryo-EM to elucidate the substrate coordination and inhibitor recognition mechanisms of TauT.
Introduction
Taurine, one of the most abundant amino acids in mammals, is widely distributed throughout various cells and tissues, including the brain, retina, leukocytes, heart, and kidney1,2. It plays a pivotal role in neural development, primarily exerting its neuronal inhibitory effect by activating postsynaptic GABAA receptors (GABAAR) and glycine receptors, albeit with relatively lower affinity for them3,4. In the brain, it is often considered as a trophic factor for brain development, promoting the proliferation of brain cells4,5. Taurine is also an endogenous substance in the retina, where its high concentration can play a neuroprotective role in preventing retinal damage1,6. In the immune system, taurine depletion can lead to T-cell exhaustion and tumor progression, while high levels of taurine are crucial for sustaining leukocyte function7,8. Taurine also participates in many essential biological processes9, serving as an indirect antioxidant10 and playing a crucial role in osmoregulation11,12, membrane stabilization13, and Ca2+ signaling regulation14. It has been well-documented to possess the key functions in maintaining metabolic homeostasis11. A recent study has unveiled the pivotal role of secondary taurine metabolites in anti-obesity effects, offering a promising pathway for the development of drugs specifically targeting obesity treatment15. Furthermore, taurine exhibits neuroprotective and antitoxic effects in various neurodegenerative diseases, such as Parkinson’s disease16,17, Alzheimer’s disease18, and Huntington’s disease19. Deficiency of taurine can cause retinal diseases and mitochondrial dysfunction, which in severe cases may lead to blindness6,20,21 and dilated cardiomyopathy in human22. Taurine also functions in diabetic nephropathy by reducing the accumulation of glycosylated products in the kidneys23,24. Notably, recent scientific investigations have identified taurine deficiency as a significant contributor to the aging, suggesting that reversing taurine deficiency during aging may represent a promising avenue for anti-aging strategies25. The uptake of taurine in human cells is mainly regulated by TauT (encoded by SLC6A6).
TauT, a member of the solute carrier 6 (SLC6) family, is a secondary active transporter that shares architectural and mechanistic properties with other SLC6 members26–28. It plays a crucial role in human physiological health, as evidenced by its protective function against oxidative stress in the Blood-Testis Barrier (BTB), where it transports taurine to the seminiferous tubules, thereby improving male infertility treatment outcomes29. In the inner blood-retinal barrier (BRB), TauT regulates retinal osmolarity by transporting taurine from circulating blood to the retina, which is essential for maintaining retinal health30,31. Furthermore, TauT has been implicated in cancer progression, with overexpression in gastric cancer cells leading to taurine deficiency in the tumor microenvironment and inducing T cell death and dysfunction8. TauT is also associated with colorectal cancer, playing a significant role in the survival and maintenance of colorectal cancer stem cells, tumor initiation, hunger tolerance, and multidrug resistance32.
TauT may serve as a pivotal target for anticancer therapies. Several compounds reported to interact with TauT are substrate analogs, such as β-alanine (β-Ala), guanidinoacetate (GAA), and γ-aminobutyric acid (GABA), as well as inhibitors, including nipecotic acid, imidazole-4-acetic acid (I4AA), piperidine-4-sulfonic acid (P4S), guanidinoethyl sulfonate (GES), 5-aminovaleric acid (5AVA), and homotaurine (Htau)33. Nipecotic acid is known to potentiate the depressant effects of taurine on the vertebrate nervous system34. I4AA, containing a flexible carboxylic acid chain and an imidazole ring, has shown potential in interacting with TauT in BRB cells35. P4S, a competitive inhibitor of TauT, exerts its inhibitory effect without exhibiting toxic side effects36. GES functions as a competitive inhibitor of TauT and has been reported to reduce taurine levels in both the brain and plasma36–38. The longer carbon chain of 5AVA may adopt a bent conformation to interact with TauT36. Htau, featuring a C3-carbon linker connecting the acidic moiety and amino group, demonstrates an inhibitory effect on TauT33,36. Although many compounds targeting TauT have been reported, determining the high resolution structure of TauT may be beneficial for the development of potent and selective inhibitors with anti-cancer properties.
In this work, we present eleven cryo-electron microscopy (cryo-EM) structures of human TauT, encompassing its apo state, the taurine-bound state, as well as states bound to taurine analogs and inhibitors, captured in distinct conformations. These structural insights advance our understanding of the substrate coordination and inhibitor recognition mechanisms of TauT.
Results
Epitope transfer facilitates TauT structure determination
The wild-type full-length TauT is a monomeric membrane protein (~70 kDa), mainly composed of transmembrane (TM) helices, which is localized at the plasma membrane. HEK293 cells expressing human wild type TauT (TauTWT) displayed robust accumulation of [3H]Taurine, which could be effectively abolished upon the addition of taurine analogs, including β-Ala, GAA, GABA, P4S, I4AA, GES, 5AVA, Htau and nipecotic acid, which exhibited varying degrees of biological activity towards TauT (Supplementary Fig. 1b). At present, small-sized membrane proteins pose a significant challenge for imaging, primarily because in numerous instances, the bulk of their organized structure is concealed within the detergent micelle, thereby hindering the alignment of projections essential to the reconstruction process. To facilitate cryo-EM reconstructions, we perform epitope transfer from the dDAT to TauT39,40, hereafter referred to as TauTEM, to facilitate the interaction between TauT and Fab 9D5 (Supplementary Fig. 1a). Notably, the engineered TauTEM construct demonstrated substantial [3H]Taurine uptake activity, albeit at a reduced level compared to the wild-type TauTWT (Supplementary Fig. 1b). Moreover, the binding affinity of TauTEM to taurine, as well as its sensitivities to taurine analogs was found to be analogous to those of TauTWT (Supplementary Fig. 1b, c). These functional studies established the utility of TauTEM for structural analysis, underscoring its functional equivalence to TauTWT.
TauTEM is expressed in mammalian cells for cryo-EM structural studies, including both the apo state and the taurine-bound state, as well as structurally diverse substrate analogs-bound states. During the sample preparation process, we choose the small-size detergents n-dodecyl-β-D-maltoside (DDM) and cholesteryl hemisuccinate (CHS), which will generate a relatively thin micelle around the TauTEM protein and can be beneficial for structure determination. The purification of TauTEM was carried out using anti-FLAG-tag G1 affinity resin, followed by size-exclusion chromatography (SEC). For the assembly of the Fab-TauTEM complex, the purified TauTEM was incubated with the Fab 9D5, and subsequently, excess Fab 9D5 was removed via SEC (Supplementary Fig. 1d–f). Finally, a total of eleven TauT structures were determined at a resolution of 2.9 − 3.4 Å (Supplementary Figs. 2 and 3 and Table 1). These high-resolution electron microscopy maps of these structures enable precise modeling of ligands and the side chains of the residues 47−584. The additional N-terminus segment (residues 38−46) was incorporated into the final models of TauTTau, TauTβ-Ala and TauTGES, based on the density map. The 585-amino-acid C terminus, which is predicted to be disordered, were not observed in all structures (Fig. 1a–c).
Fig. 1. Cryo-EM structures of human TauT complexes.
a Cryo-EM map of TauTTau complex, captured in the occluded conformation. The TauT density is colored in a rainbow pattern and contoured at 5.6 σ by ChimeraX. The dimensions of the model are labeled as indicated. b Overall structure of TauTTau in cartoon representation. TM1, TM3, TM6, TM8 and TM12 are labeled. The taurine, sodium (Na+), and chloride ion (Cl–) are depicted as cyan, purple and green spheres, respectively. c Densities of substrate taurine and substrate analogs in the structures of TauT complexes. The densities are contoured at 4.5 σ by ChimeraX. d Surface representation of the electrostatic potential of the binding cavity. Red and blue indicate negatively and positively charged regions, respectively. The structure is overlaid in rainbow ribbon and taurine is shown in cyan sphere representation.
Architecture of TauT
The distinct conformations of TauT complexes provide insights into its interactions with substrate, substrate analogs and inhibitors. The cryo-EM structures reveal TauT in various states: TauTTau, TauTβ-Ala, and TauTGES exhibit an occluded conformation, TauTGAA and TauTGABA display an intermediate conformation. The remaining structures adopt an inward-open conformation (Supplementary Fig. 3). The TauT structure, measuring 80 Å in height and 55 Å in diameter, features a canonical LeuT fold, characteristic of the GABA subfamily. It comprises 12 transmembrane helices (TMs), with both the N and C termini located on the cytoplasmic side. TMs 1−5 and TMs 6−10 form inherent pseudo-symmetry, with TM1 and TM6 divided into two segments, designated as TM1a-TM1b and TM6a-TM6b (Fig. 1a, b). The extracellular loop 2 (EL2), connecting TM3 and TM4, is unambiguously modeled into the density, with a conserved disulfide bond identified between Cys162 and Cys171 in TauTβ-Ala, playing a crucial role in maintaining the unique configuration of EL2 (Supplementary Figs. 2h and 4). A central binding cavity, positioned at the membrane’s midpoint and is found to be occupied by ligands in distinct conformations (Fig. 1c, d). Sequence alignment demonstrates a high identity among the GABA subfamily in the central binding pocket region (Supplementary Fig. 5a).
Notably, despite sharing a common folding pattern, structural differences exist between TauT and other members of the GABA subfamily (Supplementary Fig. 6). The most significant structural differences between TauT, hGAT1, and rGAT1 are observed in TM5, TM8–TM12, extracellular loop 4 (EL4), and the N terminus. TauT exhibits a greater distance between EL2 and EL6, leading to an outward shift of TM11 and TM12, positioning them further apart from TM10 (Supplementary Fig. 6b, c). Additionally, the IL2 region of TauT is significantly disrupted, enhancing the flexibility of TM4 and TM5 near the IL2 region, effectively positioning them apart from their neighboring TMs (Supplementary Fig. 6f, g). High-resolution electron microscopy maps enable precise modeling of an elongated N terminus (residues 38-46) in TauTTau, a domain not observed in the architectures of hGAT1 and rGAT1 (Supplementary Fig. 6f). The presence of taurine causes the IL4 loop region to shift towards the N terminus, resulting in an outward movement of TM8 while also enhancing the stability of the occluded state (Supplementary Fig. 6f, g).
Substrate binding of the TauT
The taurine-binding site is inaccessible from both the extracellular and intracellular sides, indicating that the TauTTau is captured in an occluded state. The central binding cavity of taurine is sculpted by TM1, TM3, TM6, and TM8. In the central binding pocket, the binding pose of taurine is stabilized through crucial interactions. Specifically, one oxygen atom of the sulfo group of taurine interacts with Na+ at the Na1 site and this same oxygen atom also forms interactions with the residues Gly57 and Phe58 in TM1. The remaining oxygen atoms of taurine engage in hydrogen-bonding interactions with the nitrogen atom of Gly62, the hydroxyl group of Tyr138, and the side chain of Ser402 (Fig. 2a). These residues are conserved in the GABA subfamily and are crucial for substrate binding (Supplementary Fig. 4). The amino group of bound taurine also contributes to binding, interacting with the carbonyl oxygen atom on the main chain of Phe300 (Fig. 2a). Moreover, taurine is encircled by surrounding residues through van der Waals forces or hydrophobic interactions, including Gly60, Leu134, Leu306 and Glu406. The key residues, such as Gly57, Phe58, Gly60, Gly62, Leu134, Tyr138, Phe300, Leu306, Ser402 and Glu406 alanine mutants drastically impaired the transport activity of taurine, consistent with our structural observations (Supplementary Fig. 5e). In vitro microscale thermophoresis (MST) assay revealed that alanine substitutions of Phe58, Phe300, and Glu406 reduced the taurine binding to varying extents, while Tyr138 and Ser402 alanine mutants exhibited undetectable binding of taurine (Fig. 2a and Supplementary Table 2).
Fig. 2. Taurine and taurine analogs binding to TauT.
a−d Left, interactions of taurine (a), taurine analogs: β-Alanine (b), guanidinoacetate (c), and γ-aminobutyric acid (d) with residues in the central binding site of TauT. Interaction distances are represented as dashed lines with values representing angstroms. Right, binding affinity of TauT mutants with four ligands measured using MST assay. Assays were conducted with three independent biological replicates (n = 3). Data are shown as mean ± s.e.m. Source data are provided as a Source Data file.
Two sodium ion binding sites (Na1 and Na2 sites) and one chloride ion binding site are discernible in TauTTau. The residues coordinating the chloride ion are strictly conserved in hGAT1 and rGAT1, but the residues participating in sodium ion binding do not exhibit such conservation (Supplementary Figs. 4 and 5b–d). To corroborate our structural observation, TauT variants with single point substitutions of the ion-interacting residues were generated, and their taurine transport activities were examined. As expected, the substitution of Asn63, Ser301, and Asn333 with either alanine or aspartate, which fails to coordinate sodium ions at the Na1 site, results in a complete loss of function. Additionally, mutations in Asp401 and Ser402, which function in chelating Na+ at the Na2 site, weaken the substrate transport function of TauT (Supplementary Fig. 5f).
The residues surrounding the central binding cavity are crucial for taurine transport, and even minor variations in the pocket components and ligand binding positions can significantly impact their preference for the GABA subfamily. To investigate the determinants of substrate specificity, we mutated non-conserved residues in the central binding site of TauT to their equivalent residues in other members of the GABA subfamily (Supplementary Fig. 7a, b). The mutants were further examined to assess their influence on the transport activity of GABA and GAA. The mutation Glu406Cys results in a significant increase in the transport activity of GABA, the mutation Leu134Cys exhibits significantly lower transport activity of taurine and greater transport activity of GAA (Supplementary Fig. 7c-e). These findings indicate that residues Leu134 and Glu406 are intricately linked to the substrate specificity exhibited by TauT, supporting the hypothesis that the reduction in the side chain bulkiness of the residues may lead to an increase in the volume of the substrate binding cavity, which can accommodate a bulkier molecular size of substrate. Moreover, amino acid substitutions in residues Gly57, Phe58, and Leu306 result in a decrease in transport activity for taurine, GABA, and GAA. We speculate that these residues are important for TauT transport activity but play a minor role in determining the substrate specificity of TauT (Supplementary Fig. 7c-e). These findings provide helpful information to increase our understanding of the substrate specificity of GABA subfamily, including GAT1, GAT2, GAT3, BGT1 and CT1. Additionally, the substrate analogs β-alanine, GABA, and GAA demonstrate potent inhibitory effects on the TauT. Determining the structures of TauT in complex with these ligands will further advance our understanding of TauT.
Substrate analogs binding of the TauT
The three TauT structures bound with substrate analogues are captured in distinct conformations. Notably, the binding sites for β-Ala in the occluded state, as well as GAA and GABA in an intermediate conformation, are all situated within the central binding pocket. This pocket is primarily surrounded by a combination of hydrophobic, polar and negatively charged residues. The carboxylate group of these ligands is positioned at the partially positively charged N-terminus of TM1b, while the interaction mode of the amino group varies significantly among them (Fig. 2b–d).
The interaction mode of β-Ala’s carboxylate group closely resembles that observed for taurine. However, the amino group of β-Ala engages in an interaction with Glu406 within TM8 instead of Phe300 located in TM6 (Fig. 2b). Additionally, β-Ala forms van der Waals forces or hydrophobic interactions with surrounding residues Gly57, Phe300, Ser301, Leu306 and Ser402 (Fig. 2b). The alanine mutations of Phe58 and Ser402 result in a 58-fold and 120-fold reduction in β-Ala binding affinity, respectively, while Tyr138Ala, Phe300Ala and Glu406Ala mutants all abolished the binding of β-Ala, indicating their important role in β-Ala recognition (Fig. 2b and Supplementary Table 2).
In the structure of TauTGAA and TauTGABA, GAA and GABA are also situated in the central binding pocket surrounded by a combination of hydrophobic, polar and negatively charged residues. The interaction mode of ligands is slightly different from that of taurine (Fig. 2c, d). Compared with taurine, in addition to interacting with the main chain nitrogen atom of Gly62 and the side chain hydroxyl group oxygen atom of Tyr138, the carboxylate group of GAA also interacts with the side chain oxygen atom of Asn63. The amino group of GAA forms hydrogen bonds with the main chain oxygen atom of Phe300 and the side chain of Glu406 (Fig. 2c). MST measurement further demonstrated a drastic loss of GAA binding affinity in alanine mutations of Gly62, Tyr138, Phe300 and Leu306, while the Glu406Ala mutant only marginally weakened the binding affinity for GAA (Fig. 2c and Supplementary Table 2).
The GABA interaction pattern in TauT also varies from that of hGAT1 in the occluded state. Specifically, the residues Gly62 and Tyr138 that interact with the carboxyl group are conserved. Further structural observation reveals that Phe58 on TM1a in TauT, which corresponds to Tyr60 in hGAT1, forms a hydrophobic interaction with GABA and is prominently displaced. Due to the presence of the Na1 site, the carboxylate group of GABA exhibits an interaction with Na+ at the Na1 site in these two structures (Fig. 2d and Supplementary Fig. 6d). In the presence of GABA, both TauT and rGAT1 exhibit similar intermediate conformations, as evidenced by varying angular displacements in TM1a tilting. The binding pose of GABA within the central pocket differs between them, with GABA in TauT moving further towards the intracellular side. Notably, the binding of GABA in rGAT1 is stabilized by more extensive interactions (Supplementary Fig. 5e). The Glu406Ala mutation reduces the binding affinity of GABA slightly, indicating a minor role for Glu406 in GABA recognition, while the alanine mutations of Gly62, Tyr138 and Phe300 lead to a complete loss of the binding of GABA (Fig. 2d and Supplementary Table 2). The variations in substrate analog binding modes underscore the complexity of TauT’s function and the potential for targeted drug design to modulate TauT activity.
Comparison of TauT structures in distinct conformations
We next proceed with a comparative analysis of TauT structures across various conformations (Fig. 3). The TauTTau complex can be superimposed on the structure of TauTβ-Ala, with root-mean-square deviation (RMSD) value of 0.86 Å. Similarly, the TauTGABA complex can be superimposed on the structure of TauTGAA, yielding an RMSD value of 1.24 Å (Supplementary Fig. 8a, d). Notably, among all the TMs of these structures, the major distinction occurs in TM1, with TM1a undergoing a significant conformational shift while TM1b remains unchanged. Specifically, the last helical turn of TM1a in the apo state, spanning amino acids 47−60, unwinds and shifts towards the membrane plane, exposing the central binding cavity towards the intracellular side. In TauTGABA, TM1a displays a distinct angular orientation (Fig. 3a). The residues Phe58 and Gly56 in TM1 undergo significant displacement (Fig. 3c, d).
Fig. 3. Comparative structural analysis across diverse conformational states.
a Superposition of TauTApo (yellow), TauTGABA (pink) and TauTTau (cyan). The TM regions remain nearly identical, except that TM1a displays variation. b Structural superposition of binding sites in TauTTau and TauTGABA. Taurine and GABA are shown as ball sticks and critical residues are represented as sticks. c-d Detailed changes of TM1. Taurine and GABA are displayed as ball sticks. Residues that undergo significant displacement are displayed as sticks. e Comparison of the Na1 and Na2 sites in TauTApo and TauTTau. f Detailed view of the N-terminus of TauTTau. The electron density is depicted as a gray mesh and contoured at 5 σ by ChimeraX. Residues within the N-terminus are visualized as sticks. g The interactions of N-terminus in TauTTau. Key residues are labeled and represented by sticks. h Extracellular gate in TauT. Arg66 and Phe300 are key residues in the extracellular gate. Arg66 forms a cation-π interaction with Phe300, as indicated by the black dashed line. Arg66 is also coordinated by Gly62, Asp459 and Ser464. Hydrogen bonds are shown.
Further inspection reveals that, owing to the absence of Na+ in TauTApo, the local structural arrangement of residues coordinating Na+ displays a notable change. Phe58 and Gly56 leave the Na1 site and Na2 site, respectively, accompanying the movement of TM1a. The side chains of Asp401 and Ser402 also swing away from the Na2 site (Fig. 3e). These rearrangements expose the central pocket intracellularly, facilitating an open intracellular conformation that allows taurine release. In the presence of β-Ala, TauT exhibits stable ion coordination similar to that in TauTTau (Supplementary Fig. 8b). Compared with TauTTau, Gly56 within the TM1a in TauTGAA and TauTGABA shifts away from the Na2 site, consequently losing its ability to effectively chelate Na+ at the Na2 site (Supplementary Fig. 8f).
Notably, when compared with the binding position of taurine, GABA and GAA extend further towards the intracellular pathway (Fig. 3b and Supplementary Fig. 8e). The extended carbon linker of GAA has the potential to adopt a bent conformation, thereby facilitating more extensive interactions with TauT (Supplementary Fig. 8e). Despite presenting different conformations, the extracellular gate, formed by residues from the TM1b, the TM3, the TM6a, and the TM10, is closed among all structures. The intricate interactions at this closed extracellular gate include a comprehensive network of hydrogen bonds. Phe300 on TM6a directly overlays the taurine and GABA molecules, with Arg66 on TM1b stabilizing the conformation of Phe300 through a cation–π interaction. In addition, Arg66 forms hydrogen bonds with Asp459, Ser464, and Gln297, further stabilizing the closed extracellular gate (Fig. 3h). Furthermore, the high-quality electron microscopy map facilitated the assignment of the N-terminal below the transmembrane domain in the occluded TauTTau and TauTβ-Ala (Fig. 3f and Supplementary Fig. 8c). Residue Arg41 potentially forms an electrostatic interaction with Asp416 in the TM8 and establishes a cation-π interaction with Tyr315 in the TM6. Residue Trp44 engages in hydrophobic interactions with Phe50 and Tyr315, causing TM1 to draw closer to TM6, ultimately resulting in an occluded conformational state (Fig. 3g). However, this N-terminal segment was unresolved in other states. In light of its intimate connection with TM1a, we posit that the interaction between the N-terminal segment and the transmembrane domain plays a pivotal role in stabilizing TauT in its occluded state. The comparative structural analysis of TauT within the context of the GABA transporter subfamily is of paramount importance for elucidating the functional plasticity inherent within this subfamily. Within this subfamily, the repetition of internal structures and hinge movements facilitates the alternating access transport mechanisms, enabling the substrate-binding site to toggle its accessibility between the extracellular and cytoplasmic membranes. The diverse conformations captured in the cryo-EM structures of TauT provide a comprehensive roadmap for understanding substrate recognition, which is crucial for the future design of innovative drug targeting TauT.
Inhibitors recognition by TauT
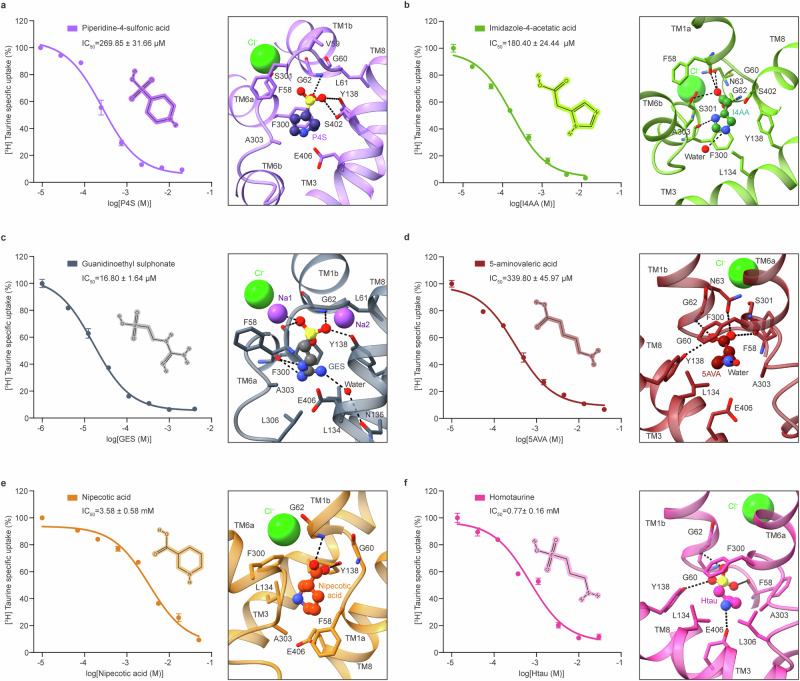
TauT inhibitors P4S, I4AA, GES, 5AVA, Htau and nipecotic acid concentration-dependently inhibited the uptake activity of TauT (Fig. 4). To investigate the molecular mechanism by which the small molecules inhibit TauT, we determined the structures of TauT in the presence of these small molecules. The structures of TauT in complex with six distinct small molecules, captured in two distinct conformations, provide insights into how these small molecules impede taurine transport. Unlike GES, which stabilizes TauT in an occluded conformation, the remaining five small molecules—P4S, I4AA, 5AVA, nipecotic acid, and Htau—stabilize TauT in an inward-open conformation. Notably, all these small molecule compounds occupy the central binding site of taurine with partially overlapping binding poses. These small molecule compounds, having a larger volume or longer connections with charged amino groups than taurine, tightly wedge into the central binding cavity, thereby blocking taurine binding (Fig. 4). Despite occupying the central binding site, subtle differences in binding poses among the six small molecules may account for their varying binding affinity towards TauT (Supplementary Fig. 9). To elucidate the specific binding patterns of these small molecule compounds, an in-depth analysis of all bound structures was performed.
Fig. 4. Recognition of small molecule compounds by TauT.
a−f Left, the concentration-dependent inhibition of six small molecule compounds on hTauT. The calculated IC50 values were 269.85 ± 31.66 µM for piperidine-4-sulfonic acid (a), 180.40 ± 24.44 µM for imidazole-4-acetatic acid (b), 16.80 ± 1.64 µM for guanidinoethyl sulphonate (c), 339.80 ± 45.97 µM for 5-aminovaleric acid (d), 3.58 ± 0.58 mM for nipecotic acid (e) and 0.77 ± 0.16 mM for homotaurine (f). Assays were conducted with three independent biological replicates (n = 3). Data are mean ± s.e.m. The 2D chemical structures of six small molecule compounds were generated using ChemDraw. Source data are provided as a Source Data file. Right, interactions of the small molecule compounds piperidine-4-sulfonic acid (a), imidazole-4-acetatic acid (b), guanidinoethyl sulphonate (c), 5-aminovaleric acid (d), nipecotic acid (e) and homotaurine (f) with residues in the central binding site of TauT. The interacting residues are shown as sticks and the dashed lines represent hydrogen bonding. The water molecules are shown as red balls.
The high-resolution cryo-EM maps revealed distinct and prominent densities of all small molecule compounds within the central binding pocket, formed by TM1, TM3, TM6, and TM8. The binding of compounds is stabilized by key interactions with TauT residues (Fig. 4). Except for I4AA, the carboxyl groups or sulfonate groups of the other ligands interact with the conserved Tyr138, irrespective of their varying conformations (Fig. 4a, c–f). The subtle variations in the interaction patterns displayed by the carboxyl or sulfonate groups of these compounds could potentially be attributed to their distinct binding poses. In the central binding pocket, the carboxyl group of I4AA fits snugly into a narrow cleft formed by TM1 and TM6, which is recognized by residues Phe58, Gly62, Asn63, and Ser301 through hydrogen bonds (Fig. 4b). In the presence of GES, distinguishable sodium ions are identified, and the sulfonate group of GES engages in an additional interaction with Na+ at the Na1 site (Fig. 4c).
In stark contrast, the interaction patterns of the amino group, piperidine ring, or imidazole ring among these small molecule compounds exhibit marked differences. The imidazole ring of I4AA and the amino group of 5AVA interact with a water molecule. The amino group of GES exhibits a water-mediated interaction with residue Asn135. Residue Glu406, located within TM8 of TauT, engages in hydrogen bonding interaction with the amino group of Htau. In contrast, the nitrogen atoms of P4S and nipecotic acid show no interaction with TauT (Fig. 4). A set of residues surrounding taurine in TauTTau also interact with small molecule compounds through van der Waals forces and hydrophobic interactions (Fig. 4). Mutations in the majority of these residues in the central binding pocket abolished the binding of six small molecule compounds, consistent with our structural observation (Supplementary Fig. 9). The similar interaction and functional analysis of small molecule compounds binding to TauT support the contention that the binding pocket formed by these residues is crucial for both the binding of small molecule compounds to TauT and the transport of taurine by TauT. This comprehensive structural analysis of TauT in complex with diverse small molecule compounds deepens our understanding of their recognition, implying significant advancements for structure-based drug design targeting TauT.
Mechanisms of inhibition by inhibitors
A comprehensive structural comparative analysis of TauT bound to six small molecule compounds and taurine, reveals distinct molecular mechanisms underlying their inhibitory effects on TauT. In the presence of I4AA, its carboxyl group extends deeper into the space between TM1 and TM6, potentially increasing contacts with residues on TM1 and TM6 of TauT (Supplementary Fig. 10b). Structural comparison also emphasizes the substantial steric hindrance between inhibitor I4AA and residue Gly57 located on TM1a in TauTTau, suggesting that I4AA may hinder the transition of TauT from the inward-open to the occluded conformation. The larger volumes of I4AA may require a more spacious binding pocket, facilitating their stabilization in the inward-open conformation (Supplementary Fig. 10b).
Structural comparison between TauTGES and TauTTau reveals that GES displays a significant overlap in the binding pose with taurine. This implies that GES blocks taurine binding through direct competition for the substrate binding site. The residues interacting with GES and taurine within the central pocket are almost identical, given the lack of interaction between the sulfonate group of GES and residue Gly57 (Figs. 2a, 4c and Supplementary Fig. 10c). Despite stabilizing TauT in a distinct conformation compared to the structure of TauTTau, P4S and 5AVA occupy the central binding site of the substrate taurine with a partially overlapping binding pose (Supplementary Fig. 10a, d). Examination of the Htau-binding pocket shows that Htau has longer carbon linkers. TM8 undergoes subtle displacement, creating space for the elongated amino group of it, facilitating its interaction with the Glu406 residue on TM8. This interaction is likely crucial for maintaining TauT in its inward-open conformation (Supplementary Fig. 10f).
Comparative analysis between TauTNipecotic acid and TauTTau indicates that nipecotic acid faces the cytosol side in the central binding pocket, and its bulkier structure moves towards TM1a, stabilizing TauT in its inward-open conformation. This slight difference may impede the necessary conformation change (Supplementary Fig. 10e). Previous studies have demonstrated that nipecotic acid exhibits varying binding affinities for hTauT and hGAT133,41. To understand the structural basis for the varying sensitivity of TauT and GAT1 to nipecotic acid, we conducted a comparative analysis of TauTNipecotic acid and GAT1N (PDB: 7Y7Y)41. Unlike the TauTNipecotic acid, nipecotic acid stabilizes hGAT1 in an occluded conformation (Supplementary Fig. 11a). The overall structures of hTauT and hGAT1 are largely superimposable, with primary deviations in TM1a and TM6b (Supplementary Fig. 11c). Regardless of the conformation, nipecotic acid occupies the central binding cavity surrounded by TMs1, 3, 6, and 8 with an indistinguishable binding pose. hTauT and hGAT1 exhibit a conserved interaction pattern with the carboxyl group of nipecotic acid, yet neither interacts with its piperidine ring (Supplementary Fig. 11b). Despite similarities in the binding poses and interaction mode of nipecotic acid in hTauT and hGAT1, subtle variations in pocket components may affect the selectivity of nipecotic acid. The residues shaping the binding pocket of nipecotic acid in hTauT and hGAT1 are not strictly conserved. We hypothesize that non-conserved residues Gly57 and Phe58 in TM1 may account for the distinct conformational differences observed between hTauT and hGAT1, and non-conserved residues Ala303 in TM6 and Glu406 in TM8 could potentially contribute to the selectivity of nipecotic acid (Supplementary Fig. 11c).
This detailed structural analysis provides a molecular framework for understanding the inhibitory mechanisms of TauT and offers a basis for the structure-based design of drugs targeting TauT, with implications for the treatment of TauT-related diseases. The variations in inhibitor binding and their impact on TauT conformation underscore the complexity of drug design and the potential for selective inhibition of TauT over other transporters.
Discussion
TauT is associated with human physiological health29–31 and various cancers42. Uncovering the atomic details of the TauT organization and its interaction with both taurine and substrate analogues is important to establish the function of TauT and develop effective drugs targeting TauT. In our research, we employed epitope transfer from dDAT to facilitate the determination of TauT structures; specifically, in the apo state, with substrate, and with substrate analogues. These structures, captured in distinct functional conformations—including occluded, inward-open, and intermediate states—provide invaluable insights into the molecular underpinnings of TauT function.
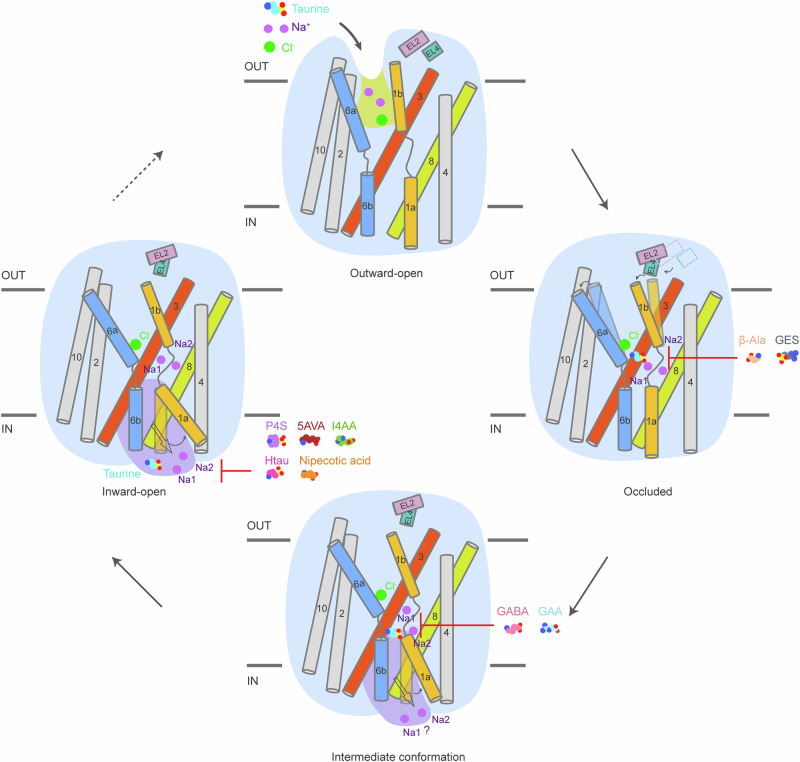
The determination of an ensemble of structures of TauT in distinct functional states enables us to outline the conformational transitions during the transport cycle (Fig. 5). A hydrophobic cavity formed by TM1, TM3, TM6, and TM8 allows taurine access and binding within the central binding site. TM1 and TM6 are particularly important for substrate recognition and conformational transitions. In the presence of taurine and β-Ala, the N-terminal residue Arg41 extensively interacts with TM6 and TM8, sealing the intracellular gate and stabilizing the occluded conformation, within which a clear visualization of two sodium ions and a chloride ion exists. In contrast, GAA and GABA stabilize TauT in an intermediate conformation, as evidenced by a distinct orientation of TM1a compared to the apo state and the solvation of sodium ions at the Na2 site. As TauT transitions to the inward-open conformation, the TM1a helix unwinds, disrupting intracellular gate interactions and leading to taurine release from the binding pocket. The chloride ion binding site density in the apo state confirms the chloride ion dependence of taurine transport. Our structures depict the transition from occluded to inward-open states, advancing our understanding of taurine release mechanism. Moreover, combined with functional analysis, our structures offer valuable insights into the factors that determine the differences in substrate specificity between TauT and other members of GABA subfamily in the central binding site, including residues Leu134 and Glu406 in TauT. It is worth noting that the substrate specificity among GABA subfamily may be influenced by the plasticity of the central binding pocket, as evidenced by the decrease of the side chain bulkiness may lead to the increase of the volume of the substrate binding pocket, which may increase the accessibility of bulkier substrates. In addition, beyond the central binding site, other regions should not be disregarded, because they might contribute to substrate binding specificity of GABA subfamily in an allosteric manner.
Fig. 5. Transport and inhibitory mechanism of human TauT.
The alternating access and inhibition mechanism of TauT. Key helices involved in the conformational changes are colored and labeled. The intracellular and extracellular cavities are depicted by purple and yellow shadows, respectively. The movement of TM1a during the transition from the occluded to the inward-open state is indicated by an ash black arrow. Sodium and chloride ions are represented as purple and green spheres, respectively. Molecules of taurine, GABA, GAA, β-Ala, P4S, I4AA, GES, 5AVA, Htau and nipecotic acid are represented as spheres. Red lines indicate that the small molecules block TauT in the current conformation.
Structural analysis of hTauT in complex with six small molecule compounds provides valuable insights into their recognition mechanisms. These structures reveal that all small molecules anchor in the central binding pocket, obstructing taurine transport in distinct conformations. Their carboxyl or sulfonate groups primarily establish polar interactions with TM1 and TM3. Compared to P4S, 5AVA and nipecotic acid, the other three small molecules exhibit additional interactions between their amino groups or imidazole rings and hTauT. GES overlaps almost completely with the taurine binding pose in the occluded conformation, while the other five ligands stabilize hTauT in an inward-open conformation. The bulkier I4AA exhibits pronounced steric hindrances with TM1a in TauTTau, potentially impeding necessary conformational changes of hTauT. Inhibitors P4S and 5AVA occupy the substrate binding site in partly overlapping poses with taurine. The binding affinity of elongated Htau may be influenced by subtle displacements of TM8, creating space to accommodate longer carbon linkers. Previous studies have shown that nipecotic acid exhibits a lower IC50 value for hGAT1 than for hTauT33,41. Despite the structures of GAT1N and TauTNipecotic acid exhibit different conformational preferences, nipecotic acid exhibits nearly identical binding poses and interaction modes with both. Sequence alignment reveals that the binding pocket for nipecotic acid in these two transporters is not strictly conserved; the slight variations may subtly affect the binding affinities of nipecotic acid for hGAT1 and hTauT. Collectively, our research offers profound insights into the recognition and inhibition mechanisms of a diverse range of small molecule compounds.
Our comprehensive structural analysis deepens the comprehension of taurine release, substrate specificity, inhibitor recognition, and inhibition mechanisms of TauT, implying significant advancements for structure-based design of drugs targeting TauT. This research underscores the potential of TauT as a therapeutic target for cancer treatment and provides a foundation for the development of inhibitors that could serve as anticancer agents, aligning with recent studies suggesting the potential of SLC6A6 inhibitors in cancer therapy.
Methods
Construct design
The full-length human SLC6A6 gene (Uniprot: P31641) used for cryo-EM analysis contains twelve epitope transfer mutations: Asp486Asn, Asn487Arg, Leu488Phe, Tyr489Ser, Asp490Glu, Gly491Asp, Glu493Arg, Tyr498Phe and Arg499Pro in the IL5 helix; as well as Lys319His, Tyr320Asn and Ser322Val in the IL3 loop. This gene was cloned into a pCAG (Invitrogen) vector and 3 × Flag tags were located at the C termini. The DNA coding sequence of heavy and light chains of Fab 9D5 was cloned into modified pCAG (Invitrogen) vector separately. A tandem Flag-His tag was fused to the C-terminal of the heavy chain.
Protein expression and purification
For protein expression, the HEK293F cells were cultured in SMM 293T-II medium (Sino Biological Inc.) at 37 °C under 5% CO2 in a shaker. A total of 1 mg plasmid and 3 mg polyethylenimines (YEASEN) were premixed in 45 mL fresh medium for 15 min at room temperature and then transiently transfected into 1 liter HEK293F cells at a cell density of 2.0-2.5 × 106 cells per mL. After 12 h, 10 mM sodium butyrate was added to the cells to enhance protein expression. The cells were further cultured for 48 h at 37 °C under 5% CO2 before being collected through centrifugation at 7510 g for 8 min. For the purification of TauTEM, the cell pellets were resuspended in the buffer containing 25 mM Tris pH 7.5, 150 mM NaCl, 1 × 10−3 mg ml−1 aprotinin, 1 × 10−3 mg ml−1 pepstatin and 1 × 10−3 mg ml−1 leupeptin. The cell membranes were extracted with 1% (w/v) n-Dodecyl-β-D-maltoside (DDM) and 0.2% (w/v) Cholesteryl Hemisuccinate Tris Salt (CHS) for 2 h at 4 °C with rotation. After high-speed centrifugation at 20,000 g for 35 min, the supernatant was incubated with the anti-DYKDDDDK G1 Affinity Resin (Genescript) at 4 °C for 1 h with rotation. The resin was washed with the washing buffer containing 25 mM Tris pH 7.5, 150 mM NaCl, 0.02% (w/v) DDM and 0.004% (w/v) CHS. The resin was then washed with the washing buffer supplemented with 2 mM ATP and 5 mM MgCl2. The TauT was then eluted with elution buffer containing 0.2 mg ml−1 FLAG peptide. Then the TauTEM was concentrated to a final volume of 1 mL and further purified through size exclusion chromatography (SEC) using a Superose 6 Increase 10/300 GL size-exclusion column equilibrated with buffer containing 25 mM Tris pH 7.5, 150 mM NaCl, 0.02% (w/v) DDM and 0.004% (w/v) CHS. The peak fractions corresponding to TauTEM were collected and concentrated.
Purification of Fab 9D5
For the purification of Fab 9D5, after centrifuging at 6000 rpm for 8 min, the supernatant was incubated with Ni Sepharose 6 Fast Flow (Cytiva) at 4 °C for 1 h. The resin was washed with the washing buffer containing 25 mM Tris pH 7.5, 150 mM NaCl and 20 mM imidazole. The Fab 9D5 was eluted with the elution buffer containing 25 mM Tris pH 7.5, 150 mM NaCl and 250 mM imidazole. Then the Fab 9D5 was concentrated to a final volume of 1 mL and further purified by size exclusion chromatography (SEC) using a Superose 6 Increase 10/300 GL size-exclusion column equilibrated with buffer containing 25 mM Tris pH 7.5, 150 mM NaCl. The peak fractions corresponding to Fab 9D5 were collected and concentrated. Then the purified TauTEM was complexed with purified Fab 9D5 on ice for 1 h at a molar ratio of 1:1.5. The excess Fab 9D5 was separated by size-exclusion column in the SEC buffer containing 25 mM Tris pH 7.5, 150 mM NaCl, 0.02% (w/v) DDM and 0.004% (w/v) CHS. The peak fractions corresponding to TauTEM-Fab 9D5 complexes were collected and concentrated for further experiments.
Cryo-EM sample preparation and data acquisition
For substrate-free form, the purified TauTEM bound with 9D5 Fab was concentrated to ~16 mg ml−1. For the substrate-bound states and inhibitor-bound states, a final concentration of 5 mM taurine, 5 mM β-alanine (β-Ala), 5 mM guanidinoacetate (GAA), 5 mM γ-aminobutyric acid (GABA), 5 mM nipecotic acid, 5 mM imidazole-4-acetic acid (I4AA), 5 mM piperidine-4-sulfonic acid (P4S), 5 mM guanidinoethyl sulfonate (GES), 5 mM 5-aminovaleric acid (5AVA) and 5 mM homotaurine were added to the cryo-EM sample of TauT, respectively, and incubated for 1 h on ice before application to grids. Quantifoil grids (1.2/1.3 Cu 300 mesh) were glow-discharged for 45 s before use. A 3 µl aliquot of the sample was applied to a glow discharged Quantifoil grid with 100% humidity at 6 °C. Then the grids were blotted for 3.5 s and subsequently flash-frozen in liquid ethane cooled by liquid nitrogen with Vitrobot Mark IV (Thermo Fisher Scientific). The prepared grids were transferred to a 300-kV Titan Krios G3 equipped with Gatan K3 detector and GIF Quantum energy filter (GIF, slit width of 20 eV). Images were automatically collected using EPU software (Thermo Fisher Scientific) at a magnification of 81,000 × under a super-resolution model with a calibrated pixel size of 0.536 Å. For the dataset of TauT bound with taurine, the calibrated pixel size is 0.548 Å. The defocus values ranging from −1.2 to − 2.0 μm. All datasets were recorded with a total dose of 50 e− Å−2 for 32 frames.
Cryo-EM data processing
The processing of datasets was illustrated in flowchart (Supplementary Fig. 2). For the dataset of TauT bound with taurine, movie stacks were corrected to beam-induced motion correction using MotionCor243 and binned twofold, resulting in pixel sizes of 1.095 Å. For the other datasets, the corresponding pixel sizes are 1.072 Å. All subsequent micrographs were imported into cryoSPARC44 and contrast transfer function parameters were calculated by Gctf45. Micrographs with a CTF resolution with a larger value than 3.8 Å were discarded. A blob picker was used to generate an initial particle-set in cryoSPARC. This particle-set was used as a template for Topaz training. Then, all micrographs were picked by Topaz Extract using the trained topaz model and extracted with a box of 256 pixels. High-quality particles were selected and subject to multiple rounds of 2D classification, ab initio reconstruction, and multiple cycles of heterogeneous refinement. Particles from the best class were utilized for non-uniform refinement and local refinement, leading to the final 3D reconstruction. The overall resolution of the final map was determined by the gold-standard Fourier shell correlation (FSC) 0.143 criterion, and the local resolution estimation was performed to determine the local resolution of the final map in cryoSPARC.
Model building and refinement
The initial structure model of TauT was generated by AlphaFold246 and then fitted into the density map in UCSF Chimera47. Then, the model was manually adjusted and rebuilt in COOT48 iteratively to closely match the density map, including the refinement of mainchain and sidechains of residues. All models were further refined using ISOLDE. For the final refinement stage, model refinement was performed using Phenix49,50 (phenix. real_space_refine) with secondary structure and geometry restraints. For the ligands bound TauT complex, the two-dimension (2D) structure of taurine, β-Ala, GAA, GABA, P4S, I4AA, 5AVA, GES, nipecotic acid and Htau were downloaded from PubChem. All figures were prepared with software UCSF ChimeraX51.
Transport assays
The substrate uptake assay was performed in HEK293T cells overexpressing TauTWT and variants as described in previous study52 with modification. Briefly, HEK293T cells were cultured on poly-D-lysine-coated 24-well plates and transfected with plasmids when cell confluence reached 80−90%. After 48 h, the intracellular amino acids were depleted by incubating the cells in HBSS containing 5.5 mM D-glucose and 0.5% FBS for 1 h. Then, the cells were washed twice with phosphate-buffered saline (PBS) and incubated in PBS containing 0.5 μCi/mL [3H]taurine (Revvity) or [3H]]γ-aminobutyric acid (Revvity) for 30 min. HEK293T cells expressing only GFP served as controls. The specific uptake of [3H]taurine or [3H]γ-aminobutyric acid by the wild-type TauT and its variants was calculated by subtracting the uptake observed in the controls. The percentage of [3H]taurine or [3H]γ-aminobutyric acid uptake by the wild-type TauT and its variants was then normalized to the indicated results. For the inhibition assay, the cells were incubated in PBS containing 0.5 μCi/mL [3H]taurine and 0.6 mM P4S (MedChemExpress), 0.8 mM I4AA (MedChemExpress), 3 mM GES (MedChemExpress), 1.5 mM 5AVA (MedChemExpress), 2 mM nipecotic acid (MedChemExpress) or 3.5 mM Htau (MedChemExpress). As for measuring the IC50 value of six inhibitors, the cells were incubated in PBS containing 0.5 μCi/mL [3H]taurine and specified concentration gradient of P4S, I4AA, GES, 5AVA, nipecotic acid or Htau. Then, the cells were washed four times with ice-cold 0.1 M MgCl2, and lysed with 1 M KOH for 2 h. The lysate was then used for liquid scintillation counting with MicroBeta JET (PerkinElmer) and for the BCA protein assay (Thermo Fisher Scientific).
For GAA uptake assay, cells were incubated in PBS containing 1 mM guanidinoacetate-15N, 13C2 (MedChemExpress) for 1 min. Then the cells were washed three times with ice-cold PBS, extracted GAA with 1 mL of pre-chilled (-80 °C) 80% (v/v) methanol solvent, and the extractions were placed at -80 °C for 30 min. Cells were then scraped down from the plate and transferred to a tube. After a centrifugation at 12,000 rpm, 4 °C for 10 min, the supernatant was transferred to a new tube for vacuum concentration and then stored at -80 °C until quantitative mass spectrometry analysis. The precipitate was lysed with 1 M KOH for the BCA protein assay.
Microscale thermophoresis
Binding of TauTWT and mutants to taurine, β-Ala, GAA, GABA and six small molecule compounds were measured by microscale thermophoresis (MST) experiments using the NanoTemper Monolith NT. 115 instrument. The His-tagged TauTWT and TauT mutants were purified by size exclusion chromatography in the assay buffer containing 25 mM Hepes, 150 mM NaCl, 0.02% DDM (w/v) and 0.004% CHS (w/v), pH 7.5. The purified TauTWT and TauT mutants were labeled with the Monolith His-Tag Labelling Kit RED-tris-NTA 2nd Generation (Cat# Mo-L018, NanoTmper). Ligand stocks (200 mM taurine, 5 M β-Ala, 24 mM GAA, 200 mM GABA, 50 mM I4AA, 200 mM P4S, 200 mM GES, 200 mM 5AVA, 200 mM Htau and 200 mM nipecotic acid) were diluted in the assay buffer at a series of gradual concentrations. For MST measurements, ligands solutions of different concentrations were mixed with the same volume of the labeled protein to final protein concentration of 50 nM and final ligand concentrations in the mM to nM range. The mixed samples were incubated in the assay buffer at room temperature for 15 min and loaded into capillaries (NanoTemper Technologies). The thermophoresis experiments were carried out using 60% light-emitting diode (LED) and medium MST power at 25 °C. The dissociation constant value (Kd) of each reaction was calculated using MO. Affinity Analysis Software (v.2.3, NanoTemper Technologies) with the Kd fit function. Capillaries displaying aggregation or adsorption were excluded. Binding affinities are mean values with standard deviation derived from at least three independent binding experiments. The binding curves were plotted by GraphPad Prism v.9.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We thank all staff members of the Cryo-EM Centre, Southern University of Science and Technology for providing data collection and computation support. We thank professor Maofu Liao from Southern University of Science and Technology and professor Yixiao Zhang from Chinese Academy of Sciences for their helpful discussions. This work was supported by National Natural Science Foundation of China (32271251 to K. Y., 32130048 and 92157301 to L. Chen), Guangdong Innovative and Entrepreneurial Research Team Program (2021ZT09Y104, 2021QN02Y429 to K. Y.), Shenzhen Science and Technology Program (No. JCYJ20220530115214033, No. KQTD20210811090115021, and No. 20231120160100001 to K. Y.), the Ministry of Science and Technology of China National Key R&D Program (2022YFA0806503 to L. Chen), and China Postdoctoral Science Foundation (2023T160351 to L. Cheng). K. Y. is an investigator of SUSTech Institute for Biological Electron Microscopy.
Author contributions
B. D. and J. X. purified the proteins and collected cryo-EM data. B. D. performed the cryo-EM analysis and the model building. L. Chen and L. Cheng designed and performed the functional analyses. B. D. and K.Y. conceived the project and wrote the paper.
Peer review
Peer review information
Nature Communications thanks Katsuhisa Inoue, Harald Sitte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Atomic model of TauTApo, TauTTau, TauTβ-Ala, TauTGAA, TauTGABA, TauTP4S, TauTI4AA, TauTGES, TauT5AVA, TauTNipecotic acid and TauTHtau have been deposited in the PDB database (http://www.rcsb.org/) and are publicly available under the accession codes 9K0C, 9K1B, 9K0N, 9K0O, 9K1F, 9K1H, 9K1I, 9K1X, 9K1V, 9K1Z and 9K21. The cryo-EM density of TauTApo, TauTTau, TauTβ-Ala, TauTGAA, TauTGABA, TauTP4S, TauTI4AA, TauTGES, TauT5AVA, TauTNipecotic acid and TauTHtau are available in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/emdb/) under the accession codes EMD-61943, EMD-61970, EMD-61948, EMD-61949, EMD-61974, EMD-61975, EMD-61976, EMD-61983, EMD-61981, EMD-61985 and EMD-61987, respectively. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bowen Du, Lili Cheng, and Jiaying Xie.
Contributor Information
Ligong Chen, Email: ligongchen@tsinghua.edu.cn.
Kaige Yan, Email: yankg@sustech.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62857-w.
References
- 1.García-Ayuso, D. et al. Taurine: a promising nutraceutic in the prevention of retinal degeneration. Neural Regen. Res.19, 606–610 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripps, H. & Shen, W. Review: taurine: a “very essential” amino acid. Mol. Vis.18, 2673–2686 (2012). [PMC free article] [PubMed] [Google Scholar]
- 3.Kilb, W. & Fukuda, A. Taurine as an essential neuromodulator during perinatal cortical development. Front Cell Neurosci.11, 328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C. et al. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci.231, 116584 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Pasantes-Morales, H. & Hernández-Benítez, R. Taurine and brain development: trophic or cytoprotective actions? Neurochem. Res.35, 1939–1943 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Castelli, V. et al. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther.27, 403–412 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemming, A. Tumour cell consumption of taurine exhausts CD8(+) T cells. Nat. Rev. Immunol.24, 306 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Cao, T. et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8(+) T cells. Cell187, 2288–2304.e2227 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Hayes, K. C. Taurine nutrition. Nutr. Res Rev.1, 99–113 (1988). [DOI] [PubMed] [Google Scholar]
- 10.Surai, P. F., Earle-Payne, K., Kidd, M. T. Taurine as a natural antioxidant: from direct antioxidant effects to protective action in various toxicological models. Antioxidants (Basel)10, 1876 (2021). [DOI] [PMC free article] [PubMed]
- 11.Baliou, S. et al. Significance of taurine transporter (TauT) in homeostasis and its layers of regulation (Review). Mol. Med. Rep.22, 2163–2173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaffer, S., Takahashi, K. & Azuma, J. Role of osmoregulation in the actions of taurine. Amino Acids19, 527–546 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Bidri, M. & Choay, P. Taurine: a particular aminoacid with multiple functions]. Ann. Pharm. Fr.61, 385–391 (2003). [PubMed] [Google Scholar]
- 14.Santulli, G. et al. Functional role of taurine in aging and cardiovascular health: an updated overview. Nutrients15, 4236 (2023). [DOI] [PMC free article] [PubMed]
- 15.Wei, W. et al. PTER is a N-acetyltaurine hydrolase that regulates feeding and obesity. Nature633, 182–188 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jangra, A. et al. Emergence of taurine as a therapeutic agent for neurological disorders. Neural Regen. Res.19, 62–68 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, K., Shi, Y., Liu, W., Liu, S. & Sun, M. Z. Taurine improves neuron injuries and cognitive impairment in a mouse Parkinson’s disease model through inhibition of microglial activation. Neurotoxicology83, 129–136 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Oh, S. J. et al. Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci. Rep.10, 15551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadros, M. G., Khalifa, A. E., Abdel-Naim, A. B. & Arafa, H. M. Neuroprotective effect of taurine in 3-nitropropionic acid-induced experimental animal model of Huntington’s disease phenotype. Pharm. Biochem. Behav.82, 574–582 (2005). [DOI] [PubMed] [Google Scholar]
- 20.García-Ayuso, D. et al. On the potential therapeutic role of taurine in retinal degenerative diseases. Acta Ophthalmologica102, S274 (2024).
- 21.Heller-Stilb, B. et al. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. Faseb j.16, 231–233 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Ito, T. & Murakami, S. Taurine deficiency associated with dilated cardiomyopathy and aging. J. Pharm. Sci.154, 175–181 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Tao, X., Zhang, Z., Yang, Z. & Rao, B. The effects of taurine supplementation on diabetes mellitus in humans: a systematic review and meta-analysis. Food Chem. (Oxf.4, 100106 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ural, C. et al. The renoprotective effects of taurine against diabetic nephropathy via the p38 MAPK and TGF-β/Smad2/3 signaling pathways. Amino Acids55, 1665–1677 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Singh, P. et al. Taurine deficiency as a driver of aging. Science380, eabn9257 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castagna, M. et al. The lepidopteran KAAT1 and CAATCH1: orthologs to understand structure-function relationships in mammalian SLC6 transporters. Neurochem Res.47, 111–126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pramod, A. B., Foster, J., Carvelli, L. & Henry, L. K. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol. Asp. Med.34, 197–219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharm. Rev.63, 585–640 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Kubo, Y. et al. Involvement of TauT/SLC6A6 in taurine transport at the blood-testis barrier. Metabolites12, 66 (2022). [DOI] [PMC free article] [PubMed]
- 30.Tomi, M., Tajima, A., Tachikawa, M. & Hosoya, K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim. Biophys. Acta1778, 2138–2142 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Kubo, Y., Akanuma, S. I. & Hosoya, K. I. Impact of SLC6A transporters in physiological taurine transport at the blood-retinal barrier and in the liver. Biol. Pharm. Bull.39, 1903–1911 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Yasunaga, M. & Matsumura, Y. Role of SLC6A6 in promoting the survival and multidrug resistance of colorectal cancer. Sci. Rep.4, 4852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stary D., Bajda M. Taurine and creatine transporters as potential drug targets in cancer therapy. Int. J. Mol. Sci.24, 3788 (2023). [DOI] [PMC free article] [PubMed]
- 34.Leach, M. J. Effect of taurine on release of 3H-GABA by depolarizing stimuli from superfused slices of rat brain cerebral cortex in vitro. J. Pharm. Pharm.31, 533–535 (1979). [DOI] [PubMed] [Google Scholar]
- 35.Valembois, S., Krall, J., Frølund, B. & Steffansen, B. Imidazole-4-acetic acid, a new lead structure for interaction with the taurine transporter in outer blood-retinal barrier cells. Eur. J. Pharm. Sci.103, 77–84 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Richter, M., Moroniak, S. J. & Michel, H. Identification of competitive inhibitors of the human taurine transporter TauT in a human kidney cell line. Pharm. Rep.71, 121–129 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Suárez, L. M. et al. The taurine transporter substrate guanidinoethyl sulfonate mimics the action of taurine on long-term synaptic potentiation. Amino Acids48, 2647–2656 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Ejiri, K., Akahori, S., Kudo, K., Sekiba, K. & Ubuka, T. Effect of guanidinoethyl sulfonate on taurine concentrations and fetal growth in pregnant rats. Biol. Neonate51, 234–240 (1987). [DOI] [PubMed] [Google Scholar]
- 39.Wang, K. H., Penmatsa, A. & Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature521, 322–327 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayak, S. R. et al. Cryo-EM structure of GABA transporter 1 reveals substrate recognition and transport mechanism. Nat. Struct. Mol. Biol.30, 1023–1032 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, A. et al. Molecular basis for substrate recognition and transport of human GABA transporter GAT1. Nat. Struct. Mol. Biol.30, 1012–1022 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Stary D., Bajda M. Structural studies of the taurine transporter: a potential biological target from the gaba transporter subfamily in cancer therapy. Int. J. Mol. Sci.25, 7339 (2024). [DOI] [PMC free article] [PubMed]
- 43.Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol.193, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput Chem.25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D. Biol. Crystallogr.66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol.74, 531–544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci.30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji, L. et al. Slc6a8-Mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity51, 272–284.e277 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic model of TauTApo, TauTTau, TauTβ-Ala, TauTGAA, TauTGABA, TauTP4S, TauTI4AA, TauTGES, TauT5AVA, TauTNipecotic acid and TauTHtau have been deposited in the PDB database (http://www.rcsb.org/) and are publicly available under the accession codes 9K0C, 9K1B, 9K0N, 9K0O, 9K1F, 9K1H, 9K1I, 9K1X, 9K1V, 9K1Z and 9K21. The cryo-EM density of TauTApo, TauTTau, TauTβ-Ala, TauTGAA, TauTGABA, TauTP4S, TauTI4AA, TauTGES, TauT5AVA, TauTNipecotic acid and TauTHtau are available in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/emdb/) under the accession codes EMD-61943, EMD-61970, EMD-61948, EMD-61949, EMD-61974, EMD-61975, EMD-61976, EMD-61983, EMD-61981, EMD-61985 and EMD-61987, respectively. Source data are provided with this paper.