Abstract
The continued development of clustered regularly interspaced short palindromic repeats (CRISPR) technology has the potential to greatly impact clinical medicine, particularly for disease diagnosis and treatment. Despite high demand for the in vivo delivery of CRISPR-based therapies, significant challenges persist. These include rapid degradation by enzymes, inefficient disease site targeting, and the risk of undesired off-target outcomes. Nanoparticulate platforms, with their tailorable properties, have been engineered to efficiently package CRISPR payloads in various formats, including as plasmid DNA, mRNA, and ribonucleoprotein complexes, for in vivo delivery. Among them, recombinant adeno-associated viruses, virus-like particles, and lipid nanoparticles have displayed exceptional promise. This review will discuss the development of these and other nanocarriers for in vivo CRISPR-based genome editing.
Keywords: clustered regularly interspaced short palindromic repeats (CRISPR), in vivo gene editing, nanocarriers, recombinant adeno-associated viruses (rAAVs), virus-like particle (VLP), lipid nanoparticle (LNP)
Graphical Abstract

1. Introduction
The ability to edit the genomic information of living cells has represented a pivotal breakthrough in scientific research [1]. This achievement has been realized through the development of three major technologies: zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the most recent innovation, clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas). ZFNs and TALENs are chimeric nucleases comprised of DNA-binding domains for precise site-specific targeting and DNA cleavage domains [2]. Unlike ZFNs and TALENs, which rely on protein-DNA interactions to determine editing positions, CRISPR systems utilize Watson-Crick base pairings facilitated by a guide RNA, either as a CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) complex or a chimeric single-guide RNA (sgRNA), to direct Cas proteins [3]. As a result, CRISPR gene editing systems have become more widely used due to the relative ease of programming guide RNAs for targeting [4]. With the advancement of this technology, different variants with unique properties have been developed [5]. For example, Cas9 is used for DNA editing, Cas13 for RNA editing, base editors for single nucleotide modifications, and prime editors for precise nucleotide substitutions. CRISPR technology has revolutionized numerous fields, ranging from biology and medicine to agriculture [6]. One of the most exciting applications is for the treatment of genetic diseases, with several ongoing clinical trials and the recent approval of the first CRISPR-based cell therapy marking significant progress in the field [7]. Needless to say, there is a high demand for the in vivo delivery of CRISPR systems, yet this demand remains largely unmet [8]. Notably, there are currently no ongoing Phase III clinical trials for in vivo CRISPR-based gene editing. Challenges with the in vivo delivery of CRISPR payloads include rapid degradation, low targeting efficiency, obstacles in cytosolic localization, potential immunogenicity, and the risk of off-target effects.
Nanosized carriers have been developed for various drug delivery applications, leveraging the unique properties of nanoparticles such as their large surface area-to-volume ratio, small and controllable size, and ability to be modified with different functionalities [9]. A variety of nanocarriers have been developed, including polymer, lipid, protein, metallic, and inorganic nanoparticles [10]. Compared to their free form, drugs encapsulated within nanocarriers oftentimes exhibit improved efficacy in vivo as a result of their increased stability, targeted delivery, and stimuli-responsive release, among other properties [11]. In addition to artificial nanocarriers, researchers have investigated those derived from cells and microorganisms, which exhibit various biomimetic functions [12, 13]. Examples of purely biological carriers include viral vectors and exosomes [13, 14]. By integrating the strengths of both artificial and natural materials, cell membrane-coated nanoparticles are imparted with cell-mimicking properties such as prolonged circulation, immune evasion, and homotypic targeting [15, 16]. The development of novel nanoparticulate platforms offers promising solutions to the challenges associated with the in vivo application of CRISPR technology.
CRISPR systems are available in three formats: DNA-based systems with plasmids encoding both Cas and sgRNA, mRNA-based systems comprising Cas mRNA and sgRNA, and ribonucleoprotein (RNP) complexes formed by Cas proteins bound to sgRNA [17]. Each of these payload formats possesses a net negative charge and is large in size, rendering it difficult to encapsulate [18]. To date, three classes of nanocarriers have demonstrated exceptional promise for the in vivo delivery of CRISPR systems: viral vectors, virus-like particles (VLPs), and lipid nanoparticles (LNPs). Among viral vectors, recombinant adeno-associated viruses (rAAVs), which encode Cas proteins and sgRNAs for CRISPR-mediated gene editing, are the most widely used in preclinical studies and clinical trials [19]. Derived from viruses, VLPs lack viral genomes while retaining the delivery capabilities of viral vectors [20]. With the appropriate modifications, CRISPR systems in either an mRNA or RNP format can be encapsulated into VLPs. LNPs are capable of encapsulating all CRISPR payload formats via electrostatic interactions with positively charged cationic and/or ionizable lipids [21]. For in vivo delivery, mRNA-based LNP formulations have been the most studied, although there has also been considerable interest in developing RNP-based LNP formulations [22].
In this review (Fig. 1), we first cover the evolution of CRISPR technology, from its discovery to its applications in biomedicine, and provide insights into challenges encountered with its in vivo application. Then, three key types of nanocarriers—viral vectors, VLPs, and LNPs—are discussed in detail.
Figure 1.
Overview of in vivo CRISPR delivery using nanocarriers. CRISPR payloads, including plasmid DNA, mRNA with sgRNA, and RNPs can be formulated into nanocarriers such as viral vectors, VLPs, and LNPs, thereby increasing their efficiency when delivered in vivo.
2. Development of CRISPR technology
2.1. Discovery
In 1987, researchers from the University of Osaka reported palindromic repeats in the genome of Escherichia coli, later dubbed CRISPR [23]. These DNA arrays, found in various prokaryotes, contained duplicates of viral sequences called spacers in between the palindromic repeats, introducing the hypothesis that they were components of an adaptive immune function against viral infection [24]. Further research would substantiate this claim, as researchers discovered that these spacer sequences would be transcribed as guide RNA, which directs Cas to precisely cleave out integrated viral sequences in the microbial genome [6].
CRISPR's advent as a gene editing tool was only recently recognized in 2012. Researchers at the University of California Berkeley elucidated the mechanisms of the type II CRISPR/Cas system and demonstrated the successful engineering of a chimeric tracrRNA and crRNA duplex to direct Cas9 endonuclease cleavage in bacterial cells [3]. The crRNA contains the targeting sequence, while the tracrRNA serves to recruit Cas proteins. The following year, the Massachusetts Institute of Technology researchers established that this system could edit genomic loci of human cells while successfully engineering the Cas9 enzyme as a nickase, introducing a pathway to homology-directed repair (HDR) [25].
Cas9 exclusively cuts DNA sequences upstream to the protospacer adjacent motif (PAM) sequence NGG, with N representing any nucleotide. This affinity towards the PAM allows Cas9 to differentiate between sequences in the spacers and integrated viral sequences in bacteria, preventing cleavage of the CRISPR array itself [26]. To cut a specific sequence of DNA, researchers synthesize a complementary sgRNA, which forms a RNP complex with the Cas9 protein [27]. The PAM-interacting region of the Cas9 protein surveys the genome for PAM sequences. Once the Cas9 latches onto a PAM sequence, it unwinds the DNA helix. If the sgRNA can bind, the Cas9 creates a double-stranded break (DSB) with its nuclease domains HNH and RuvC [28].
Cas9 is only one protein of the Cas family. Cas proteins from different species and systems, or with different modifications and PAM sequences, have been utilized in various CRISPR-based editing strategies [29, 30]. For example, Cas12a, as a type V CRISPR/Cas system, can produce sticky ends instead of the blunt ends created by Cas9 and has been utilized for multiplexed gene regulation [31]. Type I and type III systems, represented by Cas3 and Csm respectively, can produce single-stranded breaks and have been applied in human cells for broad unidirectional genome editing and high-efficiency RNA knockdown [32, 33]. Additionally, novel engineered CRISPR/Cas systems, such as base editors and prime editors, have been created for donor-free genome editing [34, 35].
2.2. Editing strategies
Traditional genome editing with CRISPR centers around two main concepts (Fig. 2(a)). When a DSB is introduced into genomic DNA, our cells naturally resort to nonhomologous end joining (NHEJ) to repair DNA without a donor DNA template. However, after Cas9 cleavage, the overhangs can be incompatible, leading to insertion–deletion mutations (indels) and a loss of function in that gene [36]. Thus, NHEJ has been used to address dominant mutant alleles such as hypermorphs and antimorphs. For example, a gain of function mutation in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene leads to overdegradation of the low-density lipoprotein (LDL) receptor, preventing the removal of LDL cholesterol. Treatments made using Cas9-packaged LNPs have been shown to successfully knock out the PCSK9 gene in vivo in primates [37].
Figure 2.
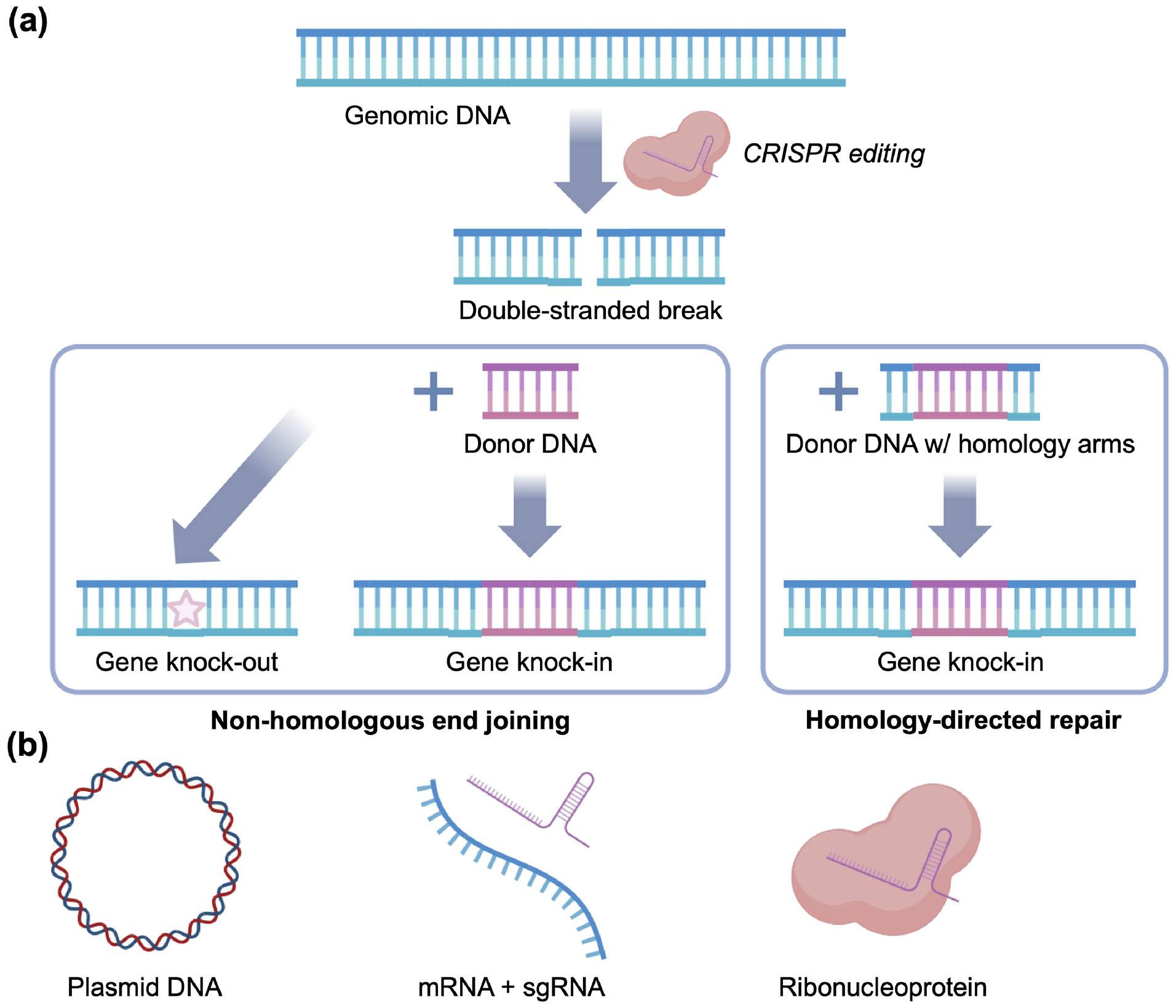
Genome editing using CRISPR/Cas. (a) Traditional CRISPR systems can knock-out or knock-in genes by two main strategies. Genomic DNA is cleaved to form a double-stranded break, which is subject to nonhomologous end joining. In the absence of donor DNA, this can create mutations that result in gene knock-out. Donor DNA with compatible overhangs can also be provided to facilitate gene knock-in. If the donor DNA is flanked by homology arms, the double-stranded break can go through the homology-directed repair pathway, which also results in gene knock-in. (b) CRISPR payloads come in three main formats: plasmid DNA, mRNA plus sgRNA, and RNPs.
For gene knock-in, homology-independent targeted integration (HITI) utilizes NHEJ but introduces a donor DNA template with Cas cleavage sites [38]. As the Cas enzyme cleaves both the target and the donor DNA with blunt DSBs, the cell can repair the DNA sequence with accuracy via NHEJ [39]. While HITI has been demonstrated with the wild-type CRISPR/Cas9 system, modifications can be made to Cas9 that allow for greater specificity and better suitability for HITI repair. For example, eSpCas9 (with a manipulated groove amino acid) and HypaCas9 (with a manipulated Rec3 domain) can provide better fidelity [40, 41].
HDR is another method for introducing genetic information and requires the co-delivery of donor DNA with homology arms matching the cleaved overhangs. This serves as a template for mending the genomic DNA, allowing precise editing of the target sequence [42]. However, this repair pathway is inefficient, making it more suitable for diseases where limited correction of cells is sufficient to alleviate the ailment [43]. HDR can be further optimized with the use of Cas12a; unlike the blunt cuts in Cas9, Cas12a creates a staggered DSB of 5 nucleotides [31]. Strategies that utilize both NHEJ and HDR at the same time have also been explored, with an example being ligation-assisted homologous recombination [44].
Biochemical modifications to Cas9 have conferred new functionalities, including the ability to make single-strand nicks [45, 46]. For example, base editors are dead Cas9 (dCas9) proteins conjugated with a cytosine or adenosine deaminase [47-49]. The former converts nucleotides on the free strand to uracil, which introduces the A-T base pair; the latter converts adenosine to inosine, introducing the C-G base pair. This engineering is further utilized in prime editors, where the nCas9 (which can only nick one strand) is fused with a reverse transcriptase that integrates the sequence of a pegRNA template into the host’s DNA [50, 51]. This system facilitates specific editing of a sequence with minimal NHEJ or HDR.
Large-scale genome editing can be achieved using CRISPR technology. For example, mutations in introns can be removed without overly disrupting the open reading frame via two guide RNAs targeting two cleavage sites spanning several mega bases apart. This has been demonstrated by using CRISPR/Cas9 to treat Duchene muscular dystrophy and Leber congenital amaurosis type 10 [52, 53]. It is also possible to achieve large genome edits using Cas3, which is a type I Cas system that can create deletions spanning several kilobases with defined boundaries via the Cascade complex [32]. Other than deletion, large-scale editing can initiate an allelic exchange between two homologous chromosomes to repair DNA mutations [54].
The editing of genomes risks off-target mutations [55]. As an alternative strategy, RNA-targeted editing provides a temporary and failsafe strategy in disease treatment. RNA-targeting Cas enzymes like Cas13 can better degrade mRNA sequences with more specificity than RNAi [56, 57]. By integrating dCas9 with a transcriptional repressor domain or an activator domain, Cas proteins can also interfere with transcription regulation [58, 59].
2.3. Formats
CRISPR-based therapy can be applied in various forms (Fig. 2(b)), mainly as either a DNA plasmid, mRNA with sgRNA, or an RNP complex. A DNA plasmid for CRISPR encodes for both the Cas protein and sgRNA [60]. While this can enable durable, long-lasting expression, the use of DNA carries an increased risk of integrative mutagenesis [61]. Its bulky size also precludes the use of some classes of delivery vehicles. The use of mRNA is another nucleic acid-based strategy for implementing CRISPR therapy [62]. Unlike plasmid DNA, mRNA does not require entrance into the cell nucleus, thereby diminishing barriers to effective expression [63]. mRNA also does not integrate into the genome and is transiently expressed, presenting a better safety outlook. However, mRNA carries its own set of challenges, such as its susceptibility to degradation [64]. Nucleic acid-based therapies do not immediately take effect, as time is required for the Cas protein to be transcribed and/or translated [65]. As such, the sgRNA also risks degradation before complexation with the Cas protein. These problems can be addressed by directly delivering an RNP complex formed by the Cas protein with an sgRNA. Although potent when properly delivered, RNP complexes are hard to package due to their large weight of approximately 160 kDa [66]. The protein form of Cas proteins can also be complex to extract and purify, which could present problems in terms of manufacturing for clinical translation [67].
2.4. Applications
The advent of CRISPR technology has enabled considerable strides to be made in biomedical research. In biology, the technology has paved the way for rapid advancements by introducing effective and straightforward screening methods that directly manipulate gene expression to unveil cellular mechanisms [68]. When it comes to the field of next-generation detection assays, CRISPR is also at the forefront. Based on the SHERLOCK technology developed to detect nucleic acids using CRISPR nucleases, a one-pot testing assay using Cas12b was shown to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), significantly streamlining the detection process and reducing the number of instruments needed [69, 70].
In therapeutics, CRISPR has been leveraged for the development of chimeric antigen receptor T cells. For example, it can be used to knock out the expression of native T cell receptors and histocompatibility antigens to provide an alternative to autologous T cells [71, 72]. This creation of off-the-shelf T cells avoids the complex and time-consuming process of extracting autologous T cells, which may already be poor in quality due to the suboptimal health condition of cancer patients.
Very recently, the Food and Drug Administration (FDA) has approved the first CRISPR-based gene therapy. In a collaboration, CRISPR Therapeutics and Vertex Pharmaceuticals developed exagamglogene autotemcel, a therapy against sickle cell disease [73]. The condition is exacerbated by a lack of fetal hemoglobin and γ-globin expression, which can be induced by the transcription factor BCL11A [74]. Treatment involves Cas9-mediated cleavage of BCL11A in CD34+ hematopoietic stem cells and progenitor cells, which are then transplanted into the patient. Clinical studies found that patients have their levels of fetal hemoglobin restored to normal after 15 to 18 months, although longer follow-ups are needed [75].
There are over 6000 monogenic diseases, and only 600 currently have a treatment, indicating boundless horizons for the expansion of gene-editing therapeutics [76]. However, the infusion of cells that are manipulated ex vivo as a mode of treatment is time-consuming and potentially immunogenic. The in vivo application of CRISPR technology to address the root cause of genetic diseases is highly desirable [77].
2.5. Clinical trials
Research on the in vivo application of CRISPR-based therapeutics is still in its preliminary stages. Currently, no in vivo CRISPR/Cas therapeutics has passed Phase III clinical trials, and there have been a number of setbacks. In an attempt to treat Duchene muscular dystrophy, the only patient of the Cure Rare Disease clinical trial died after administration of a dSaCas9 packaged within an rAAV serotype 9 vector [78]. Postmortem examination revealed that the patient suffered acute respiratory distress syndrome, and a significant amount of vector was found in the lungs compared to other tissues. As such, the patient was suspected to have passed due to an innate immune response directed towards the viral vector, necessitating further discussions on safety.
Another bottleneck of in vivo CRISPR therapy development is the profitability of treating genetic diseases that are severely crippling but affect small populations. Editas Medicine presented an AAV5-packaged dual guide RNA and SauCas9 formulation named EDIT-101 that targets intron splice donor sites in CEP290 of Leber congenital amaurosis patients [79]. However, preclinical trials showed that only 3 out of 14 subjects obtained clinically meaningful results, 2 of which had the same specific mutations [80]. Given that the pool of patients with the disease itself was already limited, the trials were paused.
Current ongoing clinical investigations for the in vivo delivery of CRISPR-based therapeutics include Intellia and Regeneron’s NTLA-2001 against transthyretin amyloidosis and Intellia’s NTLA-2002 for the treatment of hereditary angioedema [81, 82]. For NTLA-2001, the therapy directly cuts the gene encoding transthyretin, preventing both mutated and viable proteins from being made. NTLA-2002 works by downregulating the inflammatory protein bradykinin in order to establish a balance with its mutated inhibitor protein C-1. Both mRNA-based treatments utilize an LNP as the carrier, enabling them to be delivered to liver cells. Similarly, Verve Therapeutics’s VERVE-101 uses an mRNA-loaded LNP formulation to inhibit the expression of LDL cholesterol by turning off the PCSK9 gene in the liver utilizing a base editor instead of Cas9 [37, 83]. Using in vivo CRISPR formulations also extends to treating viral diseases, such as Excision’s EBT-101 formulation of an AAV-packaged CRISPR/Cas9 with dual guide RNAs that target three sites within the human immunodeficiency virus (HIV) genome [84].
Overall, for the in vivo application of CRISPR technology, there are several key factors that need to be considered for clinical success. First, any potential toxicity, both acute and long-term, needs to be carefully evaluated. Second, the performance and efficacy of these platforms in humans may differ considerably compared with what is observed in preclinical studies; as such, animal models that can better recapitulate human biological responses to treatment are in high demand. Third, any candidate formulation must ultimately be scalable and amenable to strict quality control measures.
2.6. Challenges for in vivo delivery
In vivo CRISPR therapy faces some challenges that are distinct from those of other gene therapies [85]. This is due to the fact that the approach consists of multiple components: the Cas protein, the sgRNA, and an exogenous DNA template (in the case of gene knock-in). All of these components need to be biochemically active after being transported through complex biological environments in vivo and localized within the cellular nucleus to fully exploit the utility of the CRISPR system [86]. These preconditions make CRISPR payloads extremely sensitive to degradation by various intracellular and extracellular enzymes, including RNases, DNases, and proteases [87, 88].
Furthermore, the positive charge of the Cas protein makes it susceptible to nonspecific interactions while in circulation, which can cause rapid clearance [89, 90]. The protein itself is also immunogenic, which may lead to poor performance over time and safety problems [91]. CpG motifs in oligonucleotides can trigger an anti-pathogen immune reaction, and since bacteria themselves express Cas proteins, there are oftentimes preexisting antibodies among humans. Some solutions for lowering immunogenicity include using nonviral vectors and modification of the oligo backbone [46].
Another challenge in the application of CRISPR technology is that, due to its numerous components, the payloads oftentimes exceed the loading capacity of common nanocarriers. For example, the AAV vector has a size limit of 5 kb, but a DNA plasmid encoding for all CRISPR-related components can easily reach 10 kb. A solution to this involves splitting the plasmid into two components in different delivery units or using nonviral vectors, each of which is hindered by various inefficiencies [92].
Off-target activity is another challenge associated with the in vivo application of CRISPR. sgRNA sequences are relatively short, and an increased tolerance to mismatches makes it possible for cleavage by Cas nucleases to happen away from the intended target site [93]. It has been reported that the use of CRISPR-based editing in animals can result in off-target mutations in both the treated animals and their offspring [94, 95]. Off-target prediction strategies play a crucial role in guiding sgRNA design to minimize these unwanted effects. These include alignment-based models such as CasOT and Crisflash [96, 97], as well as scoring-based models like CCTop and DeepCRISPR [98, 99]. To detect nonspecific editing, cell-free methods such as Digenome-seq and DIG-seq can be employed to directly identify genomic cleavages by CRISPR systems on extracted DNA or chromatin [100, 101]. Cell culture-based methods, including ChIP-Seq and GUIDE-seq, as well as in vivo detection methods such as Discover-Seq and GUIDE-tag, have also been developed [102-105].
A number of strategies have been reported to reduce undesired editing outcomes. One approach is to create high-fidelity versions of Cas proteins such as HypaCas9, eSpCas9, and SpCas9-HF1 [40, 41, 106]. Chemical modifications to the sgRNA backbone, including the modification of nucleotides with 2’-O-methyl-3’-phosphonoacetate and the replacement of partial ribonucleotides with deoxyribonucleotides, have also been implemented [107, 108]. In terms of payload format, RNPs have been considered a superior choice for minimizing off-target editing, which can be attributed to their heightened susceptibility to degradation [109]. Finally, paired nickase strategies can be used to improve specificity, as unpaired nicks can be repaired by the high-fidelity base excision repair pathway [110, 111].
3. Nanocarriers for in vivo CRISPR delivery
3.1. Viral vectors
3.1.1. Adeno-associated virus (AAV)
As members of the Parvoviridae family, AAVs are nonenveloped DNA viruses with a 4.7-kb single-stranded DNA genome, encapsulated by a T = 1 icosahedral capsid assembled by VP1, VP2, and VP3 (Fig. 3(a)) [112]. The genome encodes the rep and cap genes to produce proteins for replication and capsid assembly, respectively, along with two flanking T-shaped hairpin structures named inverted terminal repeats (ITRs) [113, 114]. The 145-nucleotide ITRs play a crucial role in directing the replication and packaging processes during AAV production [115, 116]. Due to the absence of rep and cap genes, rAAVs are rendered replication-deficient, exhibiting low immunogenicity and toxicity following in vivo administration [117, 118]. In contrast to wildtype AAVs, rAAVs lack the ability to actively integrate into the genome and predominantly exist in episomal form [119, 120].
Figure 3.
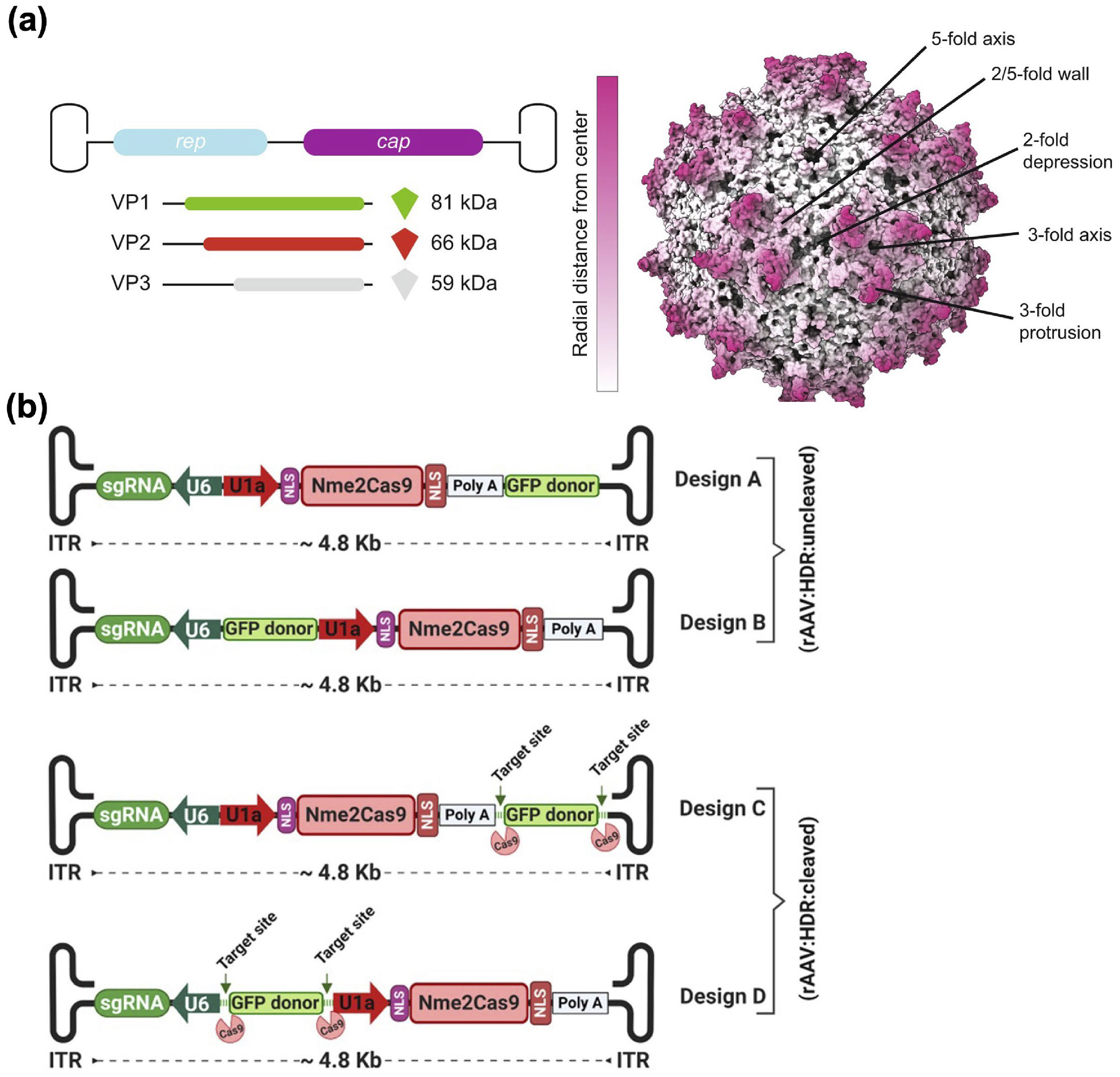
AAV vectors for CRISPR gene editing. (a) Components and structure of an AAV. Reproduced with permission from Ref. [112], © Worner, T. P. et al. 2021. (b) Examples of all-in-one rAAV vectors for CRISPR delivery. Reproduced with permission from Ref. [132], © Ibraheim, R. et al. 2021.
Diverse serotypes of AAVs with tropisms for distinct tissues have been utilized, augmenting the in vivo applicability of rAAVs for tissue-specific delivery [121, 122]. rAAVs have emerged as a leading platform for the in vivo delivery of CRISPR systems, evidenced by abundant ongoing preclinical and clinical trials targeting a spectrum of diseases, such as Leber congenital amaurosis type 10, HIV infection, and alpha-1-antitrypsin deficiency [123, 124]. Overall, developing rAAVs for CRISPR delivery poses several challenges, with size limitations, host immune responses, and undesired editing outcomes being noteworthy [125].
Limited by their capsid packaging capacity, AAVs are constrained to a genome length of approximately 5 kb [126]. In rAAVs, two ITRs with a total length of about 0.3 kb are required, leaving only approximately 4.7 kb for the transgene cassette [127]. A classical SpCas9 sequence alone occupies 4.2 kb, and when combined with the requisite sgRNA, promoters, and polyadenylation signals for CRISPR-mediated gene editing, the total package easily surpasses the 4.7-kb limit [128]. To address this limitation, numerous strategies have been explored, broadly falling into two categories: reducing the size of the CRISPR payloads and splitting the components into multiple vectors.
Many efforts have focused on reducing the size of Cas proteins [129]. Various Cas9 homologs with reduced sizes have been reported. St1Cas9, Nme2Cas9, SaCas9, and CjCas9 are compact alternatives to the classical SpCas9 with sizes more than 1 kb shorter [130, 131]. In one study, two all-in-one single rAAV vectors were engineered, incorporating Nme2Cas9 along with either (1) one sgRNA and a donor template for HDR or (2) two sgRNAs for segmental deletions (Fig. 3(b)) [132]. Following in vivo administration, both vectors demonstrated effective editing. Apart from Cas9, Cas12 nucleases have also been utilized for CRISPR-based editing, and they are generally compact with good multiplexing capabilities [133]. The hypercompact Un1Cas12f1 spans only 1.4–1.6 kb [134]. However, its broader application is hindered by low activity in eukaryotic cells. To address this limitation, a guide RNA was modified in five sites and subsequently packaged with Un1Cas12f1 into a single rAAV, resulting in an impressive 867-fold increase in indel frequency in human cells [135].
In the case of Cas-effector fusion platforms, such as base editors and primer editors, their size can exceed 5 kb with little room for minimization [50, 136]. A universal approach to accommodating large CRISPR elements involves segmenting them, with each part packaged into a separate rAAV [129]. To regenerate the complete construct in these multi-vector systems, three strategies have been utilized: genome recombination, mRNA trans-splicing, and protein trans-splicing [125].
At the genomic level, inter-vector DNA recombination can be facilitated through overlapping sequences, ITRs, or a recombinogenic sequence. This process generates recombined vector DNA within the host cell encoding the complete CRISPR construct [137]. As an example, the 6.1-kb ABE7.10 adenine base editor gene was divided into two rAAVs, one of which included the sgRNA, promoters, and N-terminal half of ABE7.10, while the other contained the C-terminal half [138]. Upon cellular delivery, the two vector genomes were recombined at the ITRs, and the intron along the ITRs was excised to generate full-length ABE7.10. At the pre-mRNA level, two transcripts from distinct genomes can undergo partial hybridization through trans-splicing, facilitated by splicing donor and acceptor sequences present in the separate transcripts [139].
Recently, a dual-rAAV technology based on mRNA trans-splicing was developed as a versatile approach for reconstituting split genes such as CRISPR [140]. Lastly, constructs translated from different genomes can be reconstituted using a pair of split inteins located at the termini of each protein, a process known as protein trans-splicing [141]. Compared to mRNA trans-splicing, the protein version does not require complex host cell machinery, making it self-sufficient and potentially applicable across various cell types [142]. In one study, split cytosine and adenine base editors delivered by dual rAAVs were reconstituted through inteins, resulting in therapeutically relevant levels of genomic editing after in vivo administration (Fig. 4) [143]. Notably, base editors reconstituted employing intein-mediated trans-splicing exhibited a 4.5-fold higher editing efficiency compared to those recombined by other methods.
Figure 4.
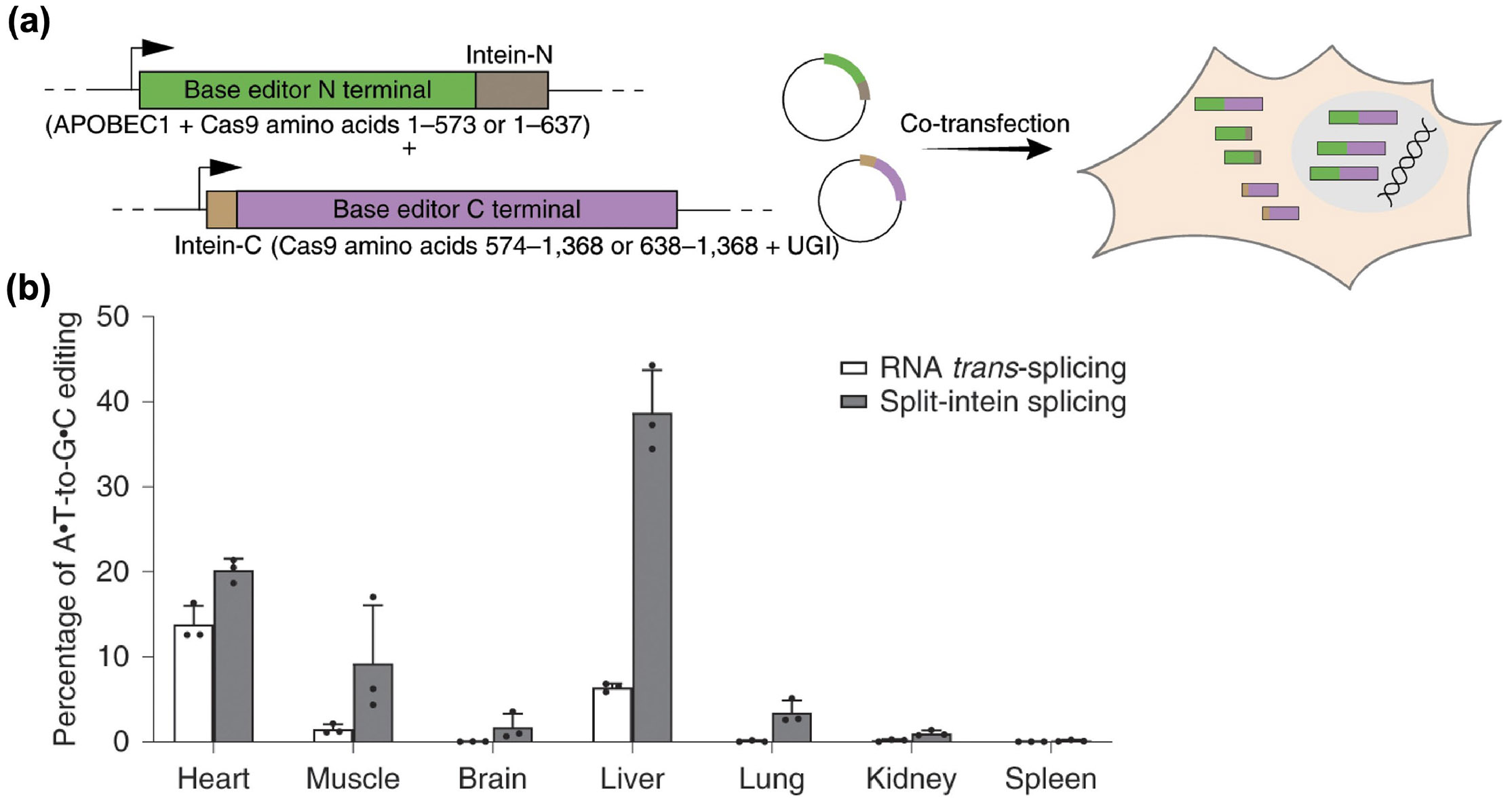
Dual-rAAV platform for base editor delivery. (a) Design of a dual-rAAV system to deliver a base editor through protein trans-splicing. (b) Comparison of in vivo editing efficiency between systems using different recombination mechanisms. Reproduced with permission from Ref. [143], © Levy, J. M. et al., under exclusive licence to Springer Nature Limited 2020.
Immunological barriers, including preexisting neutralizing antibodies, as well as adaptive and innate immune responses, constitute a significant impediment to effective rAAV-mediated CRISPR therapy. Due to the prevalence of AAV infections, it has been reported that approximately 40%–80% of the human population tests positive for neutralizing antibodies against various AAV serotypes [117, 144]. Additionally, pre-existing immunity against Cas proteins has been observed in humans [145, 146]. Neutralizing antibodies can significantly impede the in vivo delivery of CRISPR therapy mediated by rAAVs, particularly following intravenous administration [147]. To overcome this obstacle, plasmapheresis has been utilized to directly remove neutralizing antibodies from the blood [148, 149]. Additionally, empty rAAV vectors, designed to sequester neutralizing antibodies, have been utilized as decoys [150]. When coadministered systemically with functional rAAVs, this strategy was able to overcome the inhibitory effect of neutralizing antibodies. To circumvent the inhibitory impact of preexisting neutralizing antibodies in humans, researchers have explored rAAVs derived from non-human origins, including AAVrh.8, AAVrh.10, and AAVrh.43 obtained from non-human primates [117, 151]. Leveraging modern biotechnologies such as structure-guided evolution, rAAV variants devoid of epitopes recognized by preexisting neutralizing antibodies can be generated for immune evasion [152].
Upon in vivo administration, the capsids of rAAVs can elicit specific humoral and cellular immune responses, which will dramatically impact efficacy after repeated dosing [153]. Employing transient B cell depletion and utilizing immunosuppressive agents such as rapamycin can effectively inhibit adaptive immune responses, enabling the possibility of repeated rAAV administration [154, 155]. In addition, innate responses triggered by the capsids and genomes of rAAVs can hinder in vivo efficacy by inducing the production of proinflammatory cytokines and type I interferons [156]. As one of the main drivers of innate immunity against rAAVs is Toll-like receptor 9-mediated signaling, strategies have been developed to mitigate its effects by incorporating inhibitory DNA sequences and deleting CpG dinucleotide motifs in the viral genomes [157, 158].
Controlled transient expression of CRISPR machinery in target sites is preferred to minimize undesired outcomes. Various strategies such as induced expression and self-cleavage have been developed to achieve this goal [125]. For instance, researchers employed a doxycycline-inducible promoter to regulate the timing of CRISPR/Cas9 expression, allowing for inducible in vivo genome editing [159, 160]. Furthermore, researchers have developed a self-deleting AAV-based CRISPR system by incorporating an additional sgRNA targeting the Cas9 coding sequence. This innovative approach has been shown to reduce Cas9 levels by over 79% while maintaining efficient on-target editing efficiency [161]. Inspired by cell-specific expression patterns of miRNAs [162, 163], researchers have developed an miRNA-regulated rAAV platform. This system included an additional component with an anti-CRISPR protein and an miRNA binding site [164, 165]. In the cells where the appropriate miRNA was expressed, the CRISPR enzymes were protected from degradation, thus enabling them to function.
The tropisms of natural AAV serotypes have been leveraged for tissue-specific delivery. For instance, AAV1 demonstrates affinity for skeletal muscle, while AAV8 exhibits tropism for the liver [166]. However, natural tropisms typically offer only moderate specificity. To enhance targeting, various methods of capsid engineering such as directed evolution have been employed [167]. Subsequent screenings are then conducted to select rAAVs with potent targeting efficiency [168]. In addition to the genetic engineering of capsids, researchers have also explored modification with targeting ligands, such as peptides and proteins [169, 170].
3.1.2. Adenovirus (AdV)
As members of the Adenoviridae family, AdVs are non-enveloped, double-stranded DNA viruses with a genome approximately 36 kb in size [171]. Thanks to their features, such as a low off-target rate due to their lack of integration into the host genome and a large loading capacity of over 8 kb, AdVs have emerged as important viral vectors for the delivery of nucleic acids [120]. However, their in vivo application has been hindered by certain drawbacks, including the substantial immune responses elicited by AdV capsids and sequestration in the liver following systemic administration [171]. Over the course of their development, AdVs have been subjected continuous engineering, and they can be categorized into three generations [172]. A typical AdV genome comprises four early genes (E1-4) transcribed before DNA replication, five late genes (L1-5) transcribed post DNA replication, and flanking ITRs [173].
In first-generation recombinant AdVs (rAdVs), the E1 and/or E3 genes were deleted to abolish viral replication while increasing packaging capacity for foreign genes [174]. The major concern with first-generation rAdVs was the potent immune responses that they elicited in hosts. The second generation of rAdVs was developed by further deleting the E2 and E4 genes, aiming to reduce immunogenicity by minimizing the expression of viral genes [175]. In the third generation, the vector construct is now devoid of all native viral genes, leaving only the ITRs and foreign gene sequences [176]. Consequently, third-generation rAdVs exhibit significantly reduced immunogenicity, large packaging capacity, and have become the primary choice for in vivo applications.
rAdVs have been used to deliver CRISPR/Cas9 in vivo, facilitating the disruption of the PCSK9 gene and the correction of dystrophin gene mutations [177, 178]. In addition, an Ad5/35++ vector was constructed by incorporating an Ad35 fiber knob with high affinity to CD46, a receptor uniformly expressed on hematopoietic stem and progenitor cells [179]. Following systemic administration, the Ad5/35++ vector was used to express gene editing machinery within the target cells to disrupt a repressor binding region within the γ-globin promoter [180].
3.1.3. Lentivirus
Belonging to the Retroviridae family, lentiviruses are enveloped viruses with two copies of single-stranded positive-sense RNA [181]. Owing to their ability to efficiently transduce both dividing and nondividing cells, vectors derived from lentiviruses have been developed as potent gene delivery vehicles [182]. To prevent the production of replication-competent lentiviruses, third-generation self-inactivating vectors have been constructed and represent the most prevalent lentiviral platform utilized in clinical trials [183]. These vectors require the use of four plasmids for production: an Env-encoding plasmid expressing vesicular stomatitis virus glycoprotein (VSV-G), a packaging plasmid containing pol and gag genes, a plasmid encoding Rev, and a plasmid containing the gene of interest driven by a potent heterologous promoter with a deletion of the U3 region in the 3’ long terminal repeat [184]. The advantages of lentiviral vectors include their high packaging capacity of approximately of 8 kb, the ability to be engineered for various cellular tropisms, and their minimal preexisting immunity in humans [120, 185]. However, the propensity of lentiviral vectors to integrate into host genomes raises concerns regarding potential off-target events, rendering them less suitable for in vivo gene editing applications [186]. To address this issue and avoid long-term expression of Cas enzymes, integrase-deficient lentiviral vectors have been developed to prevent integration into host genomes [187]. As an example, an integrase-deficient lentiviral vector was utilized as an all-in-one platform for in vivo editing mediated by CRISPR/Cas9, demonstrating efficient editing in neurons following local injection and reduced off-target outcomes [188].
3.2. VLPs
Derived from viral vectors but lacking their genomic material, VLPs are nanosized carriers self-assembled from viral components such as capsids and envelope proteins [189]. VLPs have been developed to deliver CRISPR components in an mRNA or RNP format for transient gene editing [20]. As a result, VLPs can maintain high gene editing efficiency similar to viral vectors while minimizing off-target effects [190]. A typical CRISPR-delivering VLP contains a Gag polyprotein consisting of nucleocapsid protein, capsid protein, matrix protein, and others, which are essential for assembly. Additionally, it includes a Pol polyprotein encoding enzymes such as protease, integrase, and reverse transcriptase, an Env protein, and CRISPR components in the form of mRNA or an RNP [190, 191]. The primary focus in designing VLPs for CRISPR delivery is to encapsulate the payloads such that the gene editing components can eventually be localized and/or expressed in the cytoplasm [192].
3.2.1. mRNA delivery
To load mRNA into VLPs, researchers have utilized RNA aptamers along with aptamer-binding proteins (ABPs) due to their strong affinity for each other. Various aptamer/ABP pairs have been developed, including those utilizing λN22 peptide, Com, MS2 coat protein (MCP), and PP7 coat protein [193-196]. To load CRISPR payloads, including Cas mRNA and sgRNAs, into VLPs, two strategies have been developed. In the first strategy, Cas mRNA and the sgRNA are modified with aptamers, followed by co-packaging into VLPs self-assembled by Gag polyproteins modified with ABPs. For the second, the Cas mRNA is still modified for binding via aptamer/ABP interactions, while an sgRNA expression cassette is inserted into the viral genome RNA that carries a packaging signal for VLP encapsulation [20].
Various aptamer/ABPs pairs have been tested and optimized. For instance, in VLPs derived from gammaretroviral murine leukemia virus (MLV) and alpha-retroviral Rous sarcoma virus, the nucleocapsid domain of the Gag polyprotein was substituted with two copies of MCP. The 3’ untranslated region (UTR) of SpCas9 mRNA and the sgRNA were each modified with two copies of the corresponding aptamer [197, 198]. The resulting VLPs were able to accommodate large amounts of SpCas9 mRNA and sgRNA. Interestingly, the VLPs also contained some translated SpCas9 protein. Compared to insertion within the sgRNA sequence, placing the aptamer adjacent to the sgRNA scaffold resulted in superior editing efficacy.
Recently, a novel approach termed the selective endogenous encapsulation for cellular delivery (SEND) system was reported [199]. This system utilized PEG10, a Gag homolog, as the capsid. Leveraging the natural function of PEG10, which can bind to its own mRNA and mediate self-assembly, SpCas9 mRNA and sgRNA were flanked with the 5’ UTR and portion of the 3’ UTR of PEG10. With PEG10 as the capsid protein and VSV-G as the envelope protein, these components were assembled together to produce VLPs. Approximately 30% indels were achieved in a Neuro-2a cell line when using murine PEG10 and 40% indels were achieved in HEK293 cells using the human version.
Studies on Cas mRNA and sgRNA co-delivery have revealed that the latter may be unstable when packaged alone inside VLPs [190, 197, 199]. It is believed that, in the packing process, translation of the Cas protein results in the formation of RNP complexes that stabilize the sgRNA as it is incorporated within the VLPs. To address the inherent instability of sgRNA, a strategy involving the use of viral genomic RNA to aid in its delivery has been developed. For example, an all-in-one CRISPR lentiviral particle platform was constructed, comprising Gag polyproteins fused with one MCP in the N-terminus, SpCas9 mRNA with six copies of an MCP-binding sequence in the 3’ UTR, U6-sgRNA cassettes, and integrase-incompetent Pol polyproteins, as well as wildtype Gag, VSV-G, and Rev (Fig. 5) [200]. Following a single subretinal injection, the VLPs achieved 44% on-target editing of Vegfa in retinal pigment epithelium and reduced choroidal neovascularization area by 63% in a laser-induced wet age-related macular degeneration mouse model. Utilizing sgRNAs targeting two essential genes of herpes simplex virus type 1 (HSV-1), UL8 and UL29, the same platform effectively blocked viral replication and prevented the occurrence of herpetic stromal keratitis in various mouse models [201]. Additionally, it inhibited HSV-1 replication in human-derived corneas.
Figure 5.
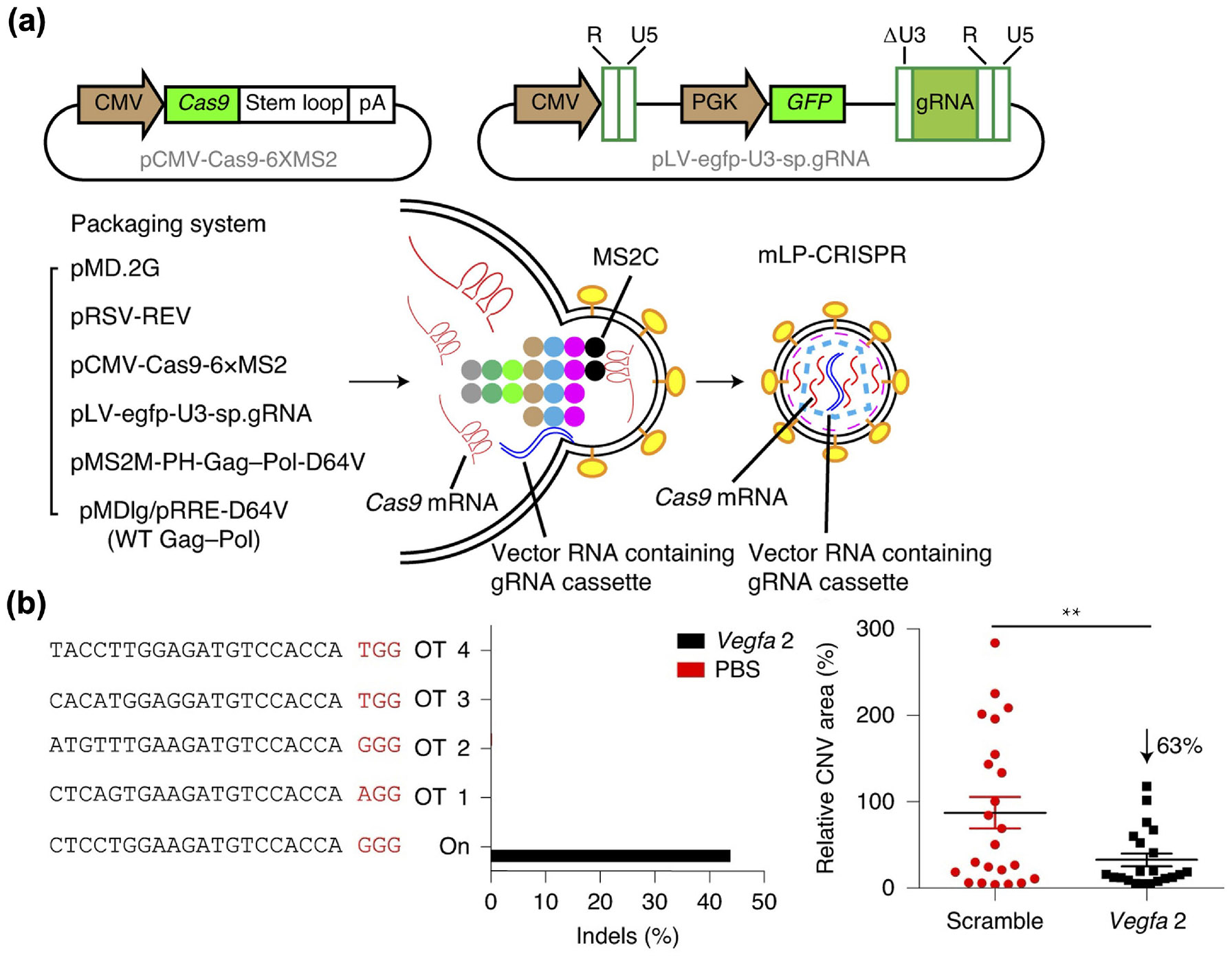
VLPs for the co-delivery of Cas9 mRNA and an sgRNA cassette. (a) Design and preparation of the all-in-one lentiviral particle CRISPR platform. (b) In a laser-induced wet age-related macular degeneration mouse model, the platform with sgRNA targeting Vegfa 2 was potent in inducing on-target indels and significantly reduced choroidal neovascularization (CNV) area. Reproduced with permission from Ref. [200], © Ling, S. et al., under exclusive licence to Springer Nature Limited 2021.
Compared to MLV and HIV, another retrovirus named foamy virus has been discovered to efficiently encapsulate and deliver nonviral RNAs both in vitro and in vivo [202]. Remarkably, the incorporation process relies solely on the Gag and Env proteins. Inspired by this discovery, a VLP/viral vector hybrid system was developed [203]. It comprised of SpCas9-delivering VLPs produced with SpCas9 mRNA, foamy virus Gag polyprotein fused with a matrix protein from HIV-1 Gag (to facilitate incorporation of non-foamy viral envelope proteins), and VSV-G as the envelope protein. The system also included integrase-deficient foamy viral vectors delivering sgRNA and/or HDR cassettes. Ultimately, the platform was able to facilitate edits such as T cell receptor knock-out in primary human T cells and the insertion of a green fluorescent protein gene (GFP) upstream of the human polo-like kinase 1 gene (PLK1) in HeLa cells.
3.2.2. RNP delivery
Avoiding the need for intracellular transcription and translation, RNP complexes have been recognized as an effective transient payload for gene editing [204]. VLPs have been developed to package and deliver RNPs, with strategies categorized by how the complexes interact with Gag.
The first strategy depends on ligand/receptor-mediated packaging of RNPs, with a common approach being the use of aptamer/ABP binding pairs. In this case, sgRNAs are modified with RNA aptamers and complexed with Cas proteins to form RNPs. These complexes then interact with ABP-modified Gag polyproteins to assemble into VLPs. In one study, an ABP was inserted after the second zinc finger domain of the viral nucleocapsid domain, and one copy of the corresponding aptamer was added to the sgRNA [205]. Different aptamer/ABP pairs and three insertion positions of the sgRNA were tested. The use of Com and its corresponding aptamer resulted in the best editing efficacy. For SaCas9 RNPs, replacing the tetraloop with the aptamer proved to be the most efficient, while for SpCas9 and adenine base editor RNPs, insertion into stem loop 2 was the most effective [205-207]. The aptamer/ABP packaging method largely preserves the structure of Gag polyproteins and has the potential to be applied to different Cas proteins, provided the appropriate sgRNA modifications.
Other methods linking RNPs with Gag polyproteins have been developed. For instance, the 12-kDa FK506 binding protein (FKBP12), the FKBP12-rapamycin binding (FRB) domain, and the rapamycin analog AP21967 can form a ternary complex with high affinity [208]. Inspired by this, HIV Gag was fused with FKBP12, and SpCas9 was fused at the N-terminus with the FRB domain [209]. In the presence of AP21967, SpCas9 could be linked onto Gag to form VLPs. To enhance the incorporation of sgRNA, it was linked with the Tat activation response element and a packaging signal, facilitating binding to Gag. Additionally, the sgRNA was flanked by self-cleaving ribozymes to aid in its release. Ultimately, use of the modified sgRNA resulted in higher gene editing efficiency compared with unmodified sgRNA. Interactions between Gag and other viral proteins have also been leveraged to package RNPs into VLPs. For example, the 14-kDa viral protein R (Vpr) of HIV can bind to the p6 region of Gag, with each lentiviral particle containing around 550 copies of Vpr [210, 211]. Inspired by this, Cas9 proteins were fused with Vpr and combined with U6-sgRNA cassettes, natural Gag and Pol polyproteins, Rev proteins, and VSV-G to form VLPs [212]. The platform demonstrated a high RNP encapsulation and achieved efficient gene editing in cell lines and primary CD4+ T cells.
In addition to ligand/receptor-mediated packaging, the direct fusion of Cas protein with Gag is another important strategy to load RNPs into VLPs. In one study, MLV Gag was fused with SpCas9 at its C-terminus [213]. A proteolytic site between Gag and SpCas9 was introduced, which could be cleaved by MLV protease. VLPs were generated by transfecting HEK293T cells with plasmids encoding Gag-SpCas9, sgRNA, unmodified MLV Gag and Pol, and VSV-G. The platform mediated genome editing in mouse embryos after injection into the perivitelline space, leading to edits that could be passed on to offspring. Furthermore, two weeks after systemic administration of the VLPs, all mice exhibited on-target editing in the liver, with efficiencies ranging between 7% and 13%.
A recent work presented a paradigm for optimizing the fusion of Gag with a base editor to produce VLPs with high efficiency (Fig. 6) [214]. In this study, MLV Gag and ABE8e were fused together. First, the cleavage site between the two components was optimized, and the sequence TSTLLMENSS was identified as promoting the best editing. To further enhance the incorporation of RNPs into VLPs, ABE8e was flanked by nuclear localization signals and modified with three copies of nuclear export signals at various sites. Finally, the ratio between the Gag-ABE8e fusion construct and unmodified Gag/Pol was optimized. The final VLPs targeting PCSK9 were injected intravenously, resulting in approximately 62% on-target editing in the liver. This led to a significant 78% reduction in serum Pcsk9 one week post injection. For prime editor applications, additional platform optimizations were explored, including engineering of pegRNAs, modifications to the prime editor, elimination of a protease recognition site at the C-terminus of MLV reverse transcriptase, positioning of nuclear export signal insertion site, and flanking of protease cleavage sites with GGS linkers [215].
Figure 6.
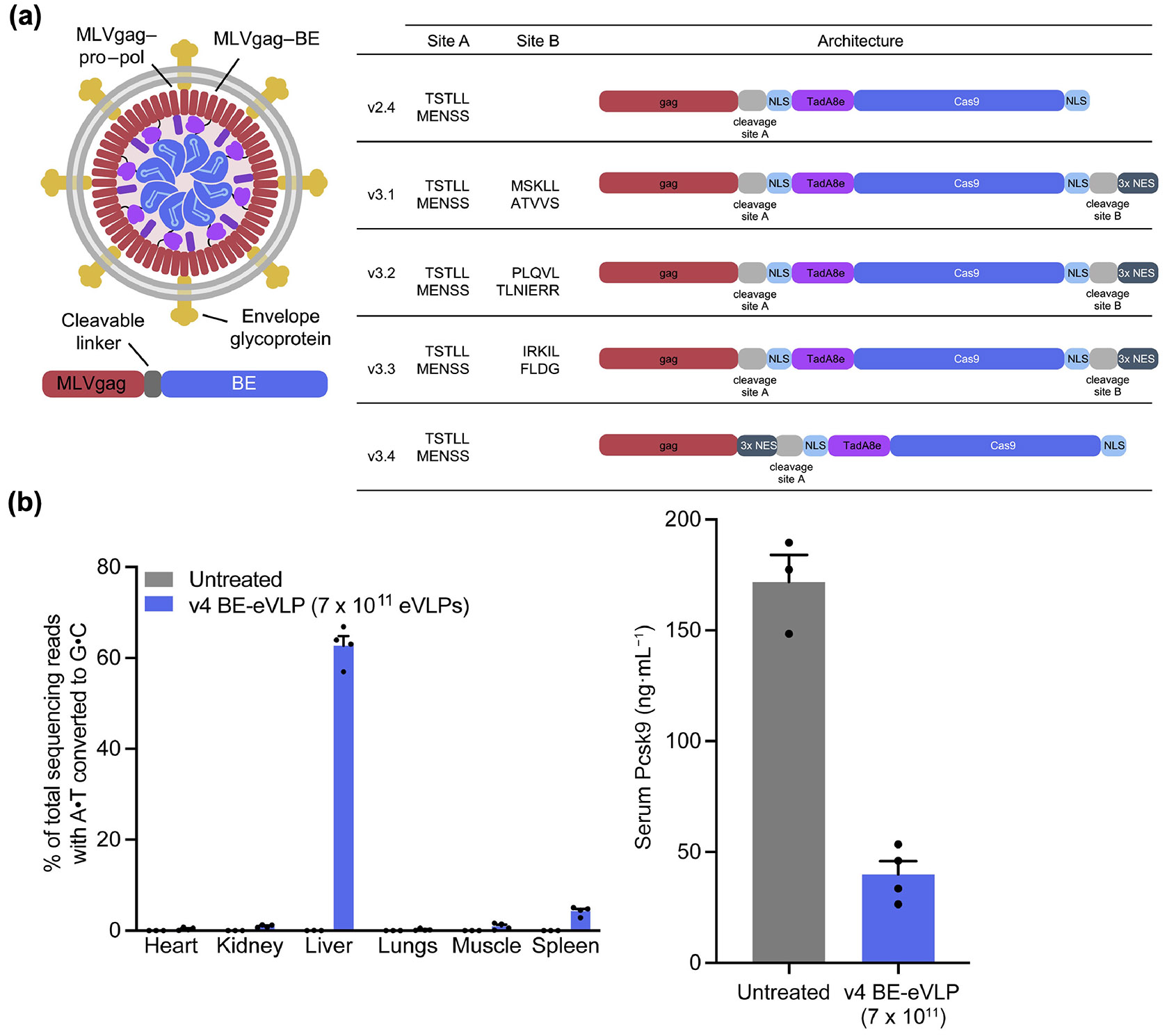
Optimization of VLPs for RNP delivery. (a) Design of a VLP for base editor delivery. Optimal placement of the cleavage site and nuclear export signal repeats is explored. (b) After systemic administration of the optimized VLP (v4 BE-eVLP) targeting PCSK9, high on-target editing efficiency and reduced serum Pcsk9 levels are observed. Reproduced with permission from Ref. [214], © Banskota, S. et al., published by Elsevier Inc. 2021.
By modifying their envelope, it is possible to engineer VLPs with unique functions, which is particularly useful for targeted delivery. In one example, a mutant VSV-G that retained endosomal fusion capabilities but lacked binding to the LDL receptor was employed. The envelope was further modified with antibody-derived single-chain variable fragments (scFvs) to confer specific targeting ability. The Gag protein was fused with Cas9 at the C-terminus, and the VLPs were constructed similarly to previous examples, comprising the Gag-Cas9 fusion, sgRNA, unmodified Gag/Pol, the mutant VSV-G, and scFvs. With T celltargeting scFvs directed against CD3, CD4, and CD28, the VLPs demonstrated the ability to perform in vivo gene editing [216].
3.3. LNPs
LNPs have been widely explored for delivering nucleic acids, with several formulations having been approved for clinical use, including Patisiran for siRNA delivery and two mRNA vaccines against SARS-CoV-2. A typical LNP consists of four components: phospholipids, cholesterol, a polyethylene glycol-linked lipid (PEG-lipid), and an ionizable or cationic lipid [217]. Phospholipids and cholesterol are seen as helper lipids that provide stability [218]. Currently approved LNPs utilize 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) as the phospholipid component; other choices include 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE). Interactions between cholesterol and phospholipids help maintain the lipid layer in an ordered phase, thereby preserving its integrity [218].
PEG-lipids consist of a PEG component, typically a linear chain with a molecular weight of several kilodaltons, attached to a lipid tail. One example used in the market is PEG-carbamate-1,2-dimyristoyl-sn-glycerol (PEG-DMG). The PEG-lipid inserts into the lipid layer of LNPs via its hydrophobic tail, and the PEG chains form a hydrophilic steric barrier on the surface to prevent aggregation [219, 220]. It has been observed that, in the absence of PEG-lipids, LNPs are unstable and polydisperse [221]. In contrast, addition of PEG-lipids in a molar ratio as low as 0.5% can lead to the formation of stable and homogenous LNPs [222]. In general, increased amounts of PEG-lipids will result in smaller LNPs, making it possible to exert some control over the final size [223]. The length of the lipid tail is correlated with the desorption rate of PEG-lipids from LNPs under biological conditions. For instance, 45% of PEG-lipids with C14 dialkyl chains dissociated from LNPs one hour after intravenous injection, whereas the dissociation rates for C16 and C18 dialkyl chains were lower at 1.3% and 0.2%, respectively [224]. In addition to increased stability, PEG-lipids can also contribute to prolonged circulation time by inhibiting recognition by the reticuloendothelial system [225].
Cationic lipids, such as 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), possess a positively charged head group and can encapsulate nucleic acids through electrostatic interactions [226]. However, it has been found that cationic lipids can induce cellular toxicity by interacting with enzymes such as protein kinase C [227]. Additionally, they may trigger immune responses, as evidenced by the release of type I and II interferons after in vivo administration [228]. As a result, ionizable lipids have been developed, which remain deprotonated under physiological conditions but present a positive charge in acidic environments below their acid dissociation constant (pKa). In general, ionizable lipids feature secondary or tertiary amines, while cationic lipids typically contain quaternary ammonium groups.
In a typical protocol for preparing nucleic acid-loaded LNPs with ionizable lipids [229], the lipid components are initially mixed in ethanol. Subsequently, this lipid mixture is combined with nucleic acids dissolved in an acidic buffer. During this process, the positively charged ionizable lipids facilitate the encapsulation of nucleic acids through electrostatic interactions. The mixture then undergoes dialysis into a near-neutral buffer like phosphate-buffered saline (PBS). During dialysis, the ionizable lipid becomes neutralized, resulting in LNPs with reduced toxicity and significantly prolonged circulation time in vivo [230]. After endosomal uptake, the acidic conditions within the endolysosomal compartment results in the protonation of the ionizable lipid, rendering it once again positively charged. This mediates endosomal escape through disruption of the endosomal membrane, allowing for cargo release into the cytosol [231]. Researchers have constructed large libraries of ionizable lipids with various combinations of hydrophilic amine heads and hydrophobic lipid tails [232]. These libraries have been subjected to large in vitro and in vivo screens to identify the most promising candidates for various applications.
3.3.1. Plasmid delivery
Plasmid-based in vivo gene editing presents several challenges, including the large size and negative charge of the payload [64]. Additionally, plasmids must be delivered into the nucleus, where they can be transcribed. In one example, a CRISPR plasmid encoding Cas9 and sgRNA sequences was mixed with chondroitin sulfate and condensed by positively charged protamine to form a stable core [233]. This complex was then further encapsulated within an LNP with DOTAP as the cationic lipid, DOPE as the phospholipid, cholesterol, and PEG-1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (PEG-DSPE) as the PEG-lipid. Intratumoral injection of a formulation targeting PLK1 resulted in effective gene knockdown in A375 cells and inhibited tumor growth.
Rather than relying on the addition of positively charged agents for plasmid condensation, there has been a shift towards screening appropriate ionizable lipids to formulate LNPs for plasmid delivery. For instance, the ionizable lipid iLY1809 was utilized in combination with DSPC, cholesterol, and PEG-DMG to encapsulate CRISPR/Cas9 plasmids [234]. After transfection with the resulting formulation, a 32% gene knock-out efficiency in HepG2-Luc cells was observed after 7 days, and the LNPs demonstrated enhanced endosomal escape. Following intravenous administration, the LNPs were primarily cleared through the liver and kidneys, and they showed prolonged retention in tumors for more than 5 days. Intratumoral injection resulted in growth inhibition in a murine HepG2-Luc model.
Presently, LNPs are not widely utilized for plasmid-based gene editing due to inefficiencies in encapsulation and delivery, as well as the relative success of viral vectors. Further explorations are needed to optimize LNPs for such applications.
3.3.2. RNP delivery
An RNP consists of a positively charged Cas protein and a negatively charged sgRNA strand, or alternatively a crRNA:tracrRNA duplex [235]. The resulting complex is overall negatively charged [17]. RNP-based gene editing offers several advantages, including its rapid action and potential for fewer off-target events due to its transient nature [236]. As a result, the utilization of LNPs for the in vivo delivery of RNPs has garnered considerable interest.
As they are negatively charged, Cas9/sgRNA complexes can be delivered both in vitro and in vivo using the cationic lipid carrier Lipofectamine RNAiMAX [237]. This led to up to 80% editing efficiency in U2OS EGFP reporter cells and up to 20% genome modification in hair cells on the inner ear of mice after local injection. Encouraged by this finding, a library of ionizable lipids was constructed through the Michael addition of (1) an acrylate with a disulfide bond and 14-carbon tail and (2) various primary or secondary amines [238]. The ionizable lipids, together with DOPE, cholesterol, and C16-PEG2000-ceramide, could encapsulate RNPs and self-assemble into LNPs. After screening in GFP-expressing HEK cells, LNPs utilizing the ionizable lipid 3-O14B demonstrated the highest efficiency.
While the typical method for preparing LNPs involves dissolving the payload in acidic buffer, the activity of Cas in the RNP complex can be affected under these conditions, suggesting the need to evaluate neutral buffers during preparation [239]. However, as ionizable lipids are deprotonated in neutral conditions and cannot effectively interact with RNPs, the inclusion of cationic lipids into the formulation has been explored. In one study, DOTAP was included as a cationic lipid alongside the ionizable lipid 5A2-SC8, DOPE, cholesterol, and PEG-DMG to successfully encapsulate RNPs into LNPs [22]. With high editing efficiency observed in HeLa-Luc and HeLa-GFP reporter cells, the LNPs were injected into a tdTomato reporter mouse model. Successful gene editing resulted in expression of the fluorescent protein. Subsequent local injections in the left leg and brain showed strong signal at the injection sites. Interestingly, by adjusting the molar ratio of DOTAP, organ-selective targeting was achieved after systemic administration, with a 10% molar ratio primarily targeting the liver and a 60% molar ratio primarily targeting the lungs.
The preparation of LNPs for RNP delivery has been evaluated in a clinically relevant microfluidic device [240]. In this case, an additional single-stranded oligonucleotide was introduced into the RNP complex to further increase its negative charge. A device with three inlets was utilized; the center inlet supplied the buffer, while the top and bottom inlets supplied lipids in ethanol and RNPs in aqueous buffer, respectively. Compared to a two-inlet device, the three-inlet version inhibited RNP aggregation at the junction. Two ionizable lipids, CL4H6 and CL15H6 [241, 242], along with the phospholipids DSPC and DOPE, were utilized in various molar ratios. Effective formulations that exhibited potent knock-out efficiency of GFP in HeLa-GFP cells were identified. After treatment using a formulation with a crRNA targeting hepatitis B, HepG2-hNTCP-30 cells infected with the virus exhibited levels of viral DNA and covalently closed circular DNA lower than the untreated group.
A direct comparison between RNPs and mRNA for CRISPR-based gene editing was conducted by using the same LNP formulation, which consisted of C12-200 as the ionizable lipid, DOTAP as a cationic lipid, DOPE, cholesterol, and PEG-DMG [243]. Compared to those loaded with mRNA, the LNPs loaded with RNPs exhibited a larger size, increased susceptibility to enzyme degradation, and lower editing efficiency in both HEK293T cells and Hepa1–6 cells. These results underscore the need to optimize LNPs specifically for RNP delivery rather than relying on formulations designed for mRNA delivery.
3.3.3. mRNA delivery
Cas mRNA with the appropriate sgRNA is the most widely employed payload format for LNP-based in vivo gene editing. An advantage of mRNA is its transient expression of Cas, which helps reduce off-target events [244]. However, mRNA can be challenging to deliver due to its susceptibility to degradation [245]. LNPs formulated to deliver Cas mRNA can protect the payload from degradation, facilitating targeted accumulation in vivo and mediating cytosolic delivery after cellular uptake.
In a notable study, a library of ionizable lipids was constructed by Michael addition of combinations of six amine heads and four acrylate tails [246]. The synthesized ionizable lipids were then combined with cholesterol, PEG-DMG, and one of three phospholipids (DSPC, DOPE, and 1,2-dioleoyl-sn-glycero-3-phosphocholine [DOPC]). Candidate LNPs were formulated with firefly luciferase (fLuc) mRNA, followed by intravenous administration to evaluate in vivo expression. Following this screening, a lead candidate was selected, which consisted of 306-O12B as the ionizable lipid and DOPC as the phospholipid, along with cholesterol and PEG-DMG. Subsequently, a CRISPR formulation to knock-out angiopoietin-like protein 3 (ANGPTL3) was injected into C57BL/6 mice. Compared to an FDA-approved LNP using DLin-MC3-DMA as the ionizable lipid [247], the optimized LNPs exhibited a higher on-target indel rate in the liver, corresponding to lower serum levels of ANGPTL3, LDL cholesterol, and triglycerides.
Chemical modifications of mRNA and sgRNA have also been extensively investigated to improve in vivo CRISPR editing efficiency. To enhance stability and reduce immunogenicity, various Cas mRNA modifications have been evaluated, including 5’ capping, 3’ polyadenylation, and the incorporation of modified nucleotides such as N1-methylpseudouridine (N1-me-Ψ), 5-methylcytidine (5meC), and 5-methyluridine (5meU) [248]. The 5’ capping of eukaryotic RNAs involves the addition of a 7-methylguanosine moiety to the first nucleotide, forming a Cap 0 structure that is crucial for recruiting translation initiation factors and preventing degradation [249]. Further methylation at the 2’ ribose position leads to the formation of a Cap 1 structure, which has been shown to reduce immunogenicity through the evasion of detection by RNA sensors [250, 251].
To enhance the stability and reduce immunogenicity of Cas9 mRNA, an improved construct was developed after evaluating various modification strategies [252]. These included 5’ capping with Cap 1, partial substitution with modified nucleotides such as N1-me-Ψ, 5meC, 5meU, and 5-methoxy uridine (5moU), uridine depletion via synonymous codons, and high-performance liquid chromatography (HPLC) purification to remove double-stranded RNA byproducts produced during in vitro transcription. Indel rates were assessed in CD34+ hematopoietic stem and progenitor cells, and immunogenicity was evaluated in the THP-1 cell line, whole human blood, and mice. Overall, uridine depletion combined with 5moU modification was the most effective strategy.
For the sgRNA component, researchers have explored various chemical modifications for enhancing its stability. Solid-phase synthesis enables the construction of heavily modified sgRNAs [253]. It is worth noting that this approach comes with higher costs and lower yields, particularly for long sgRNAs exceeding 100 nucleotides [254]. Consequently, click chemistry has been employed to ligate crRNA with tracrRNA to form a complete sgRNA [255]. A similar approach was used to combine a 20-nucleotide variable sequence with a 79-nucleotide Cas9-binding sequence to generate full-length sgRNA [256]. Chemical modifications of sgRNA can be categorized into three main groups: alterations of the linkages between nucleotides, modifications of the ribose sugar, and changes to the nucleobases [257]. To enhance enzyme resistance, alternatives to phosphodiester linkages such as phosphorothioate (PS) and phosphonoacetate linkages have been utilized [108, 258]. Modifications to the ribose sugars primarily involve substituting the 2’ hydroxyl group with alternative chemical groups, such as fluorine and methyl groups, which leads to the creation of modified nucleic acids referred as 2’-fluoro RNA (2’F RNA) and 2’-O-methyl RNA (2’OMe RNA), respectively [259]. Additionally, the modification of crRNA with bridged nucleic acids can reduce dwell time at off-target sites [260]. This behavior enhances the Cas9 specificity, thereby reducing off-target effects. Finally, the controlled activation of CRISPR systems can be achieved through nucleobase modification. 6-nitropiperonyloxymethylene can effectively suppress interactions between the sgRNA and its target site on double-stranded DNA [261]. Upon irradiation with ultraviolet light, the modified nucleobase undergoes photolysis, leading to the restoration of sgRNA function.
For LNPs, a comprehensive study was conducted to optimize sgRNA payloads for CRISPR-based gene editing in vivo (Fig. 7) [262]. The invariable region and targeting sequence of an sgRNA targeting GFP were subjected to chemical modifications, including the use of PS bonds, 2’F RNA, or 2’OMe RNA. The construct with the highest GFP knock-out efficiency, termed e-sgRNA, included 70 out of 101 nucleotides that were substituted and 34 phosphodiester bonds that were replaced with PS bonds. Cas9 mRNA and e-sgRNA targeting PCSK9 were then encapsulated into LNPs formulated using a cKK-E12 ionizable lipid, DOPE, cholesterol, and PEG2000-C14. Following intravenous injection, the formulation nearly eliminated Pcsk9 in the serum and exhibited significantly higher on-target editing efficiency compared to unmodified sgRNA [263].
Figure 7.
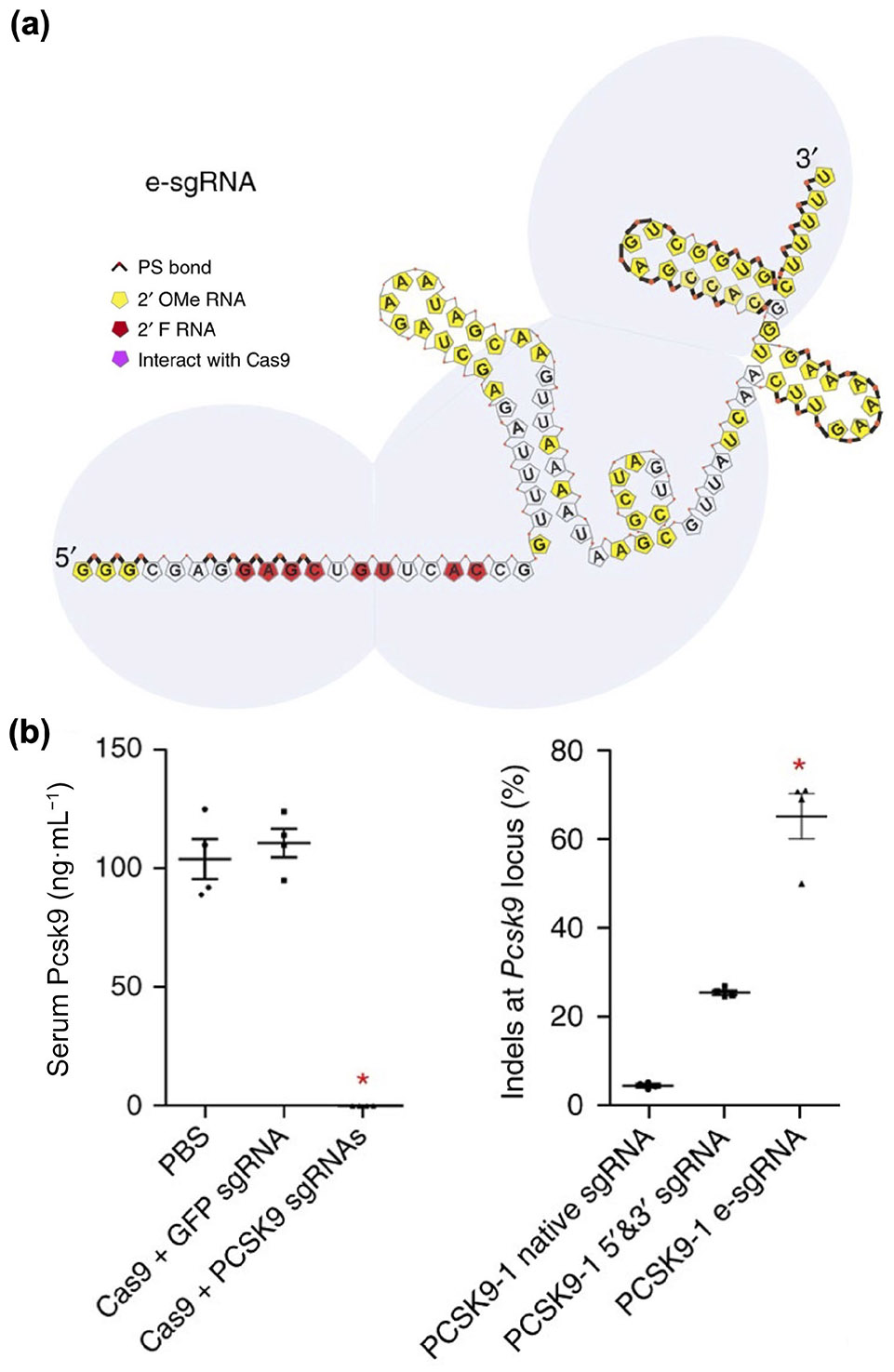
Chemically engineered sgRNA to enhance LNP-mediated CRISPR editing in vivo. (a) The optimized structure of a chemically modified sgRNA. (b) An LNP formulated with Cas9 mRNA and an optimized sgRNA targeting PCSK9 considerably reduces serum Pcsk9 levels with high on-target editing efficiency. Reproduced with permission from Ref. [262], © Springer Nature America, Inc. 2017.
3.3.4. Delivery strategies and challenges
For the majority of LNP formulations that are systemically administered, the surface will spontaneously adsorb apolipoprotein E (ApoE) along with other proteins and biomolecules to form a corona [264]. Many studies have reported that adsorbed ApoE interacts with fats such as triglycerides and cholesterol [265, 266]. Subsequently, protein-adsorbed LNPs can bind to the LDL receptor and heparan sulfate proteoglycans overexpressed on hepatocytes, thereby mediating liver accumulation [267]. The liver-targeting properties of LNPs make them suitable for treating hepatic diseases but pose challenges for the treatment of other conditions. Currently, three main strategies have been developed to deliver CRISPR payloads outside of the liver using LNPs: local injection, passive targeting by optimizing lipid components, and active targeting based on ligand/receptor interactions.
Compared to systemic injection, local administration can circumvent liver accumulation and directly deliver payloads to the disease site [268]. Furthermore, local delivery offers several advantages, including enhanced efficacy, reduced risk of off-target effects, and decreased systemic immunogenicity [85, 269]. For example, intrastromal injection of RNP-loaded LNPs has been shown to effect gene editing in the cornea of mice [270]. For Duchene muscular dystrophy, skipping exon 45 of the human version of the defective gene in the muscles of hEx45KI-mdx44 mice, whose exon 44 is missing, can restore the dystrophin reading frame and restore protein expression, thereby alleviating disease symptoms [271]. To accomplish this, an mRNA-based CRISPR LNP formulation was developed with two sgRNAs targeting the splicing acceptor site and donor site of exon 45 [272]. These sgRNAs were encapsulated with Cas9 mRNA into LNPs formulated using a TCL053 ionizable lipid, DPPC phospholipid, cholesterol, and DMG-PEG. The optimized LNPs were administered locally into the tibialis anterior muscle of hEx45KI-mdx44 mice a total of 6 times over a span of 2 weeks. Tissue samples collected 2 months post treatment showed that 38.5% of muscle fibers tested positive for dystrophin.
By refining their lipid components and preparation process, the physical properties of LNPs, including size, charge, and shape, can be tailored to facilitate organ-selective delivery [217]. It has been reported that nanoparticles smaller than 10 nm in diameter are quickly cleared by the kidney [274], while those larger than 150 nm tend to accumulate more in the spleen and lungs than in the liver [275, 276]. Different lipid components can exhibit varying affinities to serum biomolecules, thereby altering the composition of the protein corona and directing the LNPs to different organs [277]. One study revealed that LNPs incorporating DSPC as the phospholipid exhibited decreased affinity to ApoE in comparison to LNPs containing DOPE [278]. Consequently, the DSPC formulation demonstrated a tendency to accumulate more in the spleen and less in the liver compared to the DOPE formulation. Recently, a selective organ targeting (SORT) LNP platform was introduced, incorporating molecules with different charges to target distinct organs (Fig. 8) [273]. With the addition of the positively charged lipid DOTAP, the resulting mRNA-loaded LNP was able to achieve in vivo gene editing in the lungs, while the incorporation of negatively charged lipid 18PA enabled improved spleen localization.
Figure 8.
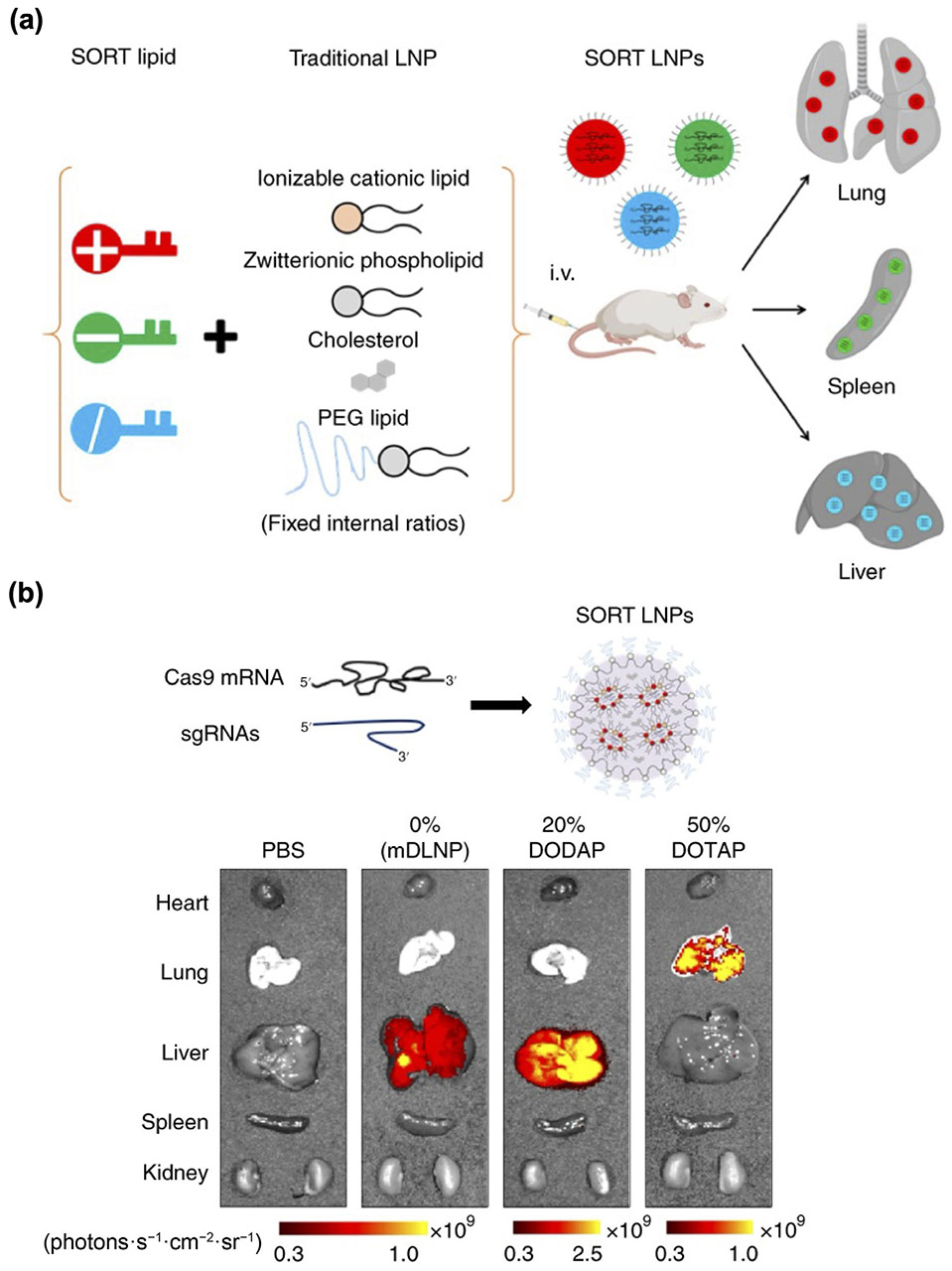
LNPs for organ-specific CRISPR delivery. (a) Addition of SORT lipids with different charges results in LNPs that can specifically deliver cargos to the lungs, spleen, or liver. (b) mRNA-based LNPs formulated with various SORT lipids demonstrate high gene editing efficiency in different organs. Reproduced with permission from Ref. [273], © Cheng, Q. et al., under exclusive licence to Springer Nature Limited 2020.
The incorporation of targeting ligands onto LNPs can facilitate receptor-mediated active delivery of CRISPR payloads. Various types of ligands, including peptides [280], antibodies [281], and aptamers [282], have been attached onto LNPs by chemical conjugation or through noncovalent methods such as lipid insertion. In one example, an anchored secondary scFv enabling targeting (ASSET) platform was constructed through the use of a membrane-anchored lipoprotein containing an scFv capable of binding to the Fc region of rat IgG2a antibodies [283]. Upon incubation with rat IgG2a antibodies with affinity for different receptors, the resulting LNPs acquired diverse targeting capabilities. Employing this ASSET platform, LNPs encapsulating Cas9 mRNA and sgRNA for PLK1 were modified to specifically target epidermal growth factor receptor (EGFR), which is highly expressed in human serous ovarian adenocarcinomas (Fig. 9) [279]. The targeted LNPs were administered via intraperitoneal injection into mice bearing OVCAR8 tumors. Compared to their untargeted counterparts, the targeted LNPs exhibited potent editing efficiency within the tumors, resulting in significant inhibition of tumor growth.
Figure 9.
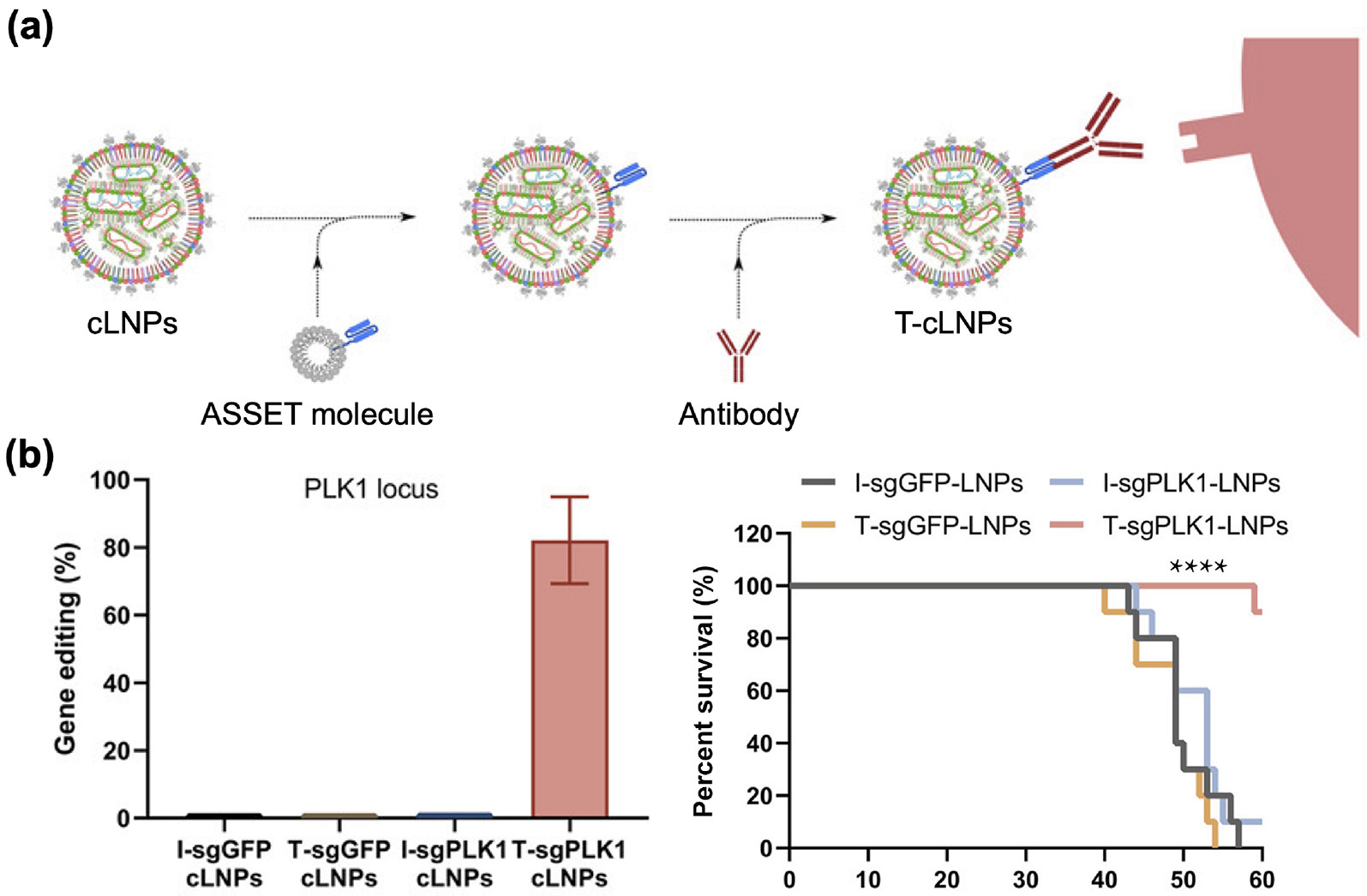
Antibody-modified LNPs for targeted CRISPR delivery. (a) LNPs loaded with CRISPR payloads (cLNPs) were modified with ASSET molecules, which enable subsequent antibody functionalization based on Fc binding. (b) Targeted cLNPs (T-cLNPs) loaded with sgRNA against PLK1 elicit potent gene editing efficiency in tumor cells and significantly improve the survival of tumor-bearing mice. Reproduced with permission from Ref. [279], © Rosenblum, D. et al., some rights reserved; exclusive licensee American Association for the Advancement of Science 2020.
4. Conclusions and outlook
In this review, we discussed the development of CRISPR technology, with a particular emphasis on three important types of nanocarriers for the in vivo delivery of CRISPR payloads (Table 1). The approval of the first CRISPR-based therapeutic, which relies on the ex vivo editing of stem cells, for treating sickle cell disease and transfusion-dependent β-thalassemia, underscores the bright future of the technology for addressing genetic diseases. However, effective in vivo CRISPR delivery has been hindered by challenges such as inefficient targeting, low efficacy, genotoxicity, and off-target editing [88]. To address these challenges, various nanoparticulate platforms have been developed, and significant efforts have been dedicated to optimizing their performance for in vivo applications [9].
Table 1.
Overview of common CRISPR nanodelivery systems
| System | Description | Advantage | Disadvantage |
|---|---|---|---|
| Viral vector | • Replication-deficient viruses for delivering CRISPR in the form of nucleic acids • Usually composed of a viral capsid, part of the viral genome, and CRISPR components |
• High in vivo editing efficiency • Natural tropism toward different cells and tissues • Endosomal escape capabilities |
• Possible immunogenicity • Limited packaging capacity • Complex production process • Possible mutagenesis |
| VLP | • Self-assembled viral particles for delivering CRISPR in the form of mRNA or RNPs • Classically composed of a Gag polyprotein for assembly, a Pol polyprotein encoding enzymes, an envelope protein, and CRISPR components |
• Relatively high editing efficiency • Modularity for enhanced functionality • Low risk of off-target edits and mutagenesis |
• Possible immunogenicity • Complex preparation process • Additional requirements for loading various CRISPR payloads |
| LNP | • Synthetic nanoparticles for delivering CRISPR in the form of a plasmid, mRNA, or RNPs • Typically consists of phospholipids, cholesterol, a PEG-lipid, an ionizable or cationic lipid, and CRISPR components |
• Low immunogenicity • High modularity for enhanced functionality • Straightforward preparation and lower cost • Low risk of mutagenesis |
• Relatively low in vivo editing efficiency • Requires the optimization of several components |
Various types of viral vectors have been utilized for CRISPR delivery, with rAAVs being the most extensively explored. However, the in vivo application of rAAVs is encumbered by three primary challenges: packaging size limitations, host immune responses, and unwanted editing events. To address these challenges, multiple strategies have been developed. For instance, researchers have explored the use of smaller Cas proteins or have split Cas genes into multiple rAAVs. Anti-vector immune responses can be addressed by removing preexisting neutralizing antibodies, employing rAAVs from non-human origins, transiently depleting B cells, and modifying genome sequences to reduce innate immunity. Furthermore, efforts have been undertaken to control the temporal expression of Cas proteins to minimize undesired editing outcomes.
VLPs have received increasing attention due to their viral vectorlike functions such as high in vivo gene editing efficacy, while lacking side effects such as genomic integration. For mRNA-based VLPs, the incorporation of sgRNAs can be unstable, and specialized loading strategies have been utilized to improve their delivery alongside Cas mRNAs. Aptamer/ABP interactions have also been leveraged to considerably improve Cas mRNA loading capacity. For RNP-based VLPs, the payload can be packaged into VLPs through various ligand/receptor interactions or through the direct fusion of Cas proteins with viral capsid proteins. Various capsid and Cas protein modifications have been explored to optimize the in vivo delivery of RNPs using VLP platforms.
LNPs are the most popular nonviral nanocarrier for delivering CRISPR payloads, including in the plasmid, mRNA, and RNP formats. A classical LNP consists of an ionizable and/or cationic lipid, phospholipid, cholesterol, and PEG-lipid. Among these components, the ionizable and/or cationic lipid plays a crucial role in payload packaging and affecting the in vivo fate of LNPs. Large libraries of ionizable lipids have been generated and screened both in vitro and in vivo for their impact on gene editing efficiency. In addition, to enhance their stability and potency, Cas mRNAs and sgRNAs have been subjected to various chemical modifications. While LNPs naturally target the liver, it is often desirable to deliver CRISPR payloads to other sites within the body. To achieve this, approaches such as local administration, passive targeting through the optimization of formulation parameters, and active targeting via ligand modification have been explored.
As researchers continue their attempts to establish the clinical feasibility of in vivo gene editing, further optimization of both CRISPR payloads and their nanocarriers is essential. Next-generation CRISPR therapies may increasingly utilize base editor, prime editor, and RNA-targeting systems, which may offer more precise gene editing with potentially lower off-target effects compared to first-generation DSB-inducing Cas9 editors [284]. The production costs of the different nanocarriers also need to be considered. For example, the large-scale production of rAAVs is currently cost-ineffective, limited by high variability during the transfection process, inefficient purification, and short shelf-life [285]. The large-scale production of VLPs as a CRISPR delivery platform is still being explored, although they will certainly benefit from the lessons learned from VLP-based vaccine production [286]. In comparison, the mass manufacturing of LNPs can be aided by microfluidic technology and has proven to be cost-effective [287, 288]. Emerging nanocarriers have the potential to improve the in vivo delivery of CRISPR payloads, offering novel functionalities that are not available to current generation platforms. For example, exosomes are natural nanovesicles that are biodegradable and can be engineered for the targeted delivery of CRISPR systems [289]. Cell membrane coating technology enables synthetic nanoparticle platforms to be camouflaged with a biocompatible layer of naturally derived cell membrane [290]. This provides the resulting core/shell nanostructure with cell-mimicking functions, including immune evasion, long circulation, and the ability to effectively biointerface with disease substrates [15, 291]. Cell membrane-coated nanoparticles have been utilized for diverse applications such as drug delivery [292], biodetoxification [293], and vaccination [294]. Along these lines, the application of biomimetic design principles and platforms [295] to CRISPR delivery could help to significantly boost in vivo gene editing efficacy in the future. Ultimately, continued research on CRISPR payload delivery will result in effective treatment options for genetic diseases, among other conditions, helping to alleviate patient suffering and improve public health.
Acknowledgements
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (No. HDTRA1-21-1-0010) and the National Institutes of Health (Nos. R21AI159492, and R21AI175904).
References
- [1].Doudna JA The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gaj T; Gersbach CA; Barbas CF III ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jinek M; Chylinski K; Fonfara I; Hauer M; Doudna JA; Charpentier E A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shamshirgaran Y; Liu J; Sumer H; Verma PJ; Taheri-Ghahfarokhi A Tools for efficient genome editing; ZFN, TALEN, and CRISPR. Methods Mol. Biol 2022, 2495, 29–46. [DOI] [PubMed] [Google Scholar]
- [5].Liu GW; Lin QP; Jin S; Gao CX The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell 2022, 82, 333–347. [DOI] [PubMed] [Google Scholar]
- [6].Wang JY; Doudna JA CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [DOI] [PubMed] [Google Scholar]
- [7].Liu WY; Li LX; Jiang JX; Wu M; Lin P Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precis. Clin. Med 2021, 4, 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosenblum D; Gutkin A; Dammes N; Peer D Progress and challenges towards CRISPR/Cas clinical translation. Adv. Drug Deliv. Rev 2020, 154–155, 176–186 [DOI] [PubMed] [Google Scholar]
- [9].Mitchell MJ; Billingsley MM; Haley RM; Wechsler ME; Peppas NA; Langer R Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov 2021, 20, 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Namiot ED; Sokolov AV; Chubarev VN; Tarasov VV; Schiöth HB Nanoparticles in clinical trials: Analysis of clinical trials, FDA approvals and use for COVID-19 vaccines. Int. J. Mol. Sci 2023, 24, 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chamundeeswari M; Jeslin J; Verma ML Nanocarriers for drug delivery applications. Environ. Chem. Lett 2019, 17, 849–865. [Google Scholar]
- [12].Meyer RA; Sunshine JC; Green JJ Biomimetic particles as therapeutics. Trends Biotechnol. 2015, 33, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davidson BL; Breakefield XO Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci 2003, 4, 353–364. [DOI] [PubMed] [Google Scholar]
- [14].Liang YJ; Duan L; Lu JP; Xia J Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu CM; Zhang L; Aryal S; Cheung C; Fang RH; Zhang LF Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U S A 2011, 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fang RH; Gao WW; Zhang LF Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol 2023, 20, 33–48. [DOI] [PubMed] [Google Scholar]
- [17].Lin Y; Wagner E; Lächelt U Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP. Biomater. Sci 2022, 10, 1166–1192. [DOI] [PubMed] [Google Scholar]
- [18].Wan T; Niu D; Wu CB; Xu FJ; Church G; Ping Y Material solutions for delivery of CRISPR/Cas-based genome editing tools: Current status and future outlook. Mater. Today 2019, 26, 40–66. [Google Scholar]
- [19].Valdmanis PN; Kay MA Future of rAAV gene therapy: Platform for RNAi, gene editing, and beyond. Hum. Gene Ther 2017, 28, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lyu P; Wang LX; Lu BS Virus-like particle mediated CRISPR/Cas9 delivery for efficient and safe genome editing. Life 2020, 10, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mukai H; Ogawa K; Kato N; Kawakami S Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics. Drug Metab. Pharmacokinet 2022, 44, 100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wei T; Cheng Q; Min YL; Olson EN; Siegwart DJ Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun 2020, 11, 3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ishino Y; Shinagawa H; Makino K; Amemura M; Nakata A Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol 1987, 169, 5429–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mojica FJM; Díez-Villaseñor C; Soria E; Juez G Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol 2000, 36, 244–246. [DOI] [PubMed] [Google Scholar]
- [25].Cong L; Ran FA; Cox D; Lin SL; Barretto R; Habib N; Hsu PD; Wu XB; Jiang WY; Marraffini LA et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gleditzsch D; Pausch P; Müller-Esparza H; Özcan A; Guo XH; Bange G; Randau L PAM identification by CRISPR-Cas effector complexes: Diversified mechanisms and structures. RNA Biol. 2019, 16, 504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Doench JG; Fusi N; Sullender M; Hegde M; Vaimberg EW; Donovan KF; Smith I; Tothova Z; Wilen C; Orchard R et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol 2016, 34, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jiang FG; Doudna JA The structural biology of CRISPR-Cas systems. Curr. Opin. Struct. Biol 2015, 30, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang JY; Pausch P; Doudna JA Structural biology of CRISPR-Cas immunity and genome editing enzymes. Nat. Rev. Microbiol 2022, 20, 641–656. [DOI] [PubMed] [Google Scholar]
- [30].Adli M The CRISPR tool kit for genome editing and beyond. Nat. Commun 2018, 9, 1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Paul B; Montoya G CRISPR-Cas12a: Functional overview and applications. Biomed. J 2020, 43, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morisaka H; Yoshimi K; Okuzaki Y; Gee P; Kunihiro Y; Sonpho E; Xu HG; Sasakawa N; Naito Y; Nakada S et al. CRISPR-Cas3 induces broad and unidirectional genome editing in human cells. Nat. Commun 2019, 10, 5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Colognori D; Trinidad M; Doudna JA Precise transcript targeting by CRISPR-Csm complexes. Nat. Biotechnol 2023, 41, 1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Porto EM; Komor AC; Slaymaker IM; Yeo GW Base editing: Advances and therapeutic opportunities. Nat. Rev. Drug Discov 2020, 19, 839–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen PJ; Liu DR Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet 2023, 24, 161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roman-Rodriguez FJ; Ugalde L; Álvarez L; Díez B; Ramirez MJ; Risueño C; Cortón M; Bogliolo M; Bernal S; March F et al. NHEJ-mediated repair of CRISPR-Cas9-induced DNA breaks efficiently corrects mutations in HSPCs from patients with Fanconi anemia. Cell Stem Cell 2019, 25, 607–621.e7. [DOI] [PubMed] [Google Scholar]
- [37].Musunuru K; Chadwick AC; Mizoguchi T; Garcia SP; DeNizio JE; Reiss CW; Wang K; Iyer S; Dutta C; Clendaniel V et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021, 593, 429–434 [DOI] [PubMed] [Google Scholar]
- [38].Stephenson AA; Nicolau S; Vetter TA; Dufresne GP; Frair EC; Sarff JE; Wheeler GL; Kelly BJ; White P; Flanigan KM CRISPR-Cas9 homology-independent targeted integration of exons 1-19 restores full-length dystrophin in mice. Mol. Ther. Methods Clin. Dev 2023, 30, 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Suzuki K; Tsunekawa Y; Hernandez-Benitez R; Wu J; Zhu J; Kim EJ; Hatanaka F; Yamamoto M; Araoka T; Li Z et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Slaymaker IM; Gao LY; Zetsche B; Scott DA; Yan WX; Zhang F Rationally engineered Cas9 nucleases with improved specificity. Science 2015, 351, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen JS; Dagdas YS; Kleinstiver BP; Welch MM; Sousa AA; Harrington LB; Sternberg SH; Joung JK; Yildiz A; Doudna JA Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 2017, 550, 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schubert MS; Thommandru B; Woodley J; Turk R; Yan SQ; Kurgan G; McNeill MS; Rettig GR Optimized design parameters for CRISPR Cas9 and Cas12a homology-directed repair. Sci. Rep 2021, 11, 19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu MJ; Rehman S; Tang XD; Gu K; Fan QL; Chen DK; Ma WT Methodologies for improving HDR efficiency. Front. Genet 2019, 9, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhao ZH; Shang P; Sage F; Geijsen N Ligation-assisted homologous recombination enables precise genome editing by deploying both MMEJ and HDR. Nucleic Acids Res. 2022, 50, e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shen B; Zhang WS; Zhang J; Zhou JK; Wang JY; Chen L; Wang L; Hodgkins A; Iyer V; Huang XX et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 2014, 11, 399–402. [DOI] [PubMed] [Google Scholar]
- [46].Mok BY; de Moraes MH; Zeng J; Bosch DE; Kotrys AV; Raguram A; Hsu F; Radey MC; Peterson SB; Mootha VK et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 2020, 583, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Komor AC; Kim YB; Packer MS; Zuris JA; Liu DR Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gaudelli NM; Komor AC; Rees HA; Packer MS; Badran AH; Bryson DI; Liu DR Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sakata RC; Ishiguro S; Mori H; Tanaka M; Tatsuno K; Ueda H; Yamamoto S; Seki M; Masuyama N; Nishida K et al. Base editors for simultaneous introduction of C-to-T and A-to-G mutations. Nat. Biotechnol 2020, 38, 865–869. [DOI] [PubMed] [Google Scholar]
- [50].Anzalone AV; Randolph PB; Davis JR; Sousa AA; Koblan LW; Levy JM; Chen PJ; Wilson C; Newby GA; Raguram A et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Adikusuma F; Lushington C; Arudkumar J; Godahewa GI; Chey YCJ; Gierus L; Piltz S; Geiger A; Jain Y; Reti D et al. Optimized nickase- and nuclease-based prime editing in human and mouse cells. Nucleic Acids Res. 2021, 49, 10785–10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nelson CE; Wu YY; Gemberling MP; Oliver ML; Waller MA; Bohning JD; Robinson-Hamm JN; Bulaklak K; Castellanos Rivera RM; Collier JH et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med 2019, 25, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ruan GX; Barry E; Yu D; Lukason M; Cheng SH; Scaria A CRISPR/Cas9-mediated genome editing as a therapeutic approach for Leber congenital amaurosis 10. Mol. Ther 2017, 25, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang D; Li J; Song CQ; Tran K; Mou HW; Wu PH; Tai PWL; Mendonca CA; Ren LZ; Wang BY et al. Cas9-mediated allelic exchange repairs compound heterozygous recessive mutations in mice. Nat. Biotechnol 2018, 36, 839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guo CT; Ma XT; Gao F; Guo YX Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol 2023, 11, 1143157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Abudayyeh OO; Gootenberg JS; Essletzbichler P; Han S; Joung J; Belanto JJ; Verdine V; Cox DBT; Kellner MJ; Regev A et al. RNA targeting with CRISPR-Cas13. Nature 2017, 550, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].East-Seletsky A; O'Connell MR; Knight SC; Burstein D; Cate JHD; Tjian R; Doudna JA Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 2016, 538, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Radzisheuskaya A; Shlyueva D; Müller I; Helin K Optimizing sgRNA position markedly improves the efficiency of CRISPR/dCas9-mediated transcriptional repression. Nucleic Acids Res. 2016, 44, e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kampmann M CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem. Biol 2018, 13, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Søndergaard JN; Geng KY; Sommerauer C; Atanasoai I; Yin XS; Kutter C Successful delivery of large-size CRISPR/Cas9 vectors in hard-to-transfect human cells using small plasmids. Commun. Biol 2020, 3, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang XM; Jin H; Huang XJ; Chaurasiya B; Dong DY; Shanley TP; Zhao YY Robust genome editing in adult vascular endothelium by nanoparticle delivery of CRISPR-Cas9 plasmid DNA. Cell Rep. 2022, 38, 110196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Miller JB; Zhang SY; Kos P; Xiong H; Zhou KJ; Perelman SS; Zhu H; Siegwart DJ Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem., Int. Ed 2017, 56, 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Luther DC; Lee YW; Nagaraj H; Scaletti F; Rotello VM Delivery approaches for CRISPR/Cas9 therapeutics in vivo: Advances and challenges. Expert Opin. Drug Deliv 2018, 15, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Xu CF; Chen GJ; Luo YL; Zhang Y; Zhao G; Lu ZD; Czarna A; Gu Z; Wang J Rational designs of in vivo CRISPR-Cas delivery systems. Adv. Drug Deliv. Rev 2021, 168, 3–29. [DOI] [PubMed] [Google Scholar]
- [65].DeWitt MA; Corn JE; Carroll D Genome. editing via delivery of Cas9 ribonucleoprotein. Methods 2017, 121–122, 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cheng H; Zhang F; Ding Y CRISPR/Cas9 delivery system engineering for genome editing in therapeutic applications. Pharmaceutics 2021, 13, 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].van Haasteren J; Li J; Scheideler OJ; Murthy N; Schaffer DV The delivery challenge: Fulfilling the promise of therapeutic genome editing. Nat. Biotechnol 2020, 38, 845–855. [DOI] [PubMed] [Google Scholar]
- [68].Bock C; Datlinger P; Chardon F; Coelho MA; Dong MB; Lawson KA; Lu T; Maroc L; Norman TM; Song B et al. High-content CRISPR screening. Nat. Rev. Methods Primers 2022, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Joung J; Ladha A; Saito M; Kim NG; Woolley AE; Segel M; Barretto RPJ; Ranu A; Macrae RK; Faure G et al. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N. Engl. J. Med 2020, 383, 1492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kellner MJ; Koob JG; Gootenberg JS; Abudayyeh OO; Zhang F SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc 2019, 14, 2986–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lee J; Sheen JH; Lim O; Lee Y; Ryu J; Shin D; Kim YY; Kim M Abrogation of HLA surface expression using CRISPR/Cas9 genome editing: A step toward universal T cell therapy. Sci. Rep 2020, 10, 17753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dimitri A; Herbst F; Fraietta JA Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022, 21, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Philippidis A CASGEVY makes history as FDA approves first CRISPR/Cas9 genome edited therapy. Hum. Gene Ther 2024, 35, 1–4. [DOI] [PubMed] [Google Scholar]
- [74].Mahmoud Ahmed NH; Lai MI The novel role of the B-cell lymphoma/leukemia 11A (BCL11A) gene in β-thalassaemia treatment. Cardiovasc. Hematol. Disord. Drug Targets 2023, 22, 226–236. [DOI] [PubMed] [Google Scholar]
- [75].Frangoul H; Locatelli F; Bhatia M; Mapara MY; Molinari L; Sharma A; Lobitz S; de Montalembert M; Rondelli D; Steinberg M et al. Efficacy and safety of a single dose of exagamglogene autotemcel for severe sickle cell disease. Blood 2022, 140, 29–31. [Google Scholar]
- [76].Condò I Rare monogenic diseases: Molecular pathophysiology and novel therapies. Int. J. Mol. Sci 2022, 23, 6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Long CZ; Amoasii L; Bassel-Duby R; Olson EN Genome editing of monogenic neuromuscular diseases: A systematic review. JAMA Neurol 2016, 73, 1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lek A; Wong B; Keeler A; Blackwood M; Ma KY; Huang SS; Sylvia K; Batista AR; Artinian R; Kokoski D et al. Death after high-dose rAAV9 gene therapy in a patient with Duchenne's muscular dystrophy. N. Engl. J. Med 2023, 389, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Maeder ML; Stefanidakis M; Wilson CJ; Baral R; Barrera LA; Bounoutas GS; Bumcrot D; Chao H; Ciulla DM; DaSilva JA et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med 2019, 25, 229–233. [DOI] [PubMed] [Google Scholar]
- [80].Gautam M; Jozic A; Su GLN; Herrera-Barrera M; Curtis A; Arrizabalaga S; Tschetter W; Ryals RC; Sahay G Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina. Nat. Commun 2023, 14, 6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gillmore JD; Gane E; Taubel J; Kao J; Fontana M; Maitland ML; Seitzer J; O'Connell D; Walsh KR; Wood K et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med 2021, 385, 493–502. [DOI] [PubMed] [Google Scholar]
- [82].Longhurst HJ; Lindsay K; Petersen RS; Fijen LM; Gurugama P; Maag D; Butler JS; Shah MY; Golden A; Xu YX et al. CRISPR-Cas9 in vivo gene editing of KLKB1 for hereditary angioedema. N. Engl. J. Med 2024, 390, 432–441. [DOI] [PubMed] [Google Scholar]
- [83].Lee RG; Mazzola AM; Braun MC; Platt C; Vafai SB; Kathiresan S; Rohde E; Bellinger AM; Khera AV Efficacy and safety of an investigational single-course CRISPR base-editing therapy targeting PCSK9 in nonhuman primate and mouse models. Circulation 2023, 147, 242–253. [DOI] [PubMed] [Google Scholar]
- [84].Kaminski R; Bella R; Yin C; Otte J; Ferrante P; Gendelman HE; Li H; Booze R; Gordon J; Hu W et al. Excision of HIV-1 DNA by gene editing: A proof-of-concept in vivo study. Gene Ther. 2016, 23, 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wei T; Cheng Q; Farbiak L; Anderson DG; Langer R; Siegwart DJ Delivery of tissue-targeted scalpels: Opportunities and challenges for in vivo CRISPR/Cas-based genome editing. ACS Nano 2020, 14, 9243–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Behr M; Zhou J; Xu B; Zhang HW In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges. Acta Pharm. Sin. B 2021, 11, 2150–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lu L; Li JR; Moussaoui M; Boix E Immune modulation by human secreted RNases at the extracellular space. Front. Immunol 2018, 9, 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rasul MF; Hussen BM; Salihi A; Ismael BS; Jalal PJ; Zanichelli A; Jamali E; Baniahmad A; Ghafouri-Fard S; Basiri A et al. Strategies to overcome the main challenges of the use of CRISPR/Cas9 as a replacement for cancer therapy. Mol. Cancer 2022, 21, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chew WL Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med 2018, 10, e1408. [DOI] [PubMed] [Google Scholar]
- [90].Li YM; Bolinger J; Yu YJ; Glass Z; Shi N; Yang L; Wang M; Xu QB Intracellular delivery and biodistribution study of CRISPR/Cas9 ribonucleoprotein loaded bioreducible lipidoid nanoparticles. Biomater. Sci 2019, 7, 596–606. [DOI] [PubMed] [Google Scholar]
- [91].Crudele JM; Chamberlain JS Cas9 immunity creates challenges for CRISPR gene editing therapies. Nat. Commun 2018, 9, 3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li L; Hu S; Chen XY Non-viral delivery systems for CRISPR/Cas9-based genome editing: Challenges and opportunities. Biomaterials 2018, 171, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Vicente MM; Chaves-Ferreira M; Jorge JMP; Proença JT; Barreto VM The off-targets of clustered regularly interspaced short palindromic repeats gene editing. Front. Cell Dev. Biol 2021, 9, 718466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Höijer I; Emmanouilidou A; Östlund R; van Schendel R; Bozorgpana S; Tijsterman M; Feuk L; Gyllensten U; den Hoed M; Ameur A CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations. Nat. Commun 2022, 13, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Garrood WT; Kranjc N; Petri K; Kim DY; Guo JA; Hammond AM; Morianou I; Pattanayak V; Joung JK; Crisanti A et al. Analysis of off-target effects in CRISPR-based gene drives in the human malaria mosquito. Proc. Natl. Acad. Sci. U S A 2021, 118, e2004838117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fu YF; Foden JA; Khayter C; Maeder ML; Reyon D; Joung JK; Sander JD High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol 2013, 31, 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gabriel R; Lombardo A; Arens A; Miller JC; Genovese P; Kaeppel C; Nowrouzi A; Bartholomae CC; Wang JB; Friedman G et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol 2011, 29, 816–823. [DOI] [PubMed] [Google Scholar]
- [98].Stemmer M; Thumberger T; Del Sol Keyer M; Wittbrodt J; Mateo JL CCTop: An intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS One 2015, 10, e0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chuai GH; Ma HH; Yan JF; Chen M; Hong NF; Xue DY; Zhou C; Zhu CY; Chen K; Duan B et al. DeepCRISPR: Optimized CRISPR guide RNA design by deep learning. Genome Biol. 2018, 19, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim D; Kim JS DIG-seq: A genome-wide CRISPR off-target profiling method using chromatin DNA. Genome Res. 2018, 28, 1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kim D; Bae S; Park J; Kim E; Kim S; Yu HR; Hwang J; Kim JI; Kim JS Digenome-seq: Genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods 2015, 12, 237–243. [DOI] [PubMed] [Google Scholar]
- [102].Park PJ ChIP-seq: Advantages and challenges of a maturing technology. Nat. Rev. Genet 2009, 10, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wienert B; Wyman SK; Richardson CD; Yeh CD; Akcakaya P; Porritt MJ; Morlock M; Vu JT; Kazane KR; Watry HL et al. Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science 2019, 364, 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tsai SQ; Zheng ZL; Nguyen NT; Liebers M; Topkar VV; Thapar V; Wyvekens N; Khayter C; Iafrate AJ; Le LP et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol 2015, 33, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liang SQ; Liu PP; Smith JL; Mintzer E; Maitland S; Dong XL; Yang QY; Lee J; Haynes CM; Zhu LJ et al. Genome-wide detection of CRISPR editing in vivo using GUIDE-tag. Nat. Commun 2022, 13, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kleinstiver BP; Pattanayak V; Prew MS; Tsai SQ; Nguyen NT; Zheng ZL; Joung JK High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ebrahimi V; Hashemi A Challenges of in vitro genome editing with CRISPR/Cas9 and possible solutions: A review. Gene 2020, 753, 144813. [DOI] [PubMed] [Google Scholar]
- [108].Ryan DE; Taussig D; Steinfeld I; Phadnis SM; Lunstad BD; Singh M; Vuong X; Okochi KD; McCaffrey R; Olesiak M et al. Improving CRISPR-Cas specificity with chemical modifications in single-guide RNAs. Nucleic Acids Res. 2018, 46, 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kim S; Kim D; Cho SW; Kim J; Kim JS Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mali P; Aach J; Stranges PB; Esvelt KM; Moosburner M; Kosuri S; Yang LH; Church GM CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol 2013, 31, 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ran FA; Hsu PD; Lin CY; Gootenberg JS; Konermann S; Trevino AE; Scott DA; Inoue A; Matoba S; Zhang Y et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wörner TP; Bennett A; Habka S; Snijder J; Friese O; Powers T; Agbandje-McKenna M; Heck AJR Adeno-associated virus capsid assembly is divergent and stochastic. Nat. Commun 2021, 12, 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sant'Anna TB; Araujo NM Adeno-associated virus infection and its impact in human health: An overview. Virol. J 2022, 19, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Naso MF; Tomkowicz B; Perry WL III; Strohl WR Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 2017, 31, 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Earley LF; Conatser LM; Lue VM; Dobbins AL; Li CW; Hirsch ML; Samulski RJ Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum. Gene Ther 2020, 31, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Berns KI The unusual properties of the AAV inverted terminal repeat. Hum. Gene Ther 2020, 31, 518–523. [DOI] [PubMed] [Google Scholar]
- [117].Wang D; Tai PWL; Gao GP Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov 2019, 18, 358–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bijlani S; Pang KM; Sivanandam V; Singh A; Chatterjee S The role of recombinant AAV in precise genome editing. Front. Genome Ed 2022, 3, 799722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sabatino DE; Bushman FD; Chandler RJ; Crystal RG; Davidson BL; Dolmetsch R; Eggan KC; Gao GP; Gil-Farina I; Kay MA et al. Evaluating the state of the science for adeno-associated virus integration: An integrated perspective. Mol. Ther 2022, 30, 2646–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Xu CL; Ruan MZC; Mahajan VB; Tsang SH Viral delivery systems for CRISPR. Viruses 2019, 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Castle MJ; Turunen HT; Vandenberghe LH; Wolfe JH Controlling AAV tropism in the nervous system with natural and engineered capsids. In Gene Therapy for Neurological Disorders Manfredsson FP, Ed.; Humana: New York, 2016, 1, pp 133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol 2016, 21, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Çerçi B; Uzay IA; Kara MK; Dinçer P Clinical trials and promising preclinical applications of CRISPR/Cas gene editing. Life Sci. 2023, 312, 121204. [DOI] [PubMed] [Google Scholar]
- [124].Shen S; Sanchez ME; Blomenkamp K; Corcoran EM; Marco E; Yudkoff CJ; Jiang HY; Teckman JH; Bumcrot D; Albright CF Amelioration of alpha-1 antitrypsin deficiency diseases with genome editing in transgenic mice. Hum. Gene Ther 2018, 29, 861–873. [DOI] [PubMed] [Google Scholar]
- [125].Wang D; Zhang F; Gao GP CRISPR-based therapeutic genome editing: Strategies and in vivo delivery by AAV vectors. Cell 2020, 181, 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wu ZJ; Yang HY; Colosi P Effect of genome size on AAV vector packaging. Mol. Ther 2010, 18, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Trapani I Adeno-associated viral vectors as a tool for large gene delivery to the retina. Genes 2019, 10, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Xu YY; Li ZJ CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J 2020, 18, 2401–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Madigan V; Zhang F; Dahlman JE Drug delivery systems for CRISPR-based genome editors. Nat. Rev. Drug Discov 2023, 22, 875–894. [DOI] [PubMed] [Google Scholar]
- [130].Tong S; Moyo B; Lee CM; Leong K; Bao G Engineered materials for in vivo delivery of genome-editing machinery. Nat. Rev. Mater 2019, 4, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chen XY; Gonçalves MAFV Engineered viruses as genome editing devices. Mol. Ther 2016, 24, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ibraheim R; Tai PWL; Mir A; Javeed N; Wang JM; Rodríguez TC; Namkung S; Nelson S; Khokhar ES; Mintzer E et al. Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo. Nat. Commun 2021, 12, 6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hillary VE; Ceasar SA A review on the mechanism and applications of CRISPR/Cas9/Cas12/Cas13/Cas14 proteins utilized for genome engineering. Mol. Biotechnol 2023, 65, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chavez M; Chen XY; Finn PB; Qi LS Advances in CRISPR therapeutics. Nat. Rev. Nephrol 2023, 19, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kim DY; Lee JM; Moon SB; Chin HJ; Park S; Lim Y; Kim D; Koo T; Ko JH; Kim YS Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat. Biotechnol 2022, 40, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Komor AC; Zhao KT; Packer MS; Gaudelli NM; Waterbury AL; Koblan LW; Kim YB; Badran AH; Liu DR Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C: G-to-T: A base editors with higher efficiency and product purity. Sci. Adv 2017, 3, eaao4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ghosh A; Yue YP; Lai Y; Duan DS A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol. Ther 2008, 16, 124–130. [DOI] [PubMed] [Google Scholar]
- [138].Ryu SM; Koo T; Kim K; Lim K; Baek G; Kim ST; Kim HS; Kim DE; Lee H; Chung E et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol 2018, 36, 536–539. [DOI] [PubMed] [Google Scholar]
- [139].Song YH; Lou HH; Boyer JL; Limberis MP; Vandenberghe LH; Hackett NR; Leopold PL; Wilson JM; Crystal RG Functional cystic fibrosis transmembrane conductance regulator expression in cystic fibrosis airway epithelial cells by AAV6.2-mediated segmental trans-splicing. Hum. Gene Ther 2009, 20, 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Riedmayr LM; Hinrichsmeyer KS; Thalhammer SB; Mittas DM; Karguth N; Otify DY; Böhm S; Weber VJ; Bartoschek MD; Splith V et al. mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy. Nat. Commun 2023, 14, 6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Villiger L; Grisch-Chan HM; Lindsay H; Ringnalda F; Pogliano CB; Allegri G; Fingerhut R; Häberle J; Matos J; Robinson MD et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med 2018, 24, 1519–1525. [DOI] [PubMed] [Google Scholar]
- [142].Aranko AS; Wlodawer A; Iwaï H Nature's recipe for splitting inteins. Protein Eng. Des. Sel 2014, 27, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Levy JM; Yeh WH; Pendse N; Davis JR; Hennessey E; Butcher R; Koblan LW; Comander J; Liu Q; Liu DR Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng 2020, 4, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Calcedo R; Morizono H; Wang LL; McCarter R; He JP; Jones D; Batshaw ML; Wilson JM Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol 2011, 18, 1586–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Charlesworth CT; Deshpande PS; Dever DP; Camarena J; Lemgart VT; Cromer MK; Vakulskas CA; Collingwood MA; Zhang LY; Bode NM et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med 2019, 25, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Simhadri VL; McGill J; McMahon S; Wang JX; Jiang HY; Sauna ZE Prevalence of pre-existing antibodies to CRISPR-associated nuclease Cas9 in the USA population. Mol. Ther. Methods Clin. Dev 2018, 10, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Manno CS; Pierce GF; Arruda VR; Glader B; Ragni M; Rasko JJE; Ozelo MC; Hoots K; Blatt P; Konkle B et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med 2006, 12, 342–347. [DOI] [PubMed] [Google Scholar]
- [148].Monteilhet V; Saheb S; Boutin S; Leborgne C; Veron P; Montus MF; Moullier P; Benveniste O; Masurier C A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol. Ther 2011, 19, 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chicoine LG; Montgomery CL; Bremer WG; Shontz KM; Griffin DA; Heller KN; Lewis S; Malik V; Grose WE; Shilling CJ et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther 2014, 22, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Mingozzi F; Anguela XM; Pavani G; Chen YF; Davidson RJ; Hui DJ; Yazicioglu M; Elkouby L; Hinderer CJ; Faella A et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med 2013, 5, 194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Mietzsch M; Barnes C; Hull JA; Chipman P; Xie J; Bhattacharya N; Sousa D; McKenna R; Gao GP; Agbandje-McKenna M Comparative analysis of the capsid structures of AAVrh.10, AAVrh.39, and AAV8. J. Virol 2020, 94, e01769–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Tse LV; Klinc KA; Madigan VJ; Castellanos Rivera RM; Wells LF; Havlik LP; Smith JK; Agbandje-McKenna M; Asokan A Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl. Acad. Sci. U S A 2017, 114, E4812–E4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Petry H; Brooks A; Orme A; Wang P; Liu P; Xie J; Kretschmer P; Qian HS; Hermiston TW; Harkins RN Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice. Gene Ther 2008, 15, 54–60. [DOI] [PubMed] [Google Scholar]
- [154].Corti M; Cleaver B; Clément N; Conlon TJ; Faris KJ; Wang GS; Benson J; Tarantal AF; Fuller D; Herzog RW et al. Evaluation of readministration of a recombinant adeno-associated virus vector expressing acid alpha-glucosidase in Pompe disease: Preclinical to clinical planning. Hum. Gene Ther. Clin. Dev 2015, 26, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Meliani A; Boisgerault F; Hardet R; Marmier S; Collaud F; Ronzitti G; Leborgne C; Costa Verdera H; Simon Sola M; Charles S et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun 2018, 9, 4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Rogers GL; Martino AT; Aslanidi GV; Jayandharan GR; Srivastava A; Herzog RW Innate immune responses to AAV vectors. Front. Microbiol 2011, 2, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Chan YK; Wang SK; Chu CJ; Copland DA; Letizia AJ; Costa Verdera H; Chiang JJ; Sethi M; Wang MK; Neidermyer WJ Jr. et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med 2021, 13, eabd3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Faust SM; Bell P; Cutler BJ; Ashley SN; Zhu YQ; Rabinowitz JE; Wilson JM CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest 2013, 123, 2994–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].de Solis CA; Ho A; Holehonnur R; Ploski JE The development of a viral mediated CRISPR/Cas9 system with doxycycline dependent gRNA expression for inducible in vitro and in vivo genome editing. Front. Mol. Neurosci 2016, 9, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zhang JF; Chen L; Zhang J; Wang Y Drug inducible CRISPR/Cas systems. Comput. Struct. Biotechnol. J 2019, 17, 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Li A; Lee CM; Hurley AE; Jarrett KE; De Giorgi M; Lu WQ; Balderrama KS; Doerfler AM; Deshmukh H; Ray A et al. A self-deleting AAV-CRISPR system for in vivo genome editing. Mol. Ther. Methods Clin. Dev 2019, 12, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lagos-Quintana M; Rauhut R; Yalcin A; Meyer J; Lendeckel W; Tuschl T Identification of tissue-specific microRNAs from mouse. Curr. Biol 2002, 12, 735–739. [DOI] [PubMed] [Google Scholar]
- [163].Chen JF; Mandel EM; Thomson JM; Wu QL; Callis TE; Hammond SM; Conlon FL; Wang DZ The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet 2006, 38, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hoffmann MD; Aschenbrenner S; Grosse S; Rapti K; Domenger C; Fakhiri J; Mastel M; Börner K; Eils R; Grimm D et al. Cell-specific CRISPR-Cas9 activation by microRNA-dependent expression of anti-CRISPR proteins. Nucleic Acids Res. 2019, 47, e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lee J; Mou HW; Ibraheim R; Liang SQ; Liu PP; Xue W; Sontheimer EJ Tissue-restricted genome editing in vivo specified by microRNA-repressible anti-CRISPR proteins. RNA 2019, 25, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Issa SS; Shaimardanova AA; Solovyeva VV; Rizvanov AA Various AAV serotypes and their applications in gene therapy: An overview. Cells 2023, 12, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Li CW; Samulski RJ Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet 2020, 21, 255–272. [DOI] [PubMed] [Google Scholar]
- [168].Jang MJ; Coughlin GM; Jackson CR; Chen XH; Chuapoco MR; Vendemiatti JL; Wang AZ; Gradinaru V Spatial transcriptomics for profiling the tropism of viral vectors in tissues. Nat. Biotechnol 2023, 41, 1272–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Lau CH; Ho JWT; Lo PK; Tin C Targeted transgene activation in the brain tissue by systemic delivery of engineered AAV1 expressing CRISPRa. Mol. Ther. Nucleic Acids 2019, 16, 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Münch RC; Janicki H; Völker I; Rasbach A; Hallek M; Büning H; Buchholz CJ Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol. Ther 2013, 21, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Boucher P; Cui XX; Curiel DT Adenoviral vectors for in vivo delivery of CRISPR-Cas gene editors. J. Control. Release 2020, 327, 788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sayedahmed EE; Kumari R; Mittal SK Current use of adenovirus vectors and their production methods. In Viral Vectors for Gene Therapy . Manfredsson FP; Benskey MJ, Eds.; Humana: New York, 2019, 1, pp, 155–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Watanabe M; Nishikawaji Y; Kawakami H; Kosai KI Adenovirus biology, recombinant adenovirus, and adenovirus usage in gene therapy. Viruses 2021, 13, 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Danthinne X; Imperiale MJ Production of first generation adenovirus vectors: A review. Gene Ther. 2000, 7, 1707–1714. [DOI] [PubMed] [Google Scholar]
- [175].Wold WSM; Toth K Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther 2013, 13, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Alba R; Bosch A; Chillon M Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005, 12, S18–S27. [DOI] [PubMed] [Google Scholar]
- [177].Ding QR; Strong A; Patel KM; Ng SL; Gosis BS; Regan SN; Cowan CA; Rader DJ; Musunuru K Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ. Res 2014, 115, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Xu L; Park KH; Zhao L; Xu J; El Refaey M; Gao YD; Zhu H; Ma JJ; Han R CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol. Ther 2016, 24, 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wang HJ; Liu Y; Li ZY; Tuve S; Stone D; Kalyushniy O; Shayakhmetov D; Verlinde CLM; Stehle T; McVey J et al. In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. J. Virol 2008, 82, 10567–10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Li C; Psatha N; Sova P; Gil S; Wang HJ; Kim J; Kulkarni C; Valensisi C; Hawkins RD; Stamatoyannopoulos G et al. Reactivation of γ-globin in adult β-YAC mice after ex vivo and in vivo hematopoietic stem cell genome editing. Blood 2018, 131, 2915–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Federico M From lentiviruses to lentivirus vectors. In Lentivirus Gene Engineering Protocols, . Federico M, Ed.; Humana: Totowa, 2003, 1, 3–15 [DOI] [PubMed] [Google Scholar]
- [182].Mátrai J; Chuah MKL; VandenDriessche T Recent advances in lentiviral vector development and applications. Mol. Ther 2010, 18, 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Milone MC; O'Doherty U Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Sakuma T; Barry MA; Ikeda Y Lentiviral vectors: Basic to translational. Biochem. J 2012, 443, 603–618. [DOI] [PubMed] [Google Scholar]
- [185].Shirley JL; de Jong YP; Terhorst C; Herzog RW Immune responses to viral gene therapy vectors. Mol. Ther 2020, 28, 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Kotterman MA; Chalberg TW; Schaffer DV Viral vectors for gene therapy: Translational and clinical outlook. Annu. Rev. Biomed. Eng 2015, 17, 63–89. [DOI] [PubMed] [Google Scholar]
- [187].Yew CHT; Gurumoorthy N; Nordin F; Tye GJ; Wan Kamarul Zaman WS; Tan JJ; Ng MH Integrase deficient lentiviral vector: Prospects for safe clinical applications. PeerJ 2022, 10, e13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Ortinski PI; O'Donovan B; Dong XY; Kantor B Integrase-deficient lentiviral vector as an all-in-one platform for highly efficient CRISPR/Cas9-mediated gene editing. Mol. Ther. Methods Clin. Dev 2017, 5, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Nooraei S; Bahrulolum H; Hoseini ZS; Katalani C; Hajizade A; Easton AJ; Ahmadian G Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol 2021, 19, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lyu P; Lu BS New advances in using virus-like particles and related technologies for eukaryotic genome editing delivery. Int. J. Mol. Sci 2022, 23, 8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Bell NM; Lever AML HIV Gag polyprotein: Processing and early viral particle assembly. Trends Microbiol. 2013, 21, 136–144. [DOI] [PubMed] [Google Scholar]
- [192].He JY; Yu LY; Lin XD; Liu XY; Zhang YM; Yang F; Deng W Virus-like particles as nanocarriers for intracellular delivery of biomolecules and compounds. Viruses 2022, 14, 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Austin RJ; Xia TB; Ren JS; Takahashi TT; Roberts RW Designed arginine-rich RNA-binding peptides with picomolar affinity. J. Am. Chem. Soc 2002, 124, 10966–10967. [DOI] [PubMed] [Google Scholar]
- [194].Wulczyn FG; Kahmann R Translational stimulation: RNA sequence and structure requirements for binding of Com protein. Cell 1991, 65, 259–269. [DOI] [PubMed] [Google Scholar]
- [195].Fouts DE; True HL; Celander DW Functional recognition of fragmented operator sites by R17/MS2 coat protein, a translational repressor. Nucleic Acids Res. 1997, 25, 4464–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Lim F; Downey TP; Peabody DS Translational repression and specific RNA binding by the coat protein of the Pseudomonas phage PP7. J. Biol. Chem 2001, 276, 22507–22513. [DOI] [PubMed] [Google Scholar]
- [197].Knopp Y; Geis FK; Heckl D; Horn S; Neumann T; Kuehle J; Meyer J; Fehse B; Baum C; Morgan M et al. Transient retrovirus-based CRISPR/Cas9 all-in-one particles for efficient, targeted gene knockout. Mol. Ther. Nucleic Acids 2018, 13, 256–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Baron Y; Sens J; Lange L; Nassauer L; Klatt D; Hoffmann D; Kleppa MJ; Barbosa PV; Keisker M; Steinberg V et al. Improved alpharetrovirus-based Gag.MS2 particles for efficient and transient delivery of CRISPR-Cas9 into target cells. Mol. Ther. Nucleic Acids 2022, 27, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Segel M; Lash B; Song JW; Ladha A; Liu CC; Jin X; Mekhedov SL; Macrae RK; Koonin EV; Zhang F Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Ling SK; Yang SQ; Hu XD; Yin D; Dai Y; Qian XQ; Wang DW; Pan XY; Hong JX; Sun XD et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng 2021, 5, 144–156. [DOI] [PubMed] [Google Scholar]
- [201].Yin D; Ling SK; Wang DW; Dai Y; Jiang H; Zhou XJ; Paludan SR; Hong JX; Cai YJ Targeting herpes simplex virus with CRISPR-Cas9 cures herpetic stromal keratitis in mice. Nat. Biotechnol 2021, 39, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hamann MV; Stanke N; Müllers E; Stirnnagel K; Hütter S; Artegiani B; Bragado Alonso S; Calegari F; Lindemann D Efficient transient genetic manipulation in vitro and in vivo by prototype foamy virus-mediated nonviral RNA transfer. Mol. Ther 2014, 22, 1460–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lindel F; Dodt CR; Weidner N; Noll M; Bergemann F; Behrendt R; Fischer S; Dietrich J; Cartellieri M; Hamann MV et al. TraFo-CRISPR: Enhanced genome engineering by transient foamy virus vector-mediated delivery of CRISPR/Cas9 components. Mol. Ther. Nucleic Acids 2019, 18, 708–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Zhang S; Shen JT; Li DL; Cheng YY Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 2021, 11, 614–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Lyu P; Javidi-Parsijani P; Atala A; Lu BS Delivering Cas9/sgRNA ribonucleoprotein (RNP) by lentiviral capsid-based bionanoparticles for efficient 'hit-and-run' genome editing. Nucleic Acids Res. 2019, 47, e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lu ZY; Yao XG; Lyu P; Yadav M; Yoo K; Atala A; Lu BS Lentiviral capsid-mediated Streptococcus pyogenes Cas9 ribonucleoprotein delivery for efficient and safe multiplex genome editing. CRISPR J. 2021, 4, 914–928. [DOI] [PubMed] [Google Scholar]
- [207].Lyu P; Lu ZY; Cho SI; Yadav M; Yoo KW; Atala A; Kim JS; Lu BS Adenine base editor ribonucleoproteins delivered by lentivirus-like particles show high on-target base editing and undetectable RNA off-target activities. CRISPR J. 2021, 4, 69–81. [DOI] [PubMed] [Google Scholar]
- [208].Banaszynski LA; Liu CW; Wandless TJ Characterization of the FKBP•rapamycin•FRB ternary complex. J. Am. Chem. Soc 2005, 127, 4715–4721. [DOI] [PubMed] [Google Scholar]
- [209].Gee P; Lung MSY; Okuzaki Y; Sasakawa N; Iguchi T; Makita Y; Hozumi H; Miura Y; Yang LF; Iwasaki M et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun 2020, 11, 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Stark LA; Hay RT Human immunodeficiency virus type 1 (HIV-1) viral protein R (Vpr) interacts with Lys-tRNA synthetase: Implications for priming of HIV-1 reverse transcription. J. Virol 1998, 72, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Müller B; Tessmer U; Schubert U; Krausslich HG Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than gag and is phosphorylated in infected cells. J. Virol 2000, 74, 9727–9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Indikova I; Indik S Highly efficient 'hit-and-run' genome editing with unconcentrated lentivectors carrying Vpr.Prot.Cas9 protein produced from RRE-containing transcripts. Nucleic Acids Res. 2020, 48, 8178–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Mangeot PE; Risson V; Fusil F; Marnef A; Laurent E; Blin J; Mournetas V; Massouridès E; Sohier TJM; Corbin A et al. Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nat. Commun 2019, 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Banskota S; Raguram A; Suh S; Du SW; Davis JR; Choi EH; Wang X; Nielsen SC; Newby GA; Randolph PB et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].An MR; Raguram A; Du SW; Banskota S; Davis JR; Newby GA; Chen PZ; Palczewski K; Liu DR Engineered virus-like particles for transient delivery of prime editor ribonucleoprotein complexes in vivo. Nat. Biotechnol, in press, DOI: 10.1038/s41587-023-02078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Hamilton JR; Chen E; Perez BS; Sandoval Espinoza CR; Kang MH; Trinidad M; Ngo W; Doudna JA In vivo human T cell engineering with enveloped delivery vehicles. Nat. Biotechnol, in press, DOI: 10.1038/s41587-023-02085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Jeong M; Lee Y; Park J; Jung H; Lee H Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv. Drug Deliv. Rev 2023, 200, 114990. [DOI] [PubMed] [Google Scholar]
- [218].Hald Albertsen C; Kulkarni JA; Witzigmann D; Lind M; Petersson K; Simonsen JB The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev 2022, 188, 114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Holland JW; Hui C; Cullis PR; Madden TD Poly(ethylene glycol)–lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry 1996, 35, 2618–2624. [DOI] [PubMed] [Google Scholar]
- [220].Suzuki T; Suzuki Y; Hihara T; Kubara K; Kondo K; Hyodo K; Yamazaki K; Ishida T; Ishihara H PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: Faster PEG shedding attenuates anti-PEG IgM production. Int. J. Pharm 2020, 588, 119792. [DOI] [PubMed] [Google Scholar]
- [221].Lokugamage MP; Vanover D; Beyersdorf J; Hatit MZC; Rotolo L; Echeverri ES; Peck HE; Ni HZ; Yoon JK; Kim Y et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng 2021, 5, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Kulkarni JA; Witzigmann D; Leung J; Tam YYC; Cullis PR On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [DOI] [PubMed] [Google Scholar]
- [223].Kauffman KJ; Dorkin JR; Yang JH; Heartlein MW; DeRosa F; Mir FF; Fenton OS; Anderson DG Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015, 15, 7300–7306. [DOI] [PubMed] [Google Scholar]
- [224].Mui BL; Tam YK; Jayaraman M; Ansell SM; Du XY; Tam YYC; Lin PJC; Chen S; Narayanannair JK; Rajeev KG et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Zhou H; Fan ZY; Li PY; Deng JJ; Arhontoulis DC; Li CY; Bowne WB; Cheng H Dense and dynamic polyethylene glycol shells cloak nanoparticles from uptake by liver endothelial cells for long blood circulation. ACS Nano 2018, 12, 10130–10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Sun D; Lu ZR Structure and function of cationic and ionizable lipids for nucleic acid delivery. Pharm. Res 2023, 40, 27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Lv HT; Zhang SB; Wang B; Cui SH; Yan J Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [DOI] [PubMed] [Google Scholar]
- [228].Ma Z; Li J; He FT; Wilson A; Pitt B; Li S Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem. Biophys. Res. Commun 2005, 330, 755–759. [DOI] [PubMed] [Google Scholar]
- [229].Wang X; Liu S; Sun YH; Yu XL; Lee SM; Cheng Q; Wei T; Gong JY; Robinson J; Zhang D et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc 2023, 18, 265–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Semple SC; Klimuk SK; Harasym TO; Dos Santos N; Ansell SM; Wong KF; Maurer N; Stark H; Cullis PR; Hope MJ et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166. [DOI] [PubMed] [Google Scholar]
- [231].Schlich M; Palomba R; Costabile G; Mizrahy S; Pannuzzo M; Peer D; Decuzzi P Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med 2021, 6, e10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Han XX; Zhang HW; Butowska K; Swingle KL; Alameh MG; Weissman D; Mitchell MJ An ionizable lipid toolbox for RNA delivery. Nat. Commun 2021, 12, 7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Zhang LM; Wang P; Feng Q; Wang NX; Chen ZT; Huang YY; Zheng WF; Jiang XY Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017, 9, e441. [Google Scholar]
- [234].Li CH; Yang TR; Weng YH; Zhang MJ; Zhao DY; Guo S; Hu B; Shao WX; Wang XX; Hussain A et al. Ionizable lipid-assisted efficient hepatic delivery of gene editing elements for oncotherapy. Bioact. Mater 2022, 9, 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Doudna JA; Charpentier E The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [DOI] [PubMed] [Google Scholar]
- [236].Liu C; Zhang L; Liu H; Cheng K Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Control. Release 2017, 266, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zuris JA; Thompson DB; Shu YL; Guilinger JP; Bessen JL; Hu JH; Maeder ML; Joung JK; Chen ZY; Liu DR Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol 2015, 33, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Wang M; Zuris JA; Meng FT; Rees H; Sun S; Deng P; Han Y; Gao X; Pouli D; Wu Q et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc. Natl. Acad. Sci. U S A 2016, 113, 2868–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Walther J; Wilbie D; Tissingh VSJ; Öktem M; van der Veen H; Lou B; Mastrobattista E Impact of formulation conditions on lipid nanoparticle characteristics and functional delivery of CRISPR RNP for gene knock-out and correction. Pharmaceutics 2022, 14, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Suzuki Y; Onuma H; Sato R; Sato Y; Hashiba A; Maeki M; Tokeshi M; Kayesh MEH; Kohara M; Tsukiyama-Kohara K et al. Lipid nanoparticles loaded with ribonucleoprotein-oligonucleotide complexes synthesized using a microfluidic device exhibit robust genome editing and hepatitis B virus inhibition. J. Control. Release 2021, 330, 61–71. [DOI] [PubMed] [Google Scholar]
- [241].Sato Y; Hashiba K; Sasaki K; Maeki M; Tokeshi M; Harashima H Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J. Control. Release 2019, 295, 140–152. [DOI] [PubMed] [Google Scholar]
- [242].Sato Y; Okabe N; Note Y; Hashiba K; Maeki M; Tokeshi M; Harashima H Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020, 102, 341–350. [DOI] [PubMed] [Google Scholar]
- [243].Walther J; Porenta D; Wilbie D; Seinen C; Benne N; Yang QB; de Jong OG; Lei ZY; Mastrobattista E Comparative analysis of lipid nanoparticle-mediated delivery of CRISPR-Cas9 RNP versus mRNA/sgRNA for gene editing in vitro and in vivo. Eur. J. Pharm. Biopharm 2024, 196, 114207. [DOI] [PubMed] [Google Scholar]
- [244].Müller JA; Schäffler N; Kellerer T; Schwake G; Ligon TS; Rädler JO Kinetics of RNA-LNP delivery and protein expression. Eur. J. Pharm. Biopharm 2024, 197, 114222. [DOI] [PubMed] [Google Scholar]
- [245].Wadhwa A; Aljabbari A; Lokras A; Foged C; Thakur A Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Qiu M; Glass Z; Chen JJ; Haas M; Jin X; Zhao XW; Rui XH; Ye ZF; Li YM; Zhang F et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. U S A 2021, 118, e2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Ingle RG; Fang WJ An overview of the stability and delivery challenges of commercial nucleic acid therapeutics. Pharmaceutics 2023, 15, 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Chen QB; Zhang Y; Yin H Recent advances in chemical modifications of guide RNA, mRNA and donor template for CRISPR-mediated genome editing. Adv. Drug Deliv. Rev 2021, 168, 246–258. [DOI] [PubMed] [Google Scholar]
- [249].Furuichi Y; Shatkin AJ Viral and cellular mRNA capping: Past and prospects. Adv. Virus Res 2000, 55, 135–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Devarkar SC; Wang C; Miller MT; Ramanathan A; Jiang FG; Khan AG; Patel SS; Marcotrigiano J Structural basis for m7G recognition and 2'-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl. Acad. Sci. U S A 2016, 113, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Züst R; Cervantes-Barragan L; Habjan M; Maier R; Neuman BW; Ziebuhr J; Szretter KJ; Baker SC; Barchet W; Diamond MS et al. Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol 2011, 12, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Vaidyanathan S; Azizian KT; Haque AKMA; Henderson JM; Hendel A; Shore S; Antony JS; Hogrefe RI; Kormann MSD; Porteus MH et al. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther. Nucleic Acids 2018, 12, 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Dellinger DJ; Timár Z; Myerson J; Sierzchala AB; Turner J; Ferreira F; Kupihar Z; Dellinger G; Hill KW; Powell JA et al. Streamlined process for the chemical synthesis of RNA using 2’-O-thionocarbamate-protected nucleoside phosphoramidites in the solid phase. J. Am. Chem. Soc 2011, 133, 11540–11556. [DOI] [PubMed] [Google Scholar]
- [254].Scaringe SA; Wincott FE; Caruthers MH Novel RNA synthesis method using 5’-O-silyl-2’-O-orthoester protecting groups. J. Am. Chem. Soc 1998, 120, 11820–11821. [Google Scholar]
- [255].He KZ; Chou ET; Begay S; Anderson EM; van Brabant Smith A Conjugation and evaluation of triazole-linked single guide RNA for CRISPR-Cas9 gene editing. ChemBioChem 2016, 17, 1809–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Taemaitree L; Shivalingam A; El-Sagheer AH; Brown T An artificial triazole backbone linkage provides a split-and-click strategy to bioactive chemically modified CRISPR sgRNA. Nat. Commun 2019, 10, 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Lennox KA; Behlke MA Chemical modifications in RNA interference and CRISPR/Cas genome editing reagents. In RNA Interference and CRISPR Technologies; Sioud M, Ed.; Humana: New York, 2020; Vol. 1, pp 23–55 [DOI] [PubMed] [Google Scholar]
- [258].Karikó K; Muramatsu H; Welsh FA; Ludwig J; Kato H; Akira S; Weissman D Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther 2008, 16, 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Mir A; Alterman JF; Hassler MR; Debacker AJ; Hudgens E; Echeverria D; Brodsky MH; Khvorova A; Watts JK; Sontheimer EJ Heavily and fully modified RNAs guide efficient SpyCas9-mediated genome editing. Nat. Commun 2018, 9, 2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Cromwell CR; Sung K; Park J; Krysler AR; Jovel J; Kim SK; Hubbard BP Incorporation of bridged nucleic acids into CRISPR RNAs improves Cas9 endonuclease specificity. Nat. Commun 2018, 9, 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Zhou WY; Brown W; Bardhan A; Delaney M; Ilk AS; Rauen RR; Kahn SI; Tsang M; Deiters A Spatiotemporal control of CRISPR/Cas9 function in cells and zebrafish using light-activated guide RNA. Angew. Chem., Int. Ed 2020, 59, 8998–9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Yin H; Song CQ; Suresh S; Wu QQ; Walsh S; Rhym LH; Mintzer E; Bolukbasi MF; Zhu LJ; Kauffman K et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol 2017, 35, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Hendel A; Bak RO; Clark JT; Kennedy AB; Ryan DE; Roy S; Steinfeld I; Lunstad BD; Kaiser RJ; Wilkens AB et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol 2015, 33, 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Kopac T Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol 2021, 169, 290–301. [DOI] [PubMed] [Google Scholar]
- [265].Akinc A; Querbes W; De S; Qin JN; Frank-Kamenetsky M; Jayaprakash KN; Jayaraman M; Rajeev KG; Cantley WL; Dorkin JR et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther 2010, 18, 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Getz GS; Reardon CA Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J. Lipid Res 2009, 50, S156–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Sato Y; Kinami Y; Hashiba K; Harashima H Different kinetics for the hepatic uptake of lipid nanoparticles between the apolipoprotein E/low density lipoprotein receptor and the N-acetyl-D-galactosamine/asialoglycoprotein receptor pathway. J. Control. Release 2020, 322, 217–226. [DOI] [PubMed] [Google Scholar]
- [268].Pardi N; Tuyishime S; Muramatsu H; Kariko K; Mui BL; Tam YK; Madden TD; Hope MJ; Weissman D Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Zhuo CY; Zhang JB; Lee JH; Jiao J; Cheng D; Liu L; Kim HW; Tao Y; Li MQ Spatiotemporal control of CRISPR/Cas9 gene editing. Signal Transduct. Target. Ther 2021, 6, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Mirjalili Mohanna SZ; Djaksigulova D; Hill AM; Wagner PK; Simpson EM; Leavitt BR LNP-mediated delivery of CRISPR RNP for wide-spread in vivo genome editing in mouse cornea. J. Control. Release 2022, 350, 401–413. [DOI] [PubMed] [Google Scholar]
- [271].Sheikh O; Yokota T Developing DMD therapeutics: A review of the effectiveness of small molecules, stop-codon readthrough, dystrophin gene replacement, and exon-skipping therapies. Expert Opin. Investig. Drugs 2021, 30, 167–176. [DOI] [PubMed] [Google Scholar]
- [272].Kenjo E; Hozumi H; Makita Y; Iwabuchi KA; Fujimoto N; Matsumoto S; Kimura M; Amano Y; Ifuku M; Naoe Y et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun 2021, 12, 7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Cheng Q; Wei T; Farbiak L; Johnson LT; Dilliard SA; Siegwart DJ Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol 2020, 15, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Huang Y; Wang J; Jiang KR; Chung EJ Improving kidney targeting: The influence of nanoparticle physicochemical properties on kidney interactions. J. Control. Release 2021, 334, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].McCright J; Naiknavare R; Yarmovsky J; Maisel K Targeting lymphatics for nanoparticle drug delivery. Front. Pharmacol 2022, 13, 887402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Guo P; Liu DX; Subramanyam K; Wang BR; Yang J; Huang J; Auguste DT; Moses MA Nanoparticle elasticity directs tumor uptake. Nat. Commun 2018, 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Park SJ Protein-nanoparticle interaction: Corona formation and conformational changes in proteins on nanoparticles. Int. J. Nanomedicine 2020, 15, 5783–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Zhang R; El-Mayta R; Murdoch TJ; Warzecha CC; Billingsley MM; Shepherd SJ; Gong NQ; Wang LL; Wilson JM; Lee D et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci 2021, 9, 1449–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Rosenblum D; Gutkin A; Kedmi R; Ramishetti S; Veiga N; Jacobi AM; Schubert MS; Friedmann-Morvinski D; Cohen ZR; Behlke MA et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv 2020, 6, eabc9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Qin JY; Xue LL; Gong NQ; Zhang HW; Shepherd SJ; Haley RM; Swingle KL; Mitchell MJ RGD peptide-based lipids for targeted mRNA delivery and gene editing applications. RSC Adv. 2022, 12, 25397–25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Ramishetti S; Kedmi R; Goldsmith M; Leonard F; Sprague AG; Godin B; Gozin M; Cullis PR; Dykxhoorn DM; Peer D Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano 2015, 9, 6706–6716. [DOI] [PubMed] [Google Scholar]
- [282].Liang C; Guo BS; Wu H; Shao NS; Li DF; Liu J; Dang L; Wang C; Li H; Li SH et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med 2015, 21, 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Kedmi R; Veiga N; Ramishetti S; Goldsmith M; Rosenblum D; Dammes N; Hazan-Halevy I; Nahary L; Leviatan-Ben-Arye S; Harlev M et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol 2018, 13, 214–219. [DOI] [PubMed] [Google Scholar]
- [284].Zeballos CMA; Gaj T Next-generation CRISPR technologies and their applications in gene and cell therapy. Trends Biotechnol. 2021, 39, 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Srivastava A; Mallela KMG; Deorkar N; Brophy G Manufacturing challenges and rational formulation development for AAV viral vectors. J. Pharm. Sci 2021, 110, 2609–2624. [DOI] [PubMed] [Google Scholar]
- [286].Vicente T; Roldão A; Peixoto C; Carrondo MJT; Alves PM Large-scale production and purification of VLP-based vaccines. J. Invertebr. Pathol 2011, 107, S42–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Maeki M; Okada Y; Uno S; Sugiura K; Suzuki Y; Okuda K; Sato Y; Ando M; Yamazaki H; Takeuchi M et al. Mass production system for RNA-loaded lipid nanoparticles using piling up microfluidic devices. Appl. Mater. Today 2023, 31, 101754. [Google Scholar]
- [288].Shepherd SJ; Han XX; Mukalel AJ; El-Mayta R; Thatte AS; Wu JY; Padilla MS; Alameh MG; Srikumar N; Lee D et al. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proc. Natl. Acad. Sci. U S A 2023, 120, e2303567120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Duan L; Ouyang K; Wang JH; Xu LM; Xu X; Wen CN; Xie YX; Liang YJ; Xia J Exosomes as targeted delivery platform of CRISPR/Cas9 for therapeutic genome editing. ChemBioChem 2021, 22, 3360–3368. [DOI] [PubMed] [Google Scholar]
- [290].Fang RH; Kroll AV; Gao WW; Zhang LF Cell membrane coating nanotechnology. Adv. Mater 2018, 30, 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Hu CM; Fang RH; Wang KC; Luk BT; Thamphiwatana S; Dehaini D; Nguyen P; Angsantikul P; Wen CH; Kroll AV et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Ying M; Zhuang J; Wei XL; Zhang XX; Zhang Y; Jiang Y; Dehaini D; Chen MC; Gu SL; Gao WW et al. Remote-loaded platelet vesicles for disease-targeted delivery of therapeutics. Adv. Funct. Mater 2018, 28, 1801032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Zhang QZ; Dehaini D; Zhang Y; Zhou JL; Chen XY; Zhang LD; Fang RH; Gao WW; Zhang LF Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol 2018, 13, 1182–1190. [DOI] [PubMed] [Google Scholar]
- [294].Guo ZY; Zhou JR; Yu YY; Krishnan N; Noh I; Zhu AT; Borum RM; Gao WW; Fang RH; Zhang LF Immunostimulatory DNA hydrogel enhances protective efficacy of nanotoxoids against bacterial infection. Adv. Mater 2023, 35, 2211717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Yoo JW; Irvine DJ; Discher DE; Mitragotri S Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov 2011, 10, 521–535. [DOI] [PubMed] [Google Scholar]


